Biological Effects of New Hydraulic Materials on Human Periodontal Ligament Stem Cells
Abstract
:1. Introduction
2. Material and Methods
2.1. Material Extracts
2.2. Ion Release Analysis and pH
2.3. Isolation and Culture of hPDLSCs
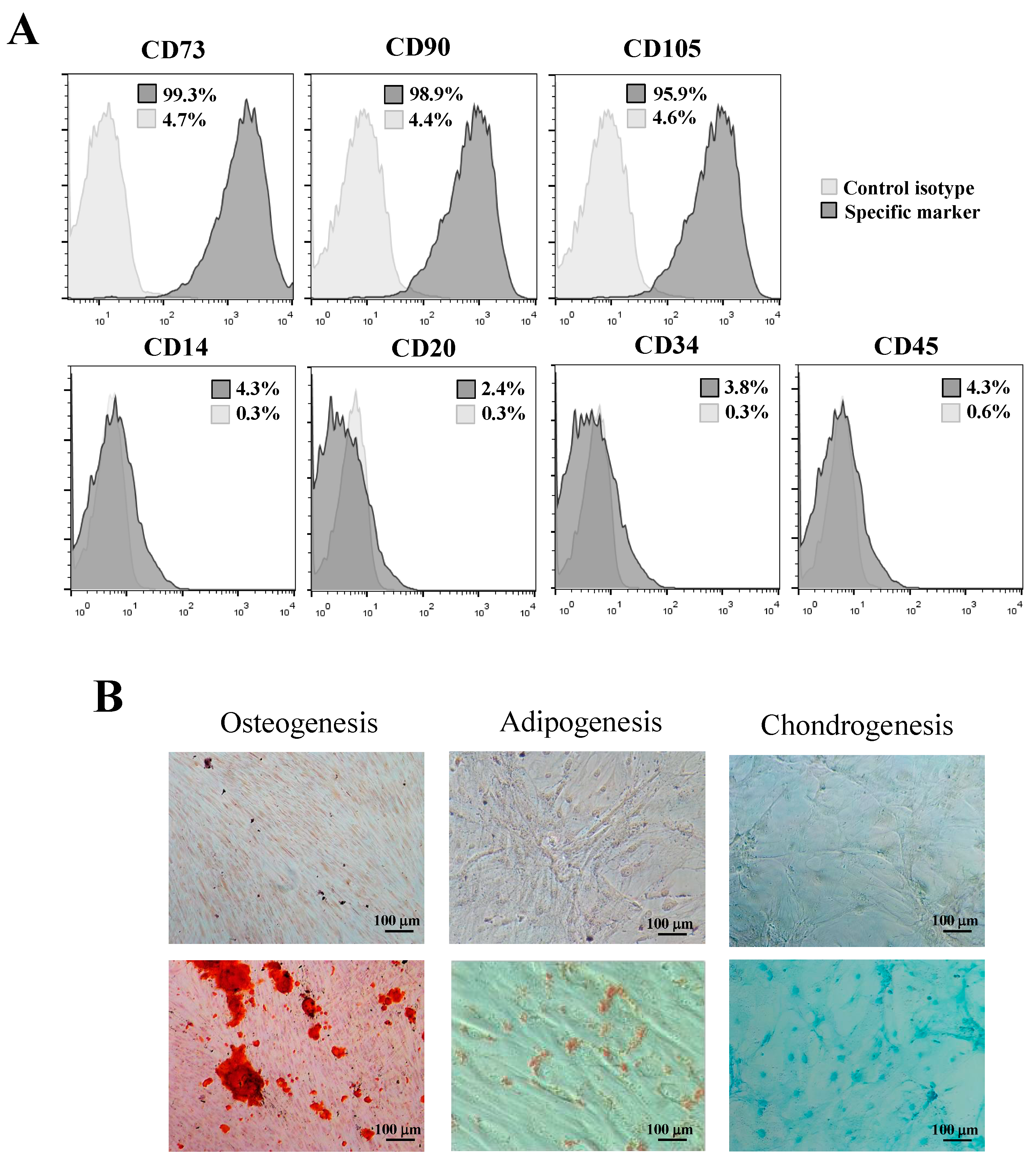
2.4. Expression of Mesenchymal Stem Cell Surface Markers
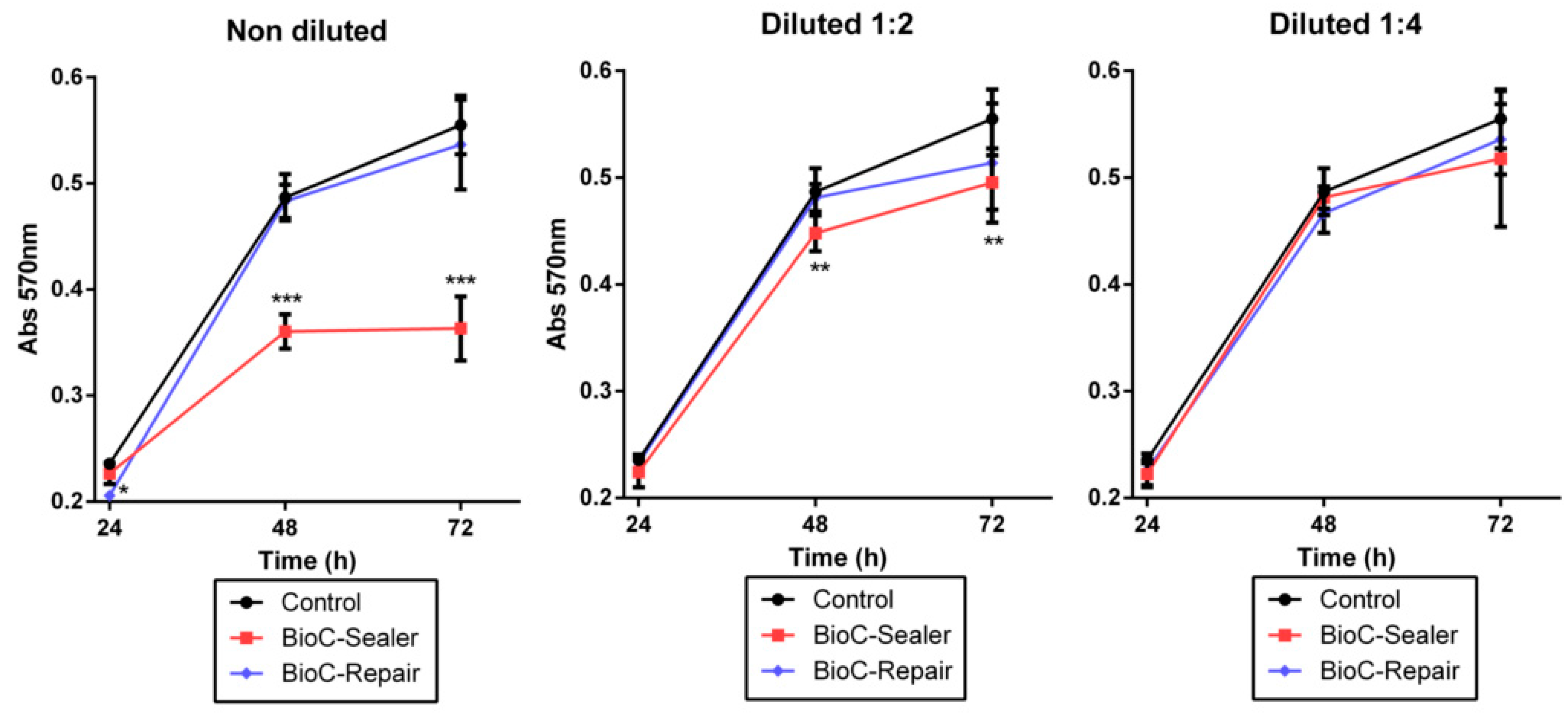
2.5. Cell Viability Assay
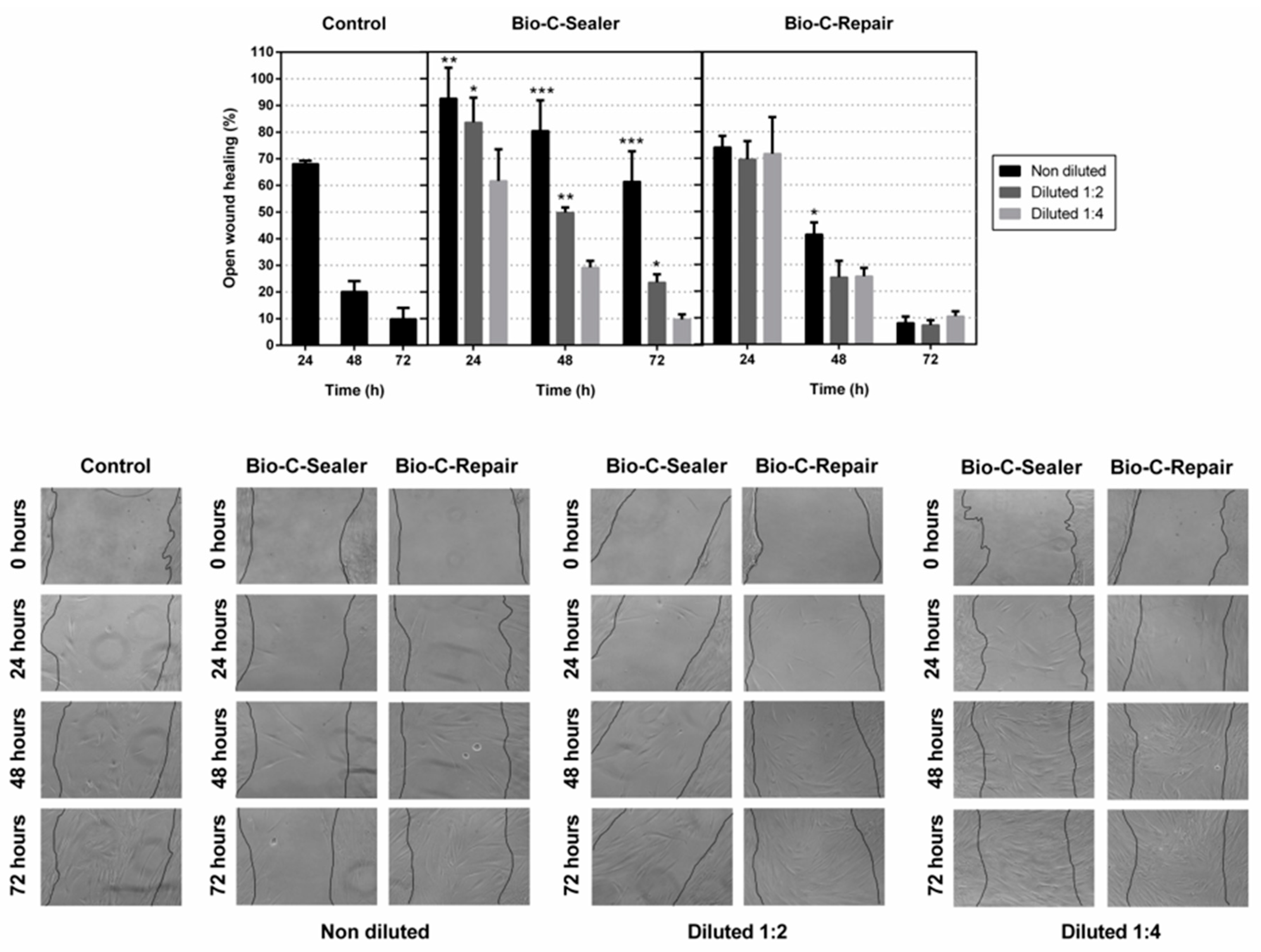
2.6. Cell Migration
2.7. Analysis of Apoptosis and Necrosis by Flow Cytometry (Annexin V/7-AAD Staining)
2.8. Scanning Electronic Microscopy and Energy-Dispersive X-Ray Analysis (SEM/EDX)
2.9. Statistical Analysis
3. Results
3.1. Ion Release and pH
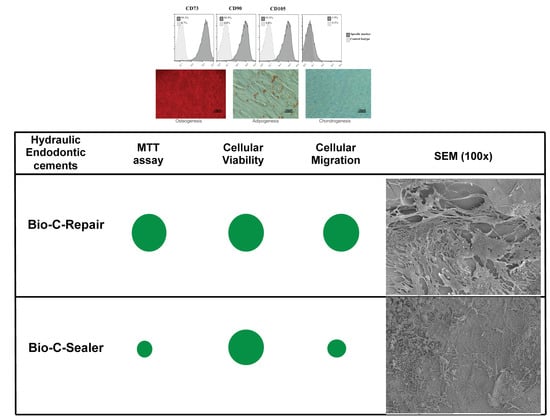
3.2. Isolation and Identification of hPDLSCs
3.3. MTT Assay
3.4. Migration Assay
3.5. Apoptosis/Necrosis Assay
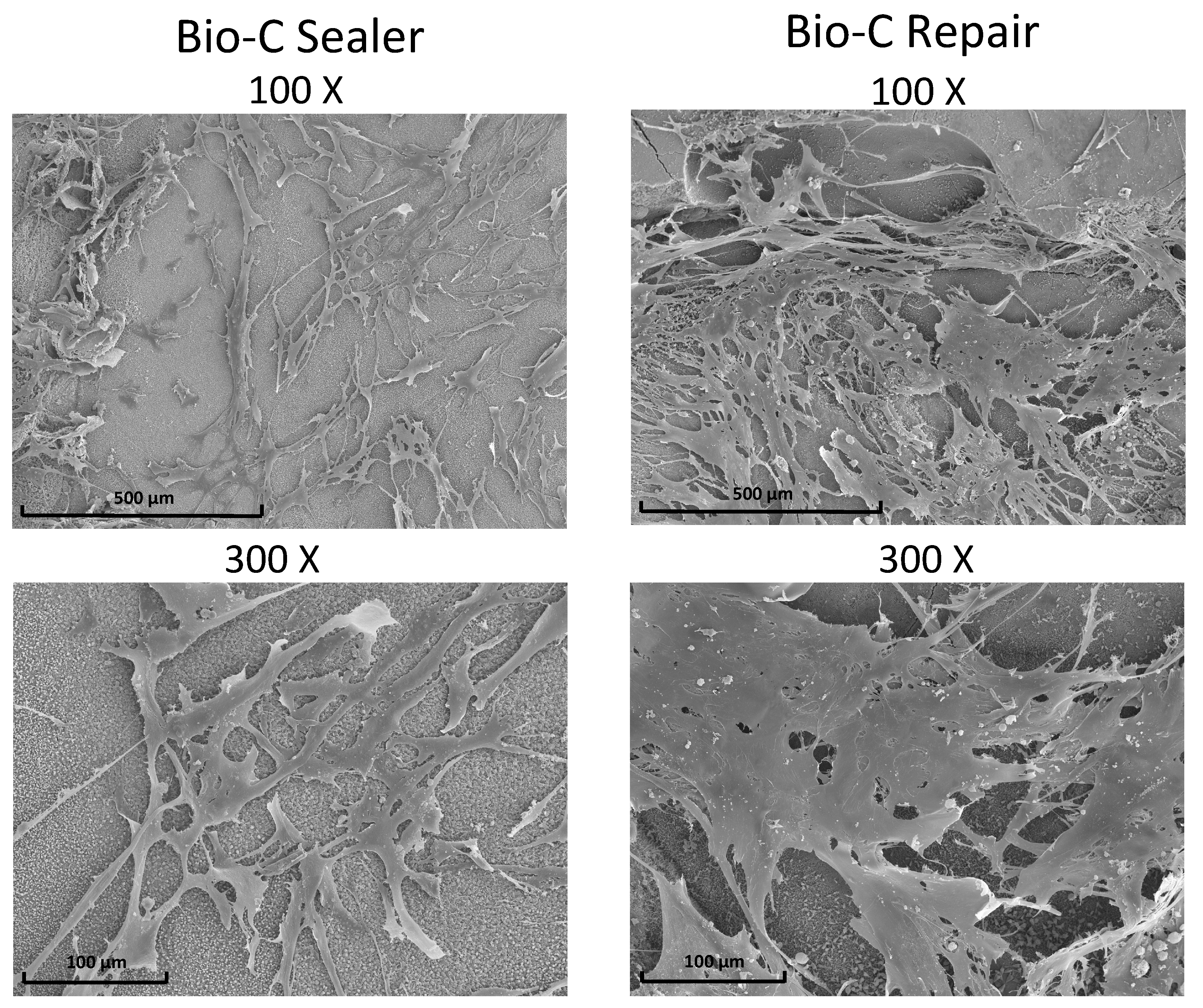
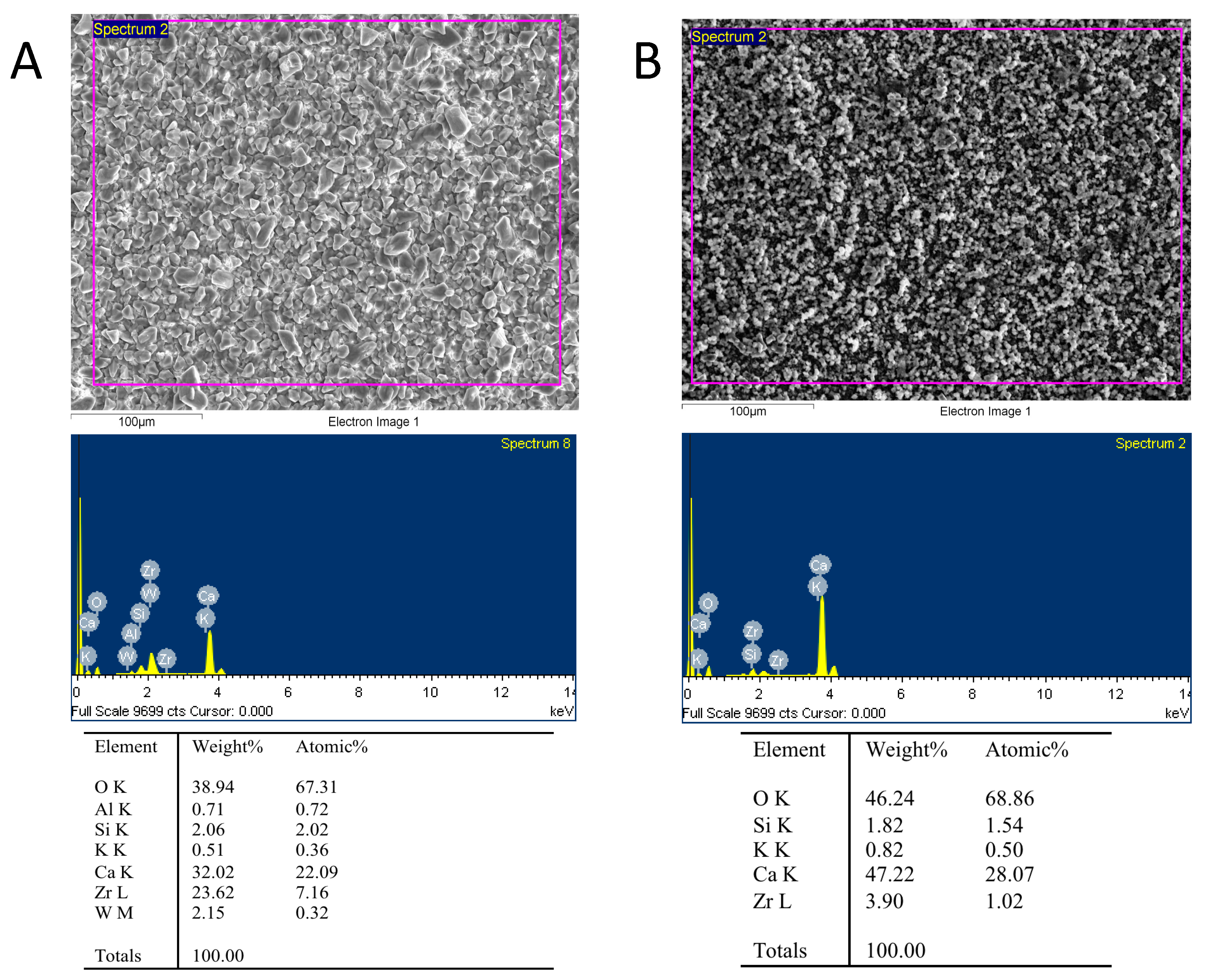
3.6. Scanning Electronic Microscopy and Energy-Dispersive X-ray Analysis (SEM/EDX)
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vallittu, P.K.; Boccaccini, A.R.; Hupa, L.; Watts, D.C. Bioactive dental materials—Do they exist and what does bioactivity mean? Dent. Mater. 2018, 34, 693–694. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pedano, M.S.; Camargo, B.; Hauben, E.; De Vleeschauwer, S.; Chen, Z.; De Munck, J.; Vandamme, K.; Van Landuyt, K.; Van Meerbeek, B. Experimental tricalcium silicate cement induces reparative dentinogenesis. Dent. Mater. 2018, 34, 1410–1423. [Google Scholar] [CrossRef] [PubMed]
- Butt, N.; Bali, A.; Talwar, S.; Chaudhry, S.; Nawal, R.; Yadav, S. Comparison of physical and mechanical properties of mineral trioxide aggregate and Biodentine. Indian J. Dent. Res. 2014, 25, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Shi, L.; Luo, J.; Engqvist, H. A novel fast-setting mineral trioxide aggregate: Its formulation, chemical-physical properties and cytocompatibility. ACS Appl. Mater. Interfaces 2018, 10, 20334–20341. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-M.; Hwang, J.; Song, J.S.; Lee, J.-H.; Choi, H.-J.; Shin, Y. Effects of Three Calcium Silicate Cements on Inflammatory Response and Mineralization-Inducing Potentials in a Dog Pulpotomy Model. Materials (Basel) 2018, 11, 899. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M.; Torabinejad, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part i: Vital pulp therapy. Int. Endod. J. 2018, 51, 177–205. [Google Scholar] [CrossRef]
- Widbiller, M.; Lindner, S.R.; Buchalla, W.; Eidt, A.; Hiller, K.A.; Schmalz, G.; Galler, K.M. Three-dimensional culture of dental pulp stem cells in direct contact to tricalcium silicate cements. Clin. Oral Investig. 2016, 20, 237–246. [Google Scholar] [CrossRef]
- Li, X.; Yoshihara, K.; De Munck, J.; Cokic, S.; Pongprueksa, P.; Putzeys, E.; Pedano, M.; Chen, Z.; Van Landuyt, K.; Van Meerbeek, B. Modified tricalcium silicate cement formulations with added zirconium oxide. Clin. Oral Investig. 2017, 21, 895–905. [Google Scholar] [CrossRef]
- Sultana, N.; Singh, M.; Nawal, R.R.; Chaudhry, S.; Yadav, S.; Mohanty, S.; Talwar, S. Evaluation of Biocompatibility and Osteogenic Potential of Tricalcium Silicate–based Cements Using Human Bone Marrow–derived Mesenchymal Stem Cells. J. Endod. 2018, 44, 446–451. [Google Scholar] [CrossRef]
- Vera-Sánchez, M.; Aznar-Cervantes, S.; Jover, E.; García-Bernal, D.; Oñate-Sánchez, R.E.; Hernández-Romero, D.; Moraleda, J.M.; Collado-González, M.; Rodríguez-Lozano, F.J.; Cenis, J.L.; et al. Silk-Fibroin and Graphene Oxide Composites Promote Human Periodontal Ligament Stem Cell Spontaneous Differentiation into Osteo/Cementoblast-Like Cells. Stem Cells Dev. 2016, 25, 1742–1754. [Google Scholar] [CrossRef]
- Akbulut, M.B.; Arpaci, P.U.; Eldeniz, A.U. Effects of novel root repair materials on attachment and morphological behaviour of periodontal ligament fibroblasts: Scanning electron microscopy observation. Microsc. Res. Tech. 2016, 79, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Küçükkaya, S.; Görduysus Ömer, M.; Zeybek, N.D.; Müftüoğlu, S.F. In Vitro Cytotoxicity of Calcium Silicate-Based Endodontic Cement as Root-End Filling Materials. Scientifica 2016, 2016, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, G.K.; Alonso-Alonso, M.L.; Fernandez-Bueno, I.; Garcia-Gutierrez, M.T.; Rull, F.; Medina, J.; Coco, R.M.; Pastor, J.C. Comparison between direct contact and extract exposure methods for PFO cytotoxicity evaluation. Sci. Rep. 2018, 8, 1425. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, J.; Xu, Y. Study of the in vitro cytotoxicity testing of medical devices. Biomed. Rep. 2015, 3, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lozano, F.J.; Garcia-Bernal, D.; Onate-Sanchez, R.E.; Ortolani-Seltenerich, P.S.; Forner, L.; Moraleda, J.M. Evaluation of cytocompatibility of calcium silicate-based endodontic sealers and their effects on the biological responses of mesenchymal dental stem cells. Int. Endod. J. 2017, 50, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Collado-Gonzalez, M.; Tomas-Catala, C.J.; Onate-Sanchez, R.E.; Moraleda, J.M.; Rodriguez-Lozano, F.J. Cytotoxicity of guttaflow bioseal, guttaflow2, mta fillapex, and ah plus on human periodontal ligament stem cells. J. Endod. 2017, 43, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Parirokh, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part ii: Other clinical applications and complications. Int. Endod. J. 2018, 51, 284–317. [Google Scholar] [CrossRef]
- Dubey, N.; Rajan, S.S.; Bello, Y.D.; Min, K.-S.; Rosa, V. Graphene Nanosheets to Improve Physico-Mechanical Properties of Bioactive Calcium Silicate Cements. Materials (Basel) 2017, 10, 606. [Google Scholar] [CrossRef]
- Jung, S.; Libricht, V.; Sielker, S.; Hanisch, M.R.; Schafer, E.; Dammaschke, T. Evaluation of the biocompatibility of root canal sealers on human periodontal ligament cells ex vivo. Odontology 2019, 107, 54–63. [Google Scholar] [CrossRef]
- Santos, J.M.; Palma, P.J.; Ramos, J.C.; Cabrita, A.S.; Friedman, S. Periapical Inflammation Subsequent to Coronal Inoculation of Dog Teeth Root Filled with Resilon/Epiphany in 1 or 2 Treatment Sessions with Chlorhexidine Medication. J. Endod. 2014, 40, 837–841. [Google Scholar] [CrossRef]
- Santos, J.M.; Pereira, S.; Sequeira, D.B.; Messias, A.L.; Martins, J.B.; Cunha, H.; Palma, P.J.; Santos, A.C. Biocompatibility of a bioceramic silicone-based sealer in subcutaneous tissue. J. Oral Sci. 2019, 61, 171–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sequeira, D.B.; Seabra, C.M.; Palma, P.J.; Cardoso, A.L.; Peça, J.; Santos, J.M. Effects of a New Bioceramic Material on Human Apical Papilla Cells. J. Funct. Biomater. 2018, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Schmalz, G.; Widbiller, M.; Galler, K. Material Tissue Interaction—From Toxicity to Tissue Regeneration. Oper. Dent. 2016, 41, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Benetti, F.; Gomes-Filho, J.E.; Lopes, J.M.A.; Barbosa, J.G.; Jacinto, R.C.; Cintra, L.T.A.; Lopes, J.M.D.A. In vivo biocompatibility and biomineralization of calcium silicate cements. Eur. J. Oral Sci. 2018, 126, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, C.; Lung, C.Y.; Wismayer, P.S.; Arias-Moliz, M.T.; Camilleri, J. The Relationship of Surface Characteristics and Antimicrobial Performance of Pulp Capping Materials. J. Endod. 2018, 44, 1115–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias-Moliz, M.T.; Farrugia, C.; Lung, C.Y.; Wismayer, P.S.; Camilleri, J. Antimicrobial and biological activity of leachate from light curable pulp capping materials. J. Dent. 2017, 64, 45–51. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standarization. 10993-5. Biological Evaluation of Medical Devices-Part 5: Test for in Vitro Cytotoxicity; International Organization for Standarization: Geneva, Switzerland, 2009. [Google Scholar]
- Candeiro, G.T.M.; Moura-Netto, C.; D’Almeida-Couto, R.S.; Azambuja-Junior, N.; Marques, M.M.; Cai, S.; Gavini, G. Cytotoxicity, genotoxicity and antibacterial effectiveness of a bioceramic endodontic sealer. Int. Endod. J. 2016, 49, 858–864. [Google Scholar] [CrossRef]
- Chakar, S.; Changotade, S.; Osta, N.; Khalil, I. Cytotoxic evaluation of a new ceramic-based root canal sealer on human fibroblasts. Eur. J. Dent. 2017, 11, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Vouzara, T.; Dimosiari, G.; Koulaouzidou, E.A.; Economides, N. Cytotoxicity of a New Calcium Silicate Endodontic Sealer. J. Endod. 2018, 44, 849–852. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, Y.; Lai, Z.; Li, M.; Huang, Y.; Jiang, H.; Wei, X. Ion release, microstructural, and biological properties of iroot bp plus and proroot mta exposed to an acidic environment. J. Endod. 2017, 43, 163–168. [Google Scholar] [CrossRef]
- Zbinden, A.; Browne, S.; Altiok, E.I.; Svedlund, F.L.; Healy, K.E.; Jackson, W.M. Multivalent conjugates of basic fibroblast growth factor enhance in vitro proliferation and migration of endothelial cells. Biomater. Sci. 2018, 6, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Pedano, M.S.; Li, X.; Li, S.; Sun, Z.; Cokic, S.M.; Putzeys, E.; Yoshihara, K.; Yoshida, Y.; Chen, Z.; Van Landuyt, K.; et al. Freshly-mixed and setting calcium-silicate cements stimulate human dental pulp cells. Dent. Mater. 2018, 34, 797–808. [Google Scholar] [CrossRef] [PubMed]
- De Souza Costa, C.A.; Hebling, J.; Scheffel, D.L.; Soares, D.G.; Basso, F.G.; Ribeiro, A.P. Methods to evaluate and strategies to improve the biocompatibility of dental materials and operative techniques. Dent. Mater. 2014, 30, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Benezra, M.K.; Wismayer, P.S.; Camilleri, J. Interfacial Characteristics and Cytocompatibility of Hydraulic Sealer Cements. J. Endod. 2018, 44, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Zamparini, F.; Siboni, F.; Prati, C.; Taddei, P.; Gandolfi, M.G. Properties of calcium silicate-monobasic calcium phosphate materials for endodontics containing tantalum pentoxide and zirconium oxide. Clin. Oral Investig. 2019, 23, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Ashofteh Yazdi, K.; Ghabraei, S.; Bolhari, B.; Kafili, M.; Meraji, N.; Nekoofar, M.H.; Dummer, P.M.H. Microstructure and chemical analysis of four calcium silicate-based cements in different environmental conditions. Clin. Oral Investig. 2019, 23, 43–52. [Google Scholar] [CrossRef] [PubMed]

| Materials | Manufacturer | Composition | Lot Number |
|---|---|---|---|
| BIO-C Sealer | Angelus, Rua Waldir Landgraf, Barrio Lindóia, Londrina, Brasil. | Powder: calcium silicate (Ca2SiO4), calcium oxide (CaO), zirconium oxide (ZrO2), iron oxide, silicon dioxide (SiO2), and dispersing agent. Dispersion agent/solid ratio = 0.52 | 43,207 |
| BIO-C Repair | Angelus, Rua Waldir Ladgraf, Barrio Lindóia, Londrina, Brasil. | Powder: calcium silicate (Ca2SiO4),calcium oxide (CaO), zirconium oxide (ZrO2), iron oxide, silicon dioxide (SiO2), and dispersing agent Dispersion agent/solid ratio = 0.46 | 43,210 |
| Concentration of Element (mg/L Solution) | ||||||
|---|---|---|---|---|---|---|
| pH | Silicon | Strontium | Calcium | Zirconium | ||
| Complete DMEM | 7.61 ± 0.03 | Milli-Q water | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Bio-C Sealer | 8.40 ± 0.05 | Bio-C Sealer | 42.01 ± 0.01 | 0.3 ± 0.04 | 63.87 ± 0.01 | 0.13 ± 0.01 |
| Bio-C Repair | 8.33 ± 0.02 | Bio-C Repair | 14.90 ± 0.1 | 0.52 ± 0.01 | 38.32 ± 0.02 | 0.12 ± 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-García, S.; Lozano, A.; García-Bernal, D.; Forner, L.; Llena, C.; Guerrero-Gironés, J.; Moraleda, J.M.; Murcia, L.; Rodríguez-Lozano, F.J. Biological Effects of New Hydraulic Materials on Human Periodontal Ligament Stem Cells. J. Clin. Med. 2019, 8, 1216. https://doi.org/10.3390/jcm8081216
López-García S, Lozano A, García-Bernal D, Forner L, Llena C, Guerrero-Gironés J, Moraleda JM, Murcia L, Rodríguez-Lozano FJ. Biological Effects of New Hydraulic Materials on Human Periodontal Ligament Stem Cells. Journal of Clinical Medicine. 2019; 8(8):1216. https://doi.org/10.3390/jcm8081216
Chicago/Turabian StyleLópez-García, Sergio, Adrián Lozano, David García-Bernal, Leopoldo Forner, Carmen Llena, Julia Guerrero-Gironés, José M. Moraleda, Laura Murcia, and Francisco J. Rodríguez-Lozano. 2019. "Biological Effects of New Hydraulic Materials on Human Periodontal Ligament Stem Cells" Journal of Clinical Medicine 8, no. 8: 1216. https://doi.org/10.3390/jcm8081216
APA StyleLópez-García, S., Lozano, A., García-Bernal, D., Forner, L., Llena, C., Guerrero-Gironés, J., Moraleda, J. M., Murcia, L., & Rodríguez-Lozano, F. J. (2019). Biological Effects of New Hydraulic Materials on Human Periodontal Ligament Stem Cells. Journal of Clinical Medicine, 8(8), 1216. https://doi.org/10.3390/jcm8081216