Systemic Oxygen Delivery during One-Lung Ventilation: Comparison between Propofol and Sevoflurane Anaesthesia in a Randomised Controlled Trial
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Population
2.2. Randomisation and Blinding Procedure
2.3. Intraoperative Management
2.4. Measurements
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ackland, G.L.; Iqbal, S.; Paredes, L.G.; Toner, A.; Lyness, C.; Jenkins, N.; Bodger, P.; Karmali, S.; Whittle, J.; Reyes, A.; et al. Individualised oxygen delivery targeted haemodynamic therapy in high-risk surgical patients: A multicentre, randomised, double-blind, controlled, mechanistic trial. Lancet Respir. Med. 2015, 3, 33–41. [Google Scholar] [CrossRef]
- Pinsky, M.R. Hemodynamic evaluation and monitoring in the ICU. Chest 2007, 132, 2020–2029. [Google Scholar] [CrossRef]
- Bihari, D.; Smithies, M.; Gimson, A.; Tinker, J. The effects of vasodilation with prostacyclin on oxygen delivery and uptake in critically ill patients. N. Engl. J. Med. 1987, 317, 397–403. [Google Scholar] [CrossRef]
- Perel, A. Non-invasive monitoring of oxygen delivery in acutely ill patients: New frontiers. Ann. Intensive Care 2015, 5, 24. [Google Scholar] [CrossRef]
- Leach, R.M.; Treacher, D.F. The pulmonary physician in critical care * 2: Oxygen delivery and consumption in the critically ill. Thorax 2002, 57, 170–177. [Google Scholar] [CrossRef]
- Shibutani, K.; Komatsu, T.; Kubal, K.; Sanchala, V.; Kumar, V.; Bizzarri, D.V. Critical level of oxygen delivery in anesthetized man. Crit. Care Med. 1983, 11, 640–643. [Google Scholar] [CrossRef]
- Sykes, O. Oxygen monitoring during low flow anaesthesia. J. Clin. Monit. Comput. 2010, 24, 141. [Google Scholar] [CrossRef]
- Sykes, O. Metabolic oxygen requirements. Anaesthesia 2017, 72, 415–416. [Google Scholar] [CrossRef] [Green Version]
- Pruszkowski, O.; Dalibon, N.; Moutafis, M.; Jugan, E.; Law-Koune, J.D.; Laloe, P.A.; Fischler, M. Effects of propofol vs. sevoflurane on arterial oxygenation during one-lung ventilation. Br. J. Anaesth. 2007, 98, 539–544. [Google Scholar] [CrossRef]
- Marshall, C.; Lindgren, L.; Marshall, B.E. Effects of halothane, enflurane, and isoflurane on hypoxic pulmonary vasoconstriction in rat lungs in vitro. Anesthesiology 1984, 60, 304–308. [Google Scholar] [CrossRef]
- Kellow, N.H.; Scott, A.D.; White, S.A.; Feneck, R.O. Comparison of the effects of propofol and isoflurane anaesthesia on right ventricular function and shunt fraction during thoracic surgery. Br. J. Anaesth. 1995, 75, 578–582. [Google Scholar] [CrossRef]
- Abe, K.; Mashimo, T.; Yoshiya, I. Arterial oxygenation and shunt fraction during one-lung ventilation: A comparison of isoflurane and sevoflurane. Anesth. Analg. 1998, 86, 1266–1270. [Google Scholar]
- Potočnik, I.; Novak Janković, V.E.S.N.A.; Štupnik, T.; Kremžar, B. Haemodynamic changes after induction of anaesthesia with sevoflurane vs. propofol. Signa Vitae J. Intesive Care Emerg. Med. 2011, 6, 52–57. [Google Scholar] [CrossRef]
- Fujita, Y.; Yamasaki, T.; Takaori, M.; Sekioka, K. Sevoflurane anaesthesia for one-lung ventilation with PEEP to the dependent lung in sheep: Effects on right ventricular function and oxygenation. Can. J. Anaesth. 1993, 40, 1195–1200. [Google Scholar] [CrossRef]
- Filipovic, M.; Wang, J.; Michaux, I.; Hunziker, P.; Skarvan, K.; Seeberger, M.D. Effects of halothane, sevoflurane and propofol on left ventricular diastolic function in humans during spontaneous and mechanical ventilation. Br. J. Anaesth. 2005, 94, 186–192. [Google Scholar] [CrossRef]
- Longnecker, D.E.; Tinker, J.H.; Morgan, G.E. Pharmacology of inhalational anesthetics. In Principles and Practice of Anesthesiology, 2nd ed.; Mosby: St. Louis, MO, USA, 1998. [Google Scholar]
- Beck, D.H.; Doepfmer, U.R.; Sinemus, C.; Bloch, A.; Schenk, M.R.; Kox, W.J. Effects of sevoflurane and propofol on pulmonary shunt fraction during one-lung ventilation for thoracic surgery. Br. J. Anaesth. 2001, 86, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.H.; Ahn, H.J.; Yi, J.W. Total Intravenous anesthesia maintained the degree of pre-existing mitral regurgitation better than isoflurane anesthesia in cardiac surgery: A randomized controlled trial. J. Clin. Med. 2019, 8, 1104. [Google Scholar] [CrossRef]
- Ritchie-McLean, S.; Shankar, R. Calculating oxygen consumption during low-flow anaesthesia. Anaesthesia 2017, 72, 789. [Google Scholar] [CrossRef]
- Jakobsson, J.; Vadman, S.; Hagel, E.; Kalman, S.; Bartha, E. The effects of general anaesthesia on oxygen consumption: A meta-analysis guiding future studies on perioperative oxygen transport. Acta Anaesthesiol. Scand. 2019, 63, 144–153. [Google Scholar] [CrossRef]
- Lugo, G.; Arizpe, D.; Dominguez, G.; Ramirez, M.; Tamariz, O. Relationship between oxygen consumption and oxygen delivery during anesthesia in high-risk surgical patients. Crit. Care Med. 1993, 21, 64–69. [Google Scholar] [CrossRef]
- DiCorte, C.J.; Latham, P.; Greilich, P.E.; Cooley, M.V.; Grayburn, P.A.; Jessen, M.E. Esophageal Doppler monitor determinations of cardiac output and preload during cardiac operations. Ann. Thorac. Surg. 2000, 69, 1782–1786. [Google Scholar] [CrossRef]
- Dark, P.M.; Singer, M. The validity of trans-esophageal Doppler ultrasonography as a measure of cardiac output in critically ill adults. Intensive Care Med. 2004, 30, 2060–2066. [Google Scholar] [CrossRef]
- Laupland, K.B.; Bands, C.J. Utility of esophageal Doppler as a minimally invasive hemodynamic monitor: A review. Can. J. Anaesth. 2002, 49, 393–401. [Google Scholar] [CrossRef]
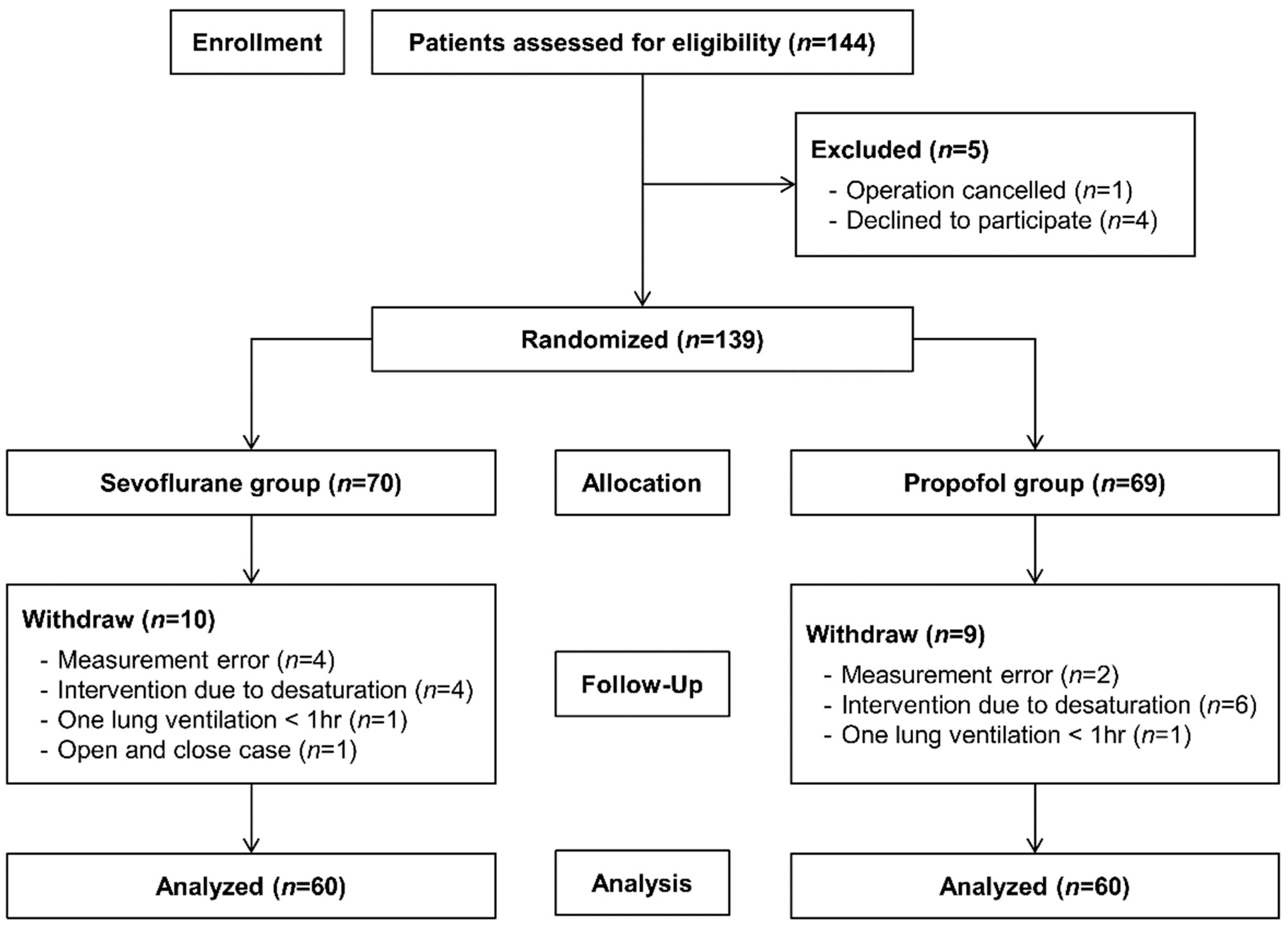

| Characteristics | Sevoflurane Group (n = 60) | Propofol Group (n = 60) |
|---|---|---|
| Age (year) | 64 (57–71) | 62 (55–68) |
| Male | 37 (61.7) | 36 (60.0) |
| Body mass index (kg/m2) | 24 (22–26) | 24 (21–25) |
| ASA physical status, 1/2/3 | 29/23/8 | 25/28/7 |
| Haemoglobin (g/dL) | 13.5 (12.4–14.4) | 13.2 (12.5–15.0) |
| Albumin (g/dL) | 4.5 (4.2–4.8) | 4.5 (4.2–4.7) |
| History of previous lung surgery | 3 (5) | 2 (3) |
| Smoking | 7 (12) | 3 (5) |
| Hypertension | 18 (30) | 16 (27) |
| Diabetes mellitus | 11 (18) | 6 (10) |
| Pulmonary comorbidities | ||
| Recent respiratory infection | 3 (5) | 1 (2) |
| History of pulmonary tuberculosis | 1 (2) | 2 (3) |
| COPD | 2 (3) | 2 (3) |
| Bronchiectasis | 0 (0) | 3 (5) |
| Cardiac disease | 3 (5) | 4 (7) |
| Liver disease | 7 (12) | 9 (15) |
| Renal disease | 4 (7) | 4 (7) |
| Previous chemotherapy and radiotherapy | 5 (8) | 4 (7) |
| Surgery, Open/VATS | 12/48 | 13/47 |
| Ventilation site, Left/Right | 40/20 | 46/14 |
| Duration of surgery (min) | 126 (93–157) | 124 (100–158) |
| Anaesthesia time (min) | 176 (142–225) | 176 (147–202) |
| Duration of one lung ventilation (min) | 100 (73–146) | 101 (78–128) |
| Intraoperative fluid amount (mL) | 900 (650–1150) | 925 (750–1150) |
| Intraoperative blood loss (mL) | 100 (50–187) | 100 (50–150) |
| Bispectral index | 45 ± 3 | 44 ± 2 |
| Variables | TLV | OLV 15 | OLV 45 |
|---|---|---|---|
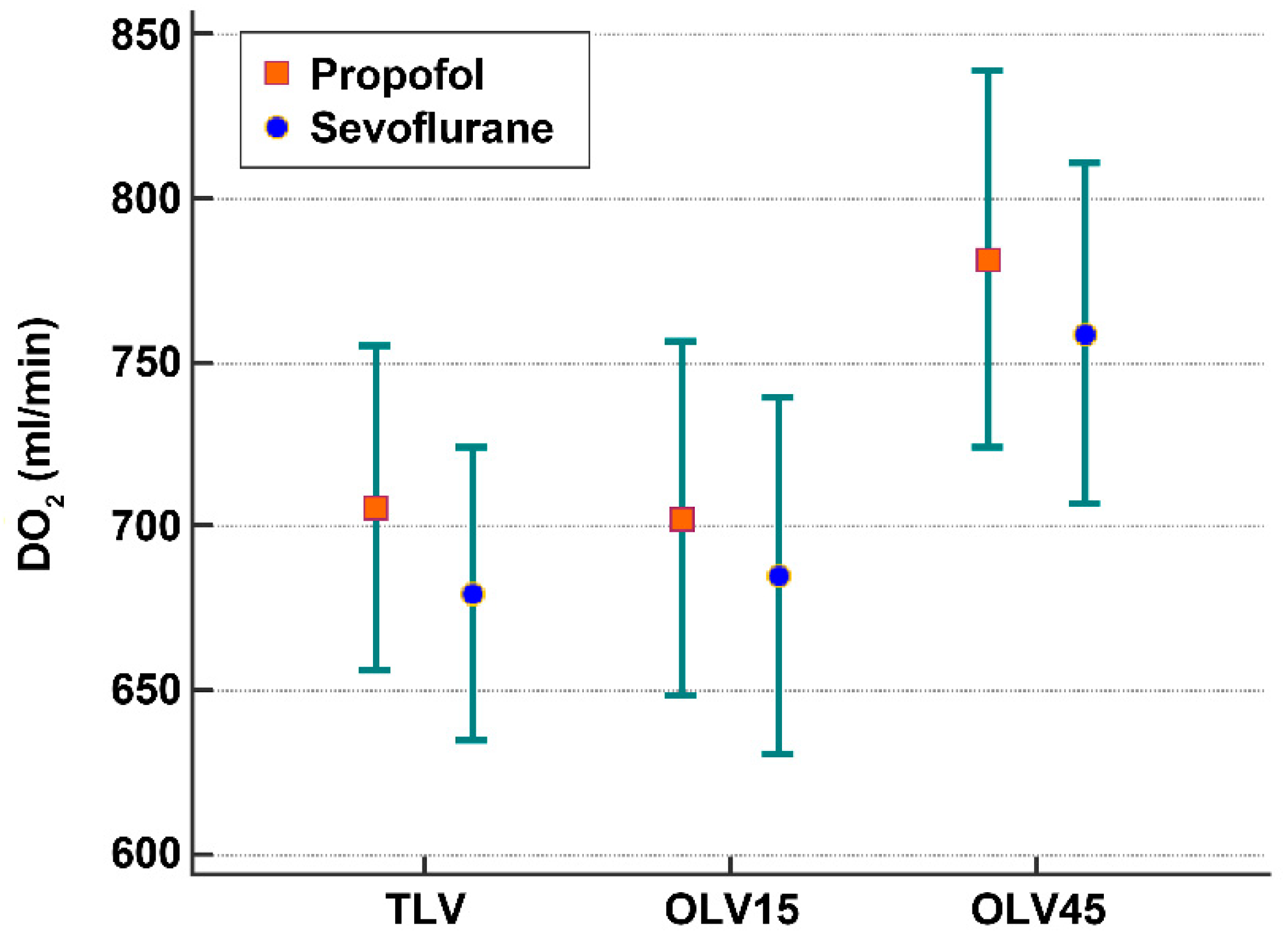
| DO2 (mL/min) | |||
| Sevoflurane | 680 ± 173 | 685 ± 209 | 759 ± 201 |
| Propofol | 706 ± 191 | 703 ± 208 | 782 ± 222 |
| SaO2 (%) | |||
| Sevoflurane | 98.8 ± 0.5 | 97.7 ± 2.0 | 97.4 ± 2.0 |
| Propofol | 98.8 ± 0.4 | 97.8 ± 1.8 | 97.8 ± 1.7 |
| Stroke volume (mL) | |||
| Sevoflurane | 54 ± 15 * | 61 ± 23 | 62 ± 22 |
| Propofol | 60 ± 14 | 63 ± 20 | 70 ± 24 |
| Heart rate (per min) | |||
| Sevoflurane | 70 ± 11 | 71 ± 11 | 75 ± 11 |
| Propofol | 68 ± 12 | 69 ± 12 | 71 ± 13 |
| Mean arterial pressure (mmHg) | |||
| Sevoflurane | 85 ± 14 | 85 ± 14 | 81 ± 11 |
| Propofol | 87 ± 14 | 87 ± 14 | 80 ± 11 |
| Cardiac output (L/min) | |||
| Sevoflurane | 3.8 ± 1.0 | 4.0 ± 1.2 | 4.4 ± 1.1 |
| Propofol | 3.9 ± 1.0 | 4.0 ± 1.0 | 4.5 ± 1.1 |
| Haemoglobin (g/dL) | |||
| Sevoflurane | 12.6 ± 1.2 | 12.6 ± 1.1 | 12.6 ± 1.2 |
| Propofol | 12.7 ± 1.3 | 12.7 ± 1.5 | 12.6 ± 1.3 |
| Alveolar-arterial O2 difference (mmHg) | |||
| Sevoflurane | 181 ± 92 | 426 ± 107 | 366 ± 146 |
| Propofol | 190 ± 101 | 418 ± 105 | 367 ± 121 |
| PaO2/FIO2 | |||
| Sevoflurane | 483 ± 88 | 235 ± 110 | 244 ± 133 |
| Propofol | 479 ± 105 | 249 ± 108 | 257 ± 121 |
| Plasma lactate (mmol/L) | |||
| Sevoflurane | 1.39 ± 0.49 | 1.40 ± 0.53 | 1.42 ± 0.48 † |
| Propofol | 1.23 ± 0.39 | 1.23 ± 0.37 | 1.21 ± 0.36 |
| Anion gap (mmol/L) | |||
| Sevoflurane | 11.4 ± 1.8 | 11.1 ± 2.0 | 11.1 ± 2.5 |
| Propofol | 11.1 ± 2.0 | 10.9 ± 1.9 | 10.3 ± 2.8 |
| Categories | Mean DO2 | Mean SaO2 |
|---|---|---|
| DO2, at <lower 10th percentile | 412 ± 52 | 97.5 ± 2.3 |
| DO2, at <500 mL min−1 cut-off | 435 ± 56 | 97.6 ± 2.2 |
| DO2, at the lowest | 255 | 98.7 |
| DO2, at SaO2 < 94% | 641 ± 203 | 92.4 ± 1.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hahm, T.S.; Jeong, H.; Ahn, H.J. Systemic Oxygen Delivery during One-Lung Ventilation: Comparison between Propofol and Sevoflurane Anaesthesia in a Randomised Controlled Trial. J. Clin. Med. 2019, 8, 1438. https://doi.org/10.3390/jcm8091438
Hahm TS, Jeong H, Ahn HJ. Systemic Oxygen Delivery during One-Lung Ventilation: Comparison between Propofol and Sevoflurane Anaesthesia in a Randomised Controlled Trial. Journal of Clinical Medicine. 2019; 8(9):1438. https://doi.org/10.3390/jcm8091438
Chicago/Turabian StyleHahm, Tae Soo, Heejoon Jeong, and Hyun Joo Ahn. 2019. "Systemic Oxygen Delivery during One-Lung Ventilation: Comparison between Propofol and Sevoflurane Anaesthesia in a Randomised Controlled Trial" Journal of Clinical Medicine 8, no. 9: 1438. https://doi.org/10.3390/jcm8091438
APA StyleHahm, T. S., Jeong, H., & Ahn, H. J. (2019). Systemic Oxygen Delivery during One-Lung Ventilation: Comparison between Propofol and Sevoflurane Anaesthesia in a Randomised Controlled Trial. Journal of Clinical Medicine, 8(9), 1438. https://doi.org/10.3390/jcm8091438





