Elevated Systemic L-Kynurenine/L-Tryptophan Ratio and Increased IL-1 Beta and Chemokine (CX3CL1, MCP-1) Proinflammatory Mediators in Patients with Long-Term Titanium Dental Implants
Abstract
:1. Introduction
Aim
Secondary Aim
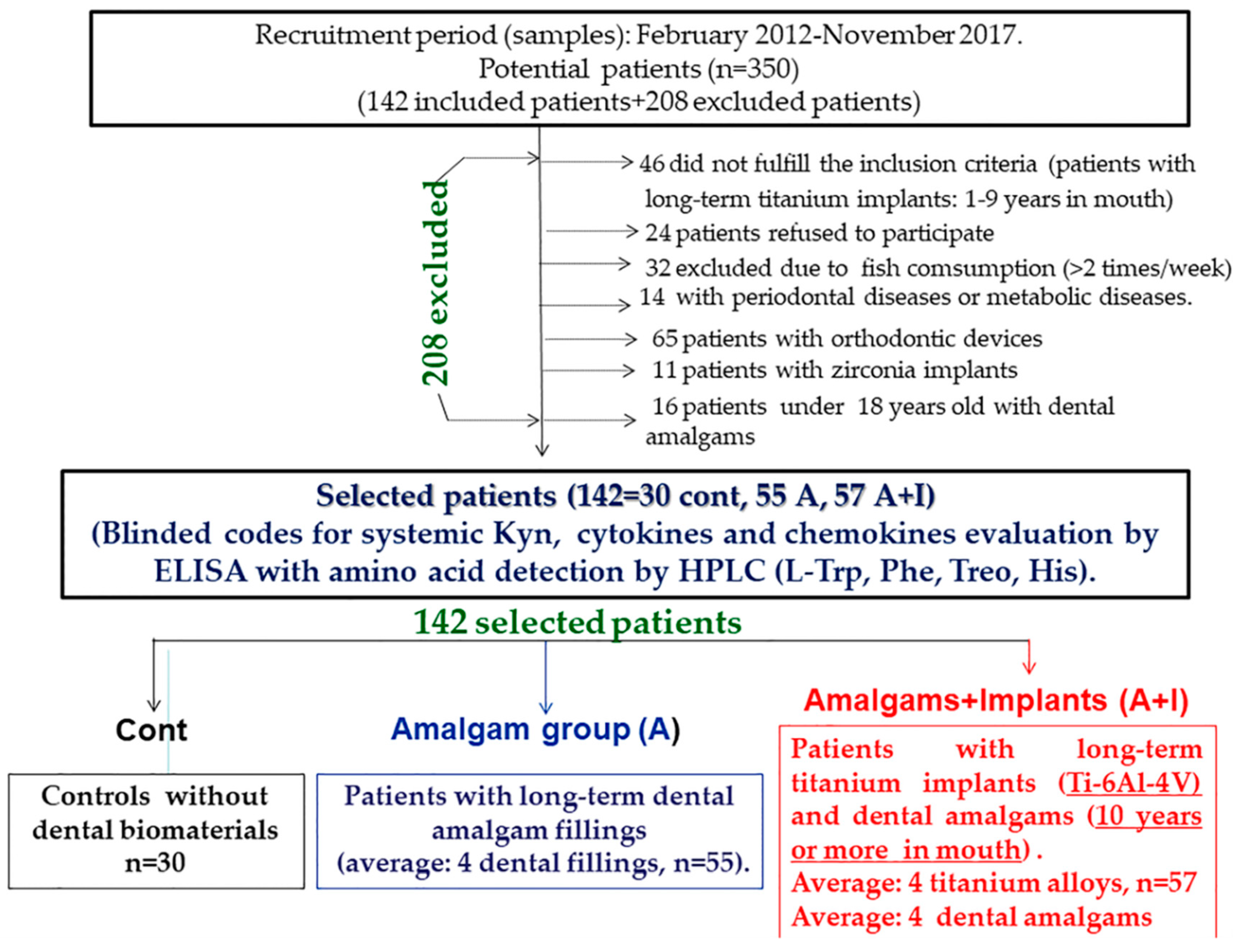
2. Materials and Methods
2.1. Patients (Inclusion Criteria)
2.2. Exclusion Criteria
2.3. ELISA for Inflammatory Mediators (IL-1 Beta, IL-4, Macrophage Colony Protein-1: MCP-1 = CCL-2, and Soluble Fractalkin Levels CX3CL1 = sFK)
2.4. L-Kynurenine (L-Kyn)
2.5. Statistical Analysis
3. Results
3.1. Figure 2: Lower Systemic Trp Levels in Patients with Long-Term Dental Titanium Amalgams as Compared to Participants with Long-Term Dental Amalgams Alone
3.2. Patients Who Had Long-Term Dental Amalgams Have Lower Systemic His Levels Than Control Subjects
3.3. L-Kynurenine
3.4. Ratio L-Kynurenine/L-Trp: An Index of IDO Activity
3.5. Proinflammatory Mediators (Cytokines and Chemokines)
3.5.1. IL-1 Beta Levels
3.5.2. MCP-1 (CCL2)
3.5.3. IL-4 Anti-Inflammatory Cytokine
3.5.4. Soluble Fractalkine (CX3CL1)
3.5.5. Ratio IL-1 Beta/IL-4
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cabaña-Muñoz, M.E.; Parmigiani-Izquierdo, J.M.; Bravo-González, L.A.; Kyung, H.M.; Merino, J.J. Increased Zn/glutathione levels and higher superoxide dismutase-1 activity as biomarkers of oxidative stress in women with long-term dental amalgam fillings: correlation between mercury/aluminium levels (in Hair) and antioxidant systems in plasma. PLoS ONE 2015, 10, e0126339. [Google Scholar] [CrossRef] [PubMed]
- Shraim, A.; Alsuhaimi, A.; Al-Thakafy, J.T. Dental clinics: A point pollution source, not only of mercury but also of other amalgam constituents. Chemosphere 2011, 84, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Bishara, S.E.; Barrett, R.D.; Selim, M.I. Biodegradation of orthodontic appliances. Part II. Changes in the blood level of nickel. Am. J. Orthod. Dentofac. Orthop. 1993, 103, 115–119. [Google Scholar] [CrossRef]
- Puchyr, R.F.; Bass, D.A.; Gajewski, R.; Calvin, M.; Marquardt, W.; Urek, K.; Druyan, M.E.; Quig, D. Preparation of hair for measurement of elements by inductively coupled plasma-mass spectrometry (ICP-MS). Biol. Trace Elem. Res. 1998, 62, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Merino, J.J.; Arce, C.; Naddaf, A.; Bellver-Landete, V.; Oset-Gasque, M.J.; González, M.P. The nitric oxide donor SNAP-induced amino acid neurotransmitter release in cortical neurons. Effects of blockers of voltage-dependent sodium and calcium channels. PLoS ONE 2014, 9, e90703. [Google Scholar] [CrossRef] [PubMed]
- Mutter, J. Is dental amalgam safe for humans? The opinion of the scientific committee of the European Commission. J. Occup. Med. Toxicol. 2011, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Park, J.D.; Zheng, W. Human exposure and health effects of inorganic and elemental mercury. J. Prev. Med. Public Health 2012, 45, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Lai, M.B.; Jandhyam, S.; Dukhande, V.V.; Bhushan, A.; Daniels, C.K.; Leung, S.W. Exposure to titanium dioxide and other metallic oxide nanoparticles induces cytotoxicity on human neural cells and fibroblasts. Int. J. Nanomed. 2008, 3, 533–545. [Google Scholar] [Green Version]
- Lim, S.D.; Takada, Y.; Kim, K.M.; Okuno, O. Ions released from dental amalgams in contact with titanium. Dent. Mater. J. 2003, 22, 96–110. [Google Scholar] [CrossRef]
- Barrett, R.D.; Bishara, S.E.; Quinn, J.K. Biodegradation of orthodontic appliances. Part, I. Biodegradation of nickel and chromium in vitro. Am. J. Orthod. Dentofac. Orthop. 1993, 103, 8–14. [Google Scholar] [CrossRef]
- Joska, L.; Fojt, J.; Cvrcek, L.; Brezina, V. Properties of titanium-alloyed DLC layers for medical applications. Biomatter 2014, 4, e29505. [Google Scholar] [CrossRef]
- Huerta-García, E.; Pérez-Arizti, J.A.; Márquez-Ramírez, S.G.; Delgado-Buenrostro, N.L.; Chirino, Y.I.; Iglesias, G.G.; López-Marure, R. Titanium dioxide nanoparticles induce strong oxidative stress and mitochondrial damage in glial cells. Free Radic. Biol. Med. 2014, 73, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Chen, Y.; Zhang, J.; Wang, J.; Shao, L.; Wei, L. Application of dental nanomaterials: Potential toxicity to the central nervous system. Int. J. Nanomed. 2015, 14, 3547–3565. [Google Scholar]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Tainaka, H.; Oba, T.; Mizuo, K.; Umezawa, M.; Takeda, K. Maternal exposure to nanoparticulate titanium dioxide during the prenatal period alters gene expression related to brain development in the mouse. Part. Fibre Toxicol. 2009, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, D.G.; Tasat, D.; Guglielmotti, M.B.; Cabrini, R.L. Titanium transport through the blood stream. An experimental study on rats. J. Mater. Sci. Mater. Med. 2003, 14, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Coccini, T.; Grandi, S.; Lonati, D.; Locatelli, C.; De Simone, U. Comparative cellular toxicity of titanium dioxide nanoparticles on human astrocyte and neuronal cells after acute and prolonged exposure. Neurotoxicology 2015, 48, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Van Donkelaar, E.L.; Blokland, A.; Ferrington, L.; Kelly, P.A.T.; Steinbusch, H.W.M.; Prickaerts, J. Mechanism of acute tryptophan depletion: Is it only serotonin? Mol. Psychiatry 2011, 16, 695–713. [Google Scholar] [CrossRef]
- Young, S.N.; Smith, S.E.; Pih, R.O.; Ervin, F.R. Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacology 1985, 87, 173–177. [Google Scholar] [CrossRef]
- Permuy, M.; López-Peña, M.; González-Cantalapiedra, A.; Muñoz, F. Melatonin: A review of its potential functions and effects on dental diseases. Int. J. Mol. Sci. 2017, 18, 865. [Google Scholar] [CrossRef]
- Jacobi-Gresser, E.; Huesker, K.; Schütt, S. Genetic and immunological markers predict titanium implant failure: A retrospective study. Int. J. Oral Maxillofac. Surg. 2013, 42, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Catena-Dell’Osso, M.; Rotella, F.; Dell’Osso, A.; Fagiolini, A.; Marazziti, D. Inflammation, serotonin and major depression. Curr. Drug Targets 2013, 14, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Oxenkrug, G.F. Tryptophan kynurenine metabolism as a common mediator of genetic and environmental impacts in major depression disorders: The serotonin hypothesis revised 40 years later. Isr. J. Psychiatry 2010, 47, 56–63. [Google Scholar]
- Mandi, Y.; Vecsei, L. The kynurenine system and immunoregulation. J. Neural Trasm. 2012, 119, 197–209. [Google Scholar] [CrossRef]
- Taylor, M.W.; Feng, G.S. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and trypthophane catabolism. FASEB J. 1991, 5, 2516–2522. [Google Scholar] [CrossRef] [PubMed]
- Fallarino, F.; Grohmann, U.; Pucceti, P. Indoleamine 2,3-dioxygenase: From catalysis to signaling function. Eur. J. Immunol. 2012, 42, 1932–1937. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Chapman, G.A.; Moores, K.E.; Gohil, J.; Berkhout, T.A.; Patel, L.; Green, P.; Macphee, C.H.; Stewart, B.R. The role of fractalkine in the recruitment of monocytes to the endothelium. Eur. J. Pharm. 2000, 31, 189–195. [Google Scholar] [CrossRef]
- Corona, A.W.; Huang, Y.; O’Connor, J.C.; Dantzer, R.; Kelley, K.W.; Popovich, P.G.; Godbout, J.P. Fractalkine receptor (CX3CR1) deficiency sensitized mice to the behaviour changes induced by lipopolysaccharide. J. Neuroinflamm. 2010, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Cabaña-Muñoz, M.E.; Parmigiani-Izquierdo, J.M.; Parmigiani-Cabaña, J.M.; Merino, J.J. Safe removal of amalgam fillings in dental clinic: Use of synergic nasal filters (active carbon) and phytonaturals. Int. J. Sci. Res. (IJSR) 2015, 4, 2393. [Google Scholar]
- Merino, J.; Aller, M.A.; Rubio, S.; Arias, N.; Nava, M.P.; Loscertales, M.; Arias, J.; Arias, J.L. Gut-brain chemokine changes in portal hypertensive rats. Dig. Dis. Sci. 2011, 56, 2309–2317. [Google Scholar] [CrossRef] [PubMed]
- Apalset, E.M.; Gjesdal, C.G.; Ueland, P.M.; Midttun, Ø.; Ulvik, A.; Eide, G.E.; Meyer, K.; Tell, G.S. Interferon (IFN)-γ-mediated inflammation and the kynurenine pathway in relation to bone mineral density: The Hordaland Health Study. Clin. Exp. Immunol. 2014, 176, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Chang, C.W.; Chou, H.C. Methoxytryptophan-dependent inhibition of oral squamous cell carcinoma metastasis. Electrophoresis 2015, 36, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Capuron, L.; Schroecksnadel, S.; Féart, C.; Aubert, A.; Higueret, D.; Barberger-Gateau, P.; Layé, S.; Fuchs, D. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: Role in neuropsychiatric symptoms. Biol. Psychiatry 2011, 70, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Tresguerres, I.F.; Clemente, C.; Blanco, L.; Khraisat, A.; Tamimi, F.; Tresguerres, J.A. Effects of local melatonin application on implant osseointegration. Clin. Implant Dent. Relat. Res. 2012, 14, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Ping, Z.; Hu, X.; Wang, L.; Shi, J.; Tao, Y.; Wu, X.; Hou, Z.; Guo, X.; Zhang, W.; Yang, H.; et al. Melatonin attenuates titanium particle-induced osteolysis via activation of Wnt/β-catenin signaling pathway. Acta Biomater. 2017, 51, 513–525. [Google Scholar] [CrossRef]
- Cutando, A.; Gómez-Moreno, G.; Arana, C.; Muñoz, F.; Lopez-Peña, M.; Stephenson, J.; Reiter, R.J. Melatonin stimulates osteointegration of dental implants. J. Pineal Res. 2008, 45, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Cutando, A.; Arana, C.; Gómez-Moreno, G.; Escaes, G.; López, A.; Ferrera, M.J.; Reiter, R.J.; Acuña-Castroviejo, D. Local application of melatonin into alveolar sockets of beagle dogs reduces tooth-removal oxidative stress. J. Periodontol. 2007, 78, 576–583. [Google Scholar] [CrossRef]
- Vidal, C.; Li, W.; Santner-Nanan, B.; Lim, C.K.; Guillemin, G.J.; Ball, H.J.; Hunt, N.H.; Nanan, R.; Duque, G. The kynurenine pathway of tryptophan degradation is activated during osteoblastogenesis. Stem Cells 2015, 33, 111–121. [Google Scholar] [CrossRef]
- Palin, L.P.; Polo, T.O.B.; Batista, F.R.S.; Gomes-Ferreira, P.H.S.; Garcia Junior, I.R.; Rossi, A.C.; Freire, A.; Faverani, L.P.; Sumida, D.H.; Okamoto, R. Daily melatonin administration improves osseointegration in pinealectomized rats. J. Appl. Oral Sci. 2018, 26, e20170470. [Google Scholar] [CrossRef] [Green Version]
- Mohammadipour, A.; Fazel, A.; Haghir, H.; Motejaded, F.; Rafatpanah, H.; Zabihi, H.; Hosseini, M.; Bideskan, A.E. Maternal exposure to titanium dioxide nanoparticles during pregnancy, impaired memory and decreased hippocampal cell proliferation in rat offspring. Environ. Toxicol. Pharm. 2014, 37, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Geraets, L.; Oomen, A.G.; Krystek, P.; Jacobsen, N.R.; Wallin, H.; Laurentie, M.; Verharen, H.W.; Brandon, E.F.; de Jong, W.H. Tissue distribution and elimination after oral and intravenous administration of different titanium dioxide nanoparticles in rats. Part. Fibre Toxicol. 2014, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Lawrence, H.; Holland, J.P.; Kirby, J.A.; Deehan, D.J.; Tyson, A.J. Effect of cobalt-mediated Toll-like receptor 4 activation on inflammatory responses in endothelial cells. Oncotarget 2016, 7, 76471–76478. [Google Scholar] [CrossRef] [PubMed]
- Cabaña-Muñoz, M.E.; Parmigiani-Izquierdo, J.M.; Camacho-Alonso, F.; Merino, J.J. Increased Systemic Malondialdehyde Levels and Decreased Mo/Co, Co/Fe2+ Ratios in Patients with Long-Term Dental Titanium Implants and Amalgams. J. Clin. Med. 2019, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N. Corrosion of metallic biomaterials: A review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Ghallab, N.A.; Hamdy, E.; Shaker, O.G. Malondialdehyde, superoxide dismutase and melatonin levels in gingival crevicular fluid of aggressive and chronic periodontitis patients. Aust. Dent. J. 2016, 61, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, H.; Deehan, D.; Holland, J.; Kirby, J.; Tyson-Capper, A. The immunobiology of cobalt: Demonstration of a potential aetiology for inflammatory pseudotumours after metal-on-metal replacement of the hip. Bone Jt. J. 2014, 96, 1172–1177. [Google Scholar] [CrossRef]
- Lechner, J.; Noumbissi, S.; von Baehr, V. Titanium implants and silent inflammation in jawbone-a critical interplay of dissolved titanium particles and cytokines TNF-α and RANTES/CCL5 on overall health? EPMA J. 2018, 9, 331–343. [Google Scholar] [CrossRef]
- Corona, A.W.; Norden, D.M.; Skendelas, J.P.; Huang, Y.; O’Connor, J.C.; Lawson, M.; Dantzer, R.; Kelly, K.; Godbout, J.P. Indoleamine 2,3-dioxygenase inhibition attenuates lipopolysaccharide induced persistent microglial activation and depressive-like complications in fractalkine receptor (CX3CR1)-deficient mice. Brain Behav. Immun. 2013, 31, 134–142. [Google Scholar] [CrossRef]
- Vallés, G.; González-Melendi, P.; González-Carrasco, J.L.; Saldaña, L.; Sánchez-Sabaté, E.; Munuera, L.; Villaboa, N. Differential inflammatory macrophage response to rutile and titanium particles. Biomaterials 2006, 27, 5199–5211. [Google Scholar]
- Lechner, J.; von Baehr, V. RANTES and fibroblast growth factor 2 in jawbone cavitations: Triggers for systemic disease? Int. J. Gen. Med. 2013, 6, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Merino, J.J.; Parmigiani-Izquierdo, J.M.; Toledano Gasca, A.; Cabaña-Muñoz, M.E. The Long-Term Algae Extract (Chlorella and Fucus sp) and Aminosulphurate Supplementation Modulate SOD-1 Activity and Decrease Heavy Metals (Hg++, Sn) Levels in Patients with Long-Term Dental Titanium Implants and Amalgam Fillings Restorations. Antioxidants 2019, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Gostner, J.M.; Geiser, S.; Stoning, M.; Mair, L.; Spencer-Unterweger, B.; Fuchs, D. Tryptophan metabolism and related pathways in psychoneuroimmunology: The impact of nutrition and lifestyle. Neuropsychobiology 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sas, K.; Robotka, H.; Toldi, J.; Vécsei, L. Mitochondria, metabolic disturbances, oxidative stress and the kynurenin system, with focus on neurodegenerative disorsers. J. Neurol. Sci. 2007, 257, 221–239. [Google Scholar] [CrossRef] [PubMed]
- Blankfield, A. Kynurenine Pathway Pathologies: Do Nicotinamide and Other Pathway Co-Factors have a Therapeutic Role in Reduction of Symptom Severity, Including Chronic Fatigue Syndrome (CFS) and Fibromyalgia (FM). Int. J. Tryptophan Res. 2013, 6, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Bortolato, B.; Berk, M.; Maes, M.; McIntyre, R.S.; Carvalho, A.F. Fibromyalgia and bipolar disorder: Emerging epidemiological associations and shared pathophysiology. Curr. Mol. Med. 2016, 16, 119–136. [Google Scholar] [CrossRef] [PubMed]






| Cont | Amalgam (A) | Amalgam + Implants (A + I) | KW | |
|---|---|---|---|---|
| IL-1 β (pg/mL) | 11.03 ± 0.48 | 11.03 ± 0.32 | 12.66 ± 0.52 # | H = 9.37, p = 0.009 |
| MCP-1 (pg/mL) | 9.48 ± 0.7 | 10.39 ± 0.24 | 11.76 ± 0.14 *,# | H = 22.88, p < 0.001 |
| CX3CL1 (pg/mL) | 30.02 ± 2.7 | 34.3 ± 7.1 | 98 ± 3.6 *,# | F (2, 140) = 5, p = 0.01 |
| IL-4 (pg/mL) | 40 ± 1.3 | 42 ± 1.1 | 37.8 ± 1.7 # | F (2, 140) = 2.63, p = 0.082 |
| IL 1 Beta/Il-4 ratio | 0.28 ± 0.01 | 0.26 ± 0.011 | 0.32 ± 0.02 # | H (4, 140), p = 0.091, n.s |
| Kyn | 1.47 ± 0.09 | 1.68 ± 0.1 | 2.09 ± 0.14 *,# | F (2, 140) = 5.96, p = 0.035 |
| Kyn/L-Trp ratio | 31 ± 2.3 | 27 ± 2.5 | 47 ± 3.3 *,# | F (2, 140) = 14.6, p < 0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merino, J.J.; Cabaña-Muñoz, M.E.; Toledano Gasca, A.; Garcimartín, A.; Benedí, J.; Camacho-Alonso, F.; Parmigiani-Izquierdo, J.M. Elevated Systemic L-Kynurenine/L-Tryptophan Ratio and Increased IL-1 Beta and Chemokine (CX3CL1, MCP-1) Proinflammatory Mediators in Patients with Long-Term Titanium Dental Implants. J. Clin. Med. 2019, 8, 1368. https://doi.org/10.3390/jcm8091368
Merino JJ, Cabaña-Muñoz ME, Toledano Gasca A, Garcimartín A, Benedí J, Camacho-Alonso F, Parmigiani-Izquierdo JM. Elevated Systemic L-Kynurenine/L-Tryptophan Ratio and Increased IL-1 Beta and Chemokine (CX3CL1, MCP-1) Proinflammatory Mediators in Patients with Long-Term Titanium Dental Implants. Journal of Clinical Medicine. 2019; 8(9):1368. https://doi.org/10.3390/jcm8091368
Chicago/Turabian StyleMerino, José Joaquín, María Eugenia Cabaña-Muñoz, Adolfo Toledano Gasca, Alba Garcimartín, Juana Benedí, Fabio Camacho-Alonso, and José María Parmigiani-Izquierdo. 2019. "Elevated Systemic L-Kynurenine/L-Tryptophan Ratio and Increased IL-1 Beta and Chemokine (CX3CL1, MCP-1) Proinflammatory Mediators in Patients with Long-Term Titanium Dental Implants" Journal of Clinical Medicine 8, no. 9: 1368. https://doi.org/10.3390/jcm8091368
APA StyleMerino, J. J., Cabaña-Muñoz, M. E., Toledano Gasca, A., Garcimartín, A., Benedí, J., Camacho-Alonso, F., & Parmigiani-Izquierdo, J. M. (2019). Elevated Systemic L-Kynurenine/L-Tryptophan Ratio and Increased IL-1 Beta and Chemokine (CX3CL1, MCP-1) Proinflammatory Mediators in Patients with Long-Term Titanium Dental Implants. Journal of Clinical Medicine, 8(9), 1368. https://doi.org/10.3390/jcm8091368





