Longitudinal Asthma Patterns in Italian Adult General Population Samples: Host and Environmental Risk Factors
Abstract
:1. Introduction
2. Materials and Methods
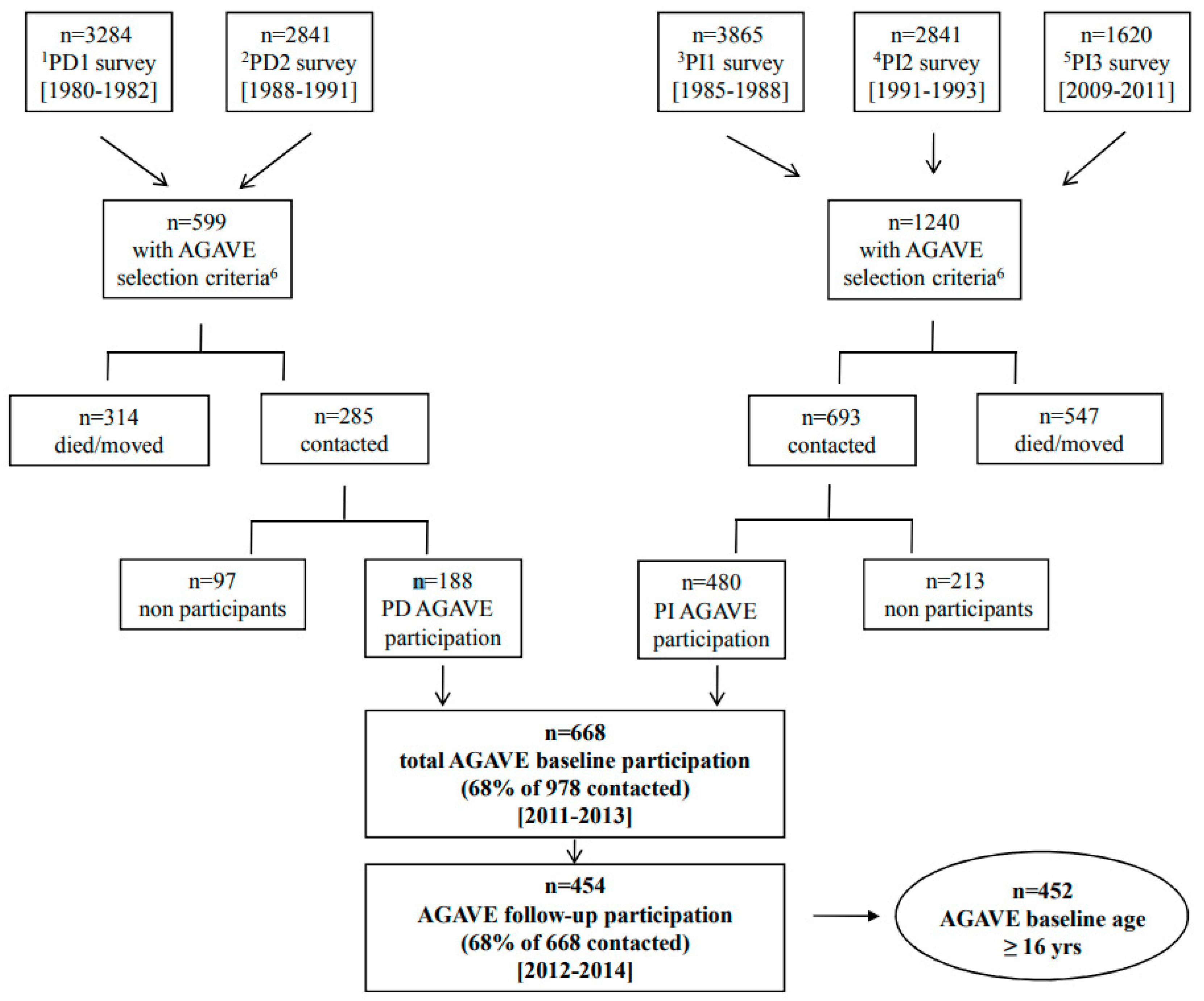
2.1. Study Population
2.2. Data Collection Tool
2.3. Statistical Analyses
3. Results
3.1. Baseline Subject Characteristics
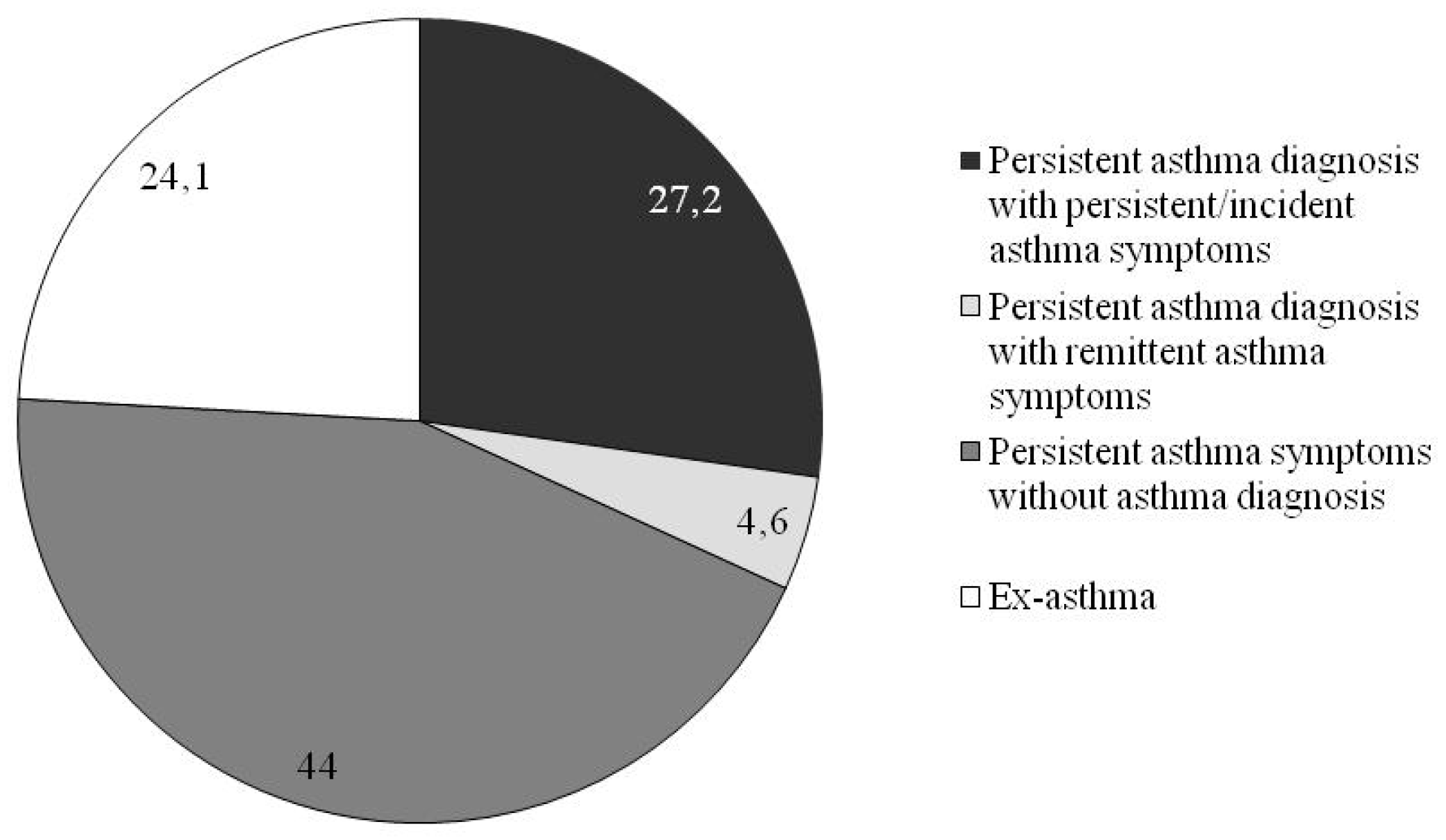
3.2. Asthma Phenotypes
3.3. Subject Characteristics by Asthma Patterns
3.4. Asthma-Related Indicators by Longitudinal Asthma Patterns
3.5. Baseline Host and Environmental Risk Factors for Longitudinal Asthma Patterns
4. Discussion
4.1. Comorbidities
4.2. Environmental Risk Factors
4.3. Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Update Accessed January, 2019. Available online: https://ginasthma.org/2018-gina-report-global-strategy-for-asthma-management-and-prevention/ (accessed on 18 September 2020).
- Maio, S.; Baldacci, S.; Simoni, M.; Angino, A.; Martini, F.; Cerrai, S.; Sarno, G.; Pala, A.; Bresciani, M.; ARGA Study Group; et al. Impact of Asthma and Comorbid Allergic Rhinitis on Quality of Life and Control in Patients of Italian General Practitioners. J. Asthma 2012, 49, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Von Mutius, E.; Smits, H.H. Primary prevention of asthma: From risk and protective factors to targeted strategies for prevention. Lancet 2020. [Google Scholar] [CrossRef]
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [Green Version]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators: Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- Guerra, S. Clinical remission of asthma: What lies beyond? Thorax 2005, 60, 5–6. [Google Scholar] [CrossRef] [Green Version]
- Gershon, A.; Guan, J.; Victor, J.; Wang, C.; To, T. The course of asthma activity: A population study. J. Allergy Clin. Immunol. 2012, 129, 679–686. [Google Scholar] [CrossRef]
- Trivedi, M.; Denton, E. Asthma in Children and Adults—What Are the Differences and What Can They Tell us about Asthma? Front. Pediatr. 2019, 7, 256. [Google Scholar] [CrossRef] [Green Version]
- To, M.; Tsuzuki, R.; Katsube, O.; Yamawaki, S.; Soeda, S.; Kono, Y.; Honda, N.; Kano, I.; Haruki, K.; To, Y. Persistent Asthma from Childhood to Adulthood Presents a Distinct Phenotype of Adult Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 1921–1927. [Google Scholar] [CrossRef]
- Yavuz, S.T.; Civelek, E.; Comert, S.; Sahiner, U.M.; Buyuktiryaki, B.; Tuncer, A.; Kalyoncu, A.F.; Sekerel, B.E. Development of rhinitis may be an indicator for the persistence of childhood asthma. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 843–849. [Google Scholar] [CrossRef]
- Papaioannou, A.I.; Kostikas, K.; Zervas, E.; Kolilekas, L.; Papiris, S.; Gaga, M. Control of asthma in real life: Still a valuable goal? Eur. Respir. Rev. 2015, 24, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Garden, F.L.; Simpson, J.M.; Mellis, C.M.; Marks, G.B. For the CAPS Investigator. Change in the manifestations of asthma and asthma-related traits in childhood: A latent transition analysis. Eur. Respir. J. 2016, 47, 499–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brew, B.K.; Chiesa, F.; Lundholm, C.; Örtqvist, A.; Almqvist, C. A modern approach to identifying and characterizing child asthma and wheeze phenotypes based on clinical data. PLoS ONE 2019, 14, e0227091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto-Ramírez, N.; Ziyab, A.H.; Karmaus, W.; Zhang, H.; Kurukulaaratchy, R.J.; Ewart, S.; Arshad, S.H. Epidemiologic Methods of Assessing Asthma and Wheezing Episodes in Longitudinal Studies: Measures of Change and Stability. J. Epidemiol. 2013, 23, 399–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaral, R.; Pereira, A.M.; Jacinto, T.; Malinovschi, A.; Janson, C.; Alving, K.; Fonseca, J.A. Comparison of hypothesis- and data-driven asthma phenotypes in NHANES 2007–2012: The importance of comprehensive data availability. Clin. Transl. Allergy 2019, 9, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maio, S.; Baldacci, S.; Bresciani, M.; Simoni, M.; Latorre, M.; Murgia, N.; Spinozzi, F.; Braschi, M.; Antonicelli, L.; Brunetto, B.; et al. RItA: The Italian severe/uncontrolled asthma registry. Allergy 2018, 73, 683–695. [Google Scholar] [CrossRef]
- Viegi, G.; Pedreschi, M.; Baldacci, S.; Chiaffi, L.; Pistelli, F.; Modena, P.; Vellutini, M.; Di Pede, F.; Carrozzi, L. Prevalence rates of respiratory symptoms and diseases in general population samples of North and Central Italy. Int. J. Tuberc. Lung Dis. 1999, 3, 1034–1042. [Google Scholar]
- Maio, S.; Baldacci, S.; Carrozzi, L.; Pistelli, F.; Angino, A.; Simoni, M.; Sarno, G.; Cerrai, S.; Martini, F.; Fresta, M.; et al. Respiratory symptoms/diseases prevalence is still increasing: A 25-yr population study. Respir. Med. 2016, 110, 58–68. [Google Scholar] [CrossRef] [Green Version]
- The European Community Respiratory Health Survey II Steering Committee. The European Community Respiratory Health Survey II. Eur. Respir. J. 2002, 20, 1071–1079. [Google Scholar] [CrossRef] [Green Version]
- Fazzi, P.; Viegi, G.; Paoletti, P.; Giuliano, G.; Begliomini, E.; Fornai, E.; Giuntini, C. Comparison between two standardized questionnaires in a group of workers. Eur. J. Respir. Dis. 1982, 63, 168–169. [Google Scholar]
- Viegi, G.; Paoletti, P.; Prediletto, R.; Carrozzi, L.; Fazzi, P.; Di Pede, F.; Pistelli, G.; Giuntini, C.; Lebowitz, M.D. Prevalence of respiratory symptoms in an unpolluted area of northern Italy. Eur. Respir. J. 1988, 1, 311–318. [Google Scholar]
- Viegi, G. Indicators and biological mechanisms of impairment of the respiratory system by environmental pollutants. An operational example: Epidemiological studies of the delta of Po and the region of Pisa. Epidemiol. Prev. 1995, 19, 66–75. [Google Scholar] [PubMed]
- Weinmayr, G.; Keller, F.; Kleiner, A.; du Prel, J.B.; Garcia-Marcos, L.; Batllés-Garrido, J.; Garcia-Hernandez, G.; Suarez-Varela, M.M.; Strachan, D.P.; Nagel, G. Asthma phenotypes identified by latent class analysis in the ISAAC phase II Spain study. Clin. Exp. Allergy 2013, 43, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Boudier, A.; Curjuric, I.; Basagaña, X.; Hazgui, H.; Anto, J.M.; Bousquet, J.; Bridevaux, P.O.; Dupuis-Lozeron, E.; Garcia-Aymerich, J.; Heinrich, J.; et al. Ten-Year Follow-up of Cluster-based Asthma Phenotypes in Adults. A Pooled Analysis of Three Cohorts. Am. J. Respir. Crit. Care Med. 2013, 188, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.M.; Lanza, S.T. Latent Class and Latent Transition Analysis with Applications in the Social, Behavioral, and Health Sciences; Wiley Series in Probability and Statistics 2010; Balding, D., Cressie, N.A.C., Fitzmaurice, G.M., Johnstone, I.M., Molenberghs, G., Scott, L., Smith, A.F.M., Tsay, R.S., Weisberg, S.P., Eds.; A John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 1–283. [Google Scholar]
- Hsu, J.; Chen, J.; Mirabelli, M.C. Asthma Morbidity, Comorbidities, and Modifiable Factors among Older Adults. J. Allergy Clin. Immunol. Pract. 2018, 6, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.M.; Jackson, D.J. Microbes, allergic sensitization, and the natural history of asthma. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 116–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, G.E.; Sherrill, D.L.; Guerra, S.; Barbee, R.A. Asthma as a risk factor for COPD in a longitudinal study. Chest 2004, 126, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.-X.; Xiao, Y. Asthma and Obstructive Sleep Apnea. Chin. Med. J. 2015, 128, 2798–2804. [Google Scholar] [CrossRef]
- Prasad, B.; Nyenhuis, S.M.; Imayama, I.; Siddiqi, A.; Teodorescu, M. Asthma and Obstructive Sleep Apnea Overlap: What Has the Evidence Taught Us? Am. J. Respir. Crit. Care Med. 2020, 201, 1345–1357. [Google Scholar] [CrossRef]
- Dixit, R.; Verma, S.; Gupta, N.; Sharma, A.; Chandran, A. Obstructive Sleep Apnea in Bronchial Asthma Patients: Assessment of Prevalence and Risk Factors. J. Assoc. Physicians India 2018, 66, 45–48. [Google Scholar]
- Teodorescu, M.; Polomis, D.A.; Teodorescu, M.C.; Gangnon, R.E.; Peterson, A.G.; Consens, F.B.; Chervin, R.D.; Jarjour, N.N. Association of obstructive sleep apnea risk or diagnosis with daytime asthma in adults. J. Asthma 2012, 49, 620–628. [Google Scholar] [CrossRef]
- Prüss-Ustün, A.; Wolf, J.; Corvalán, C.; Bos, R.; Neira, M. Preventing Disease through Healthy Environments. A Global Assessment of the Burden of Disease from Environmental Risks. WHO. 2016. Available online: http://www.who.int/quantifying_ehimpacts/publications/preventing-disease/en/ (accessed on 18 September 2020).
- Thurston, G.D.; Kipen, H.; Annesi-Maesano, I.; Balmes, J.; Brook, R.D.; Cromar, K.; De Matteis, S.; Forastiere, F.; Forsberg, B.; Frampton, M.W.; et al. A joint ERS/ATS policy statement: What constitutes an adverse health effect of air pollution? An analytical framework. Eur. Respir. J. 2017, 49, 1600419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thurston, G.D.; Balmes, J.R.; Garcia, E.; Gilliland, F.D.; Rice, M.B.; Schikowski, T.; Van Winkle, L.S.; Annesi-Maesano, I.; Burchard, E.G.; Carlsten, C.; et al. Outdoor Air Pollution and New-Onset Airway Disease. An Official American Thoracic Society Workshop Report. Ann. Am. Thorac. Soc. 2020, 17, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Bowatte, G.; Lodge, C.J.; Knibbs, L.D.; Erbas, B.; Perret, J.L.; Jalaludin, B.; Morgan, G.G.; Bui, D.S.; Giles, G.G.; Hamilton, G.S.; et al. Traffic related air pollution and development and persistence of asthma and low lung function. Environ. Int. 2018, 113, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Hegseth, M.N.; Oftedal, B.M.; Höper, A.C.; Aminoff, A.L.; Thomassen, M.R.; Svendsen, M.V.; Fell, A.K.M. Self-reported traffic-related air pollution and respiratory symptoms among adults in an area with modest levels of traffic. PLoS ONE 2019, 14, e0226221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maio, S.; Baldacci, S.; Carrozzi, L.; Polverino, E.; Angino, A.; Pistelli, F.; Di Pede, F.; Simoni, M.; Sherrill, D.; Viegi, G. Urban residence is associated with bronchial hyperresponsiveness in Italian general population samples. Chest 2009, 135, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Raza, A.; Kurukulaaratchy, R.J.; Grundy, J.D.; Clayton, C.B.; Mitchell, F.A.; Roberts, G.; Ewart, S.; Sadeghnejad, A.; Arshad, S.H. What does adolescent undiagnosed wheeze represent? Findings from the Isle of Wight Cohort. Eur. Respir. J. 2012, 40, 580–588. [Google Scholar] [CrossRef] [Green Version]
- Accordini, S.; Cappa, V.; Braggion, M.; Corsico, A.G.; Bugiani, M.; Pirina, P.; Verlato, G.; Villani, S.; de Marco, R. The impact of diagnosed and undiagnosed current asthma in the general adult population. Int. Arch. Allergy Immunol. 2011, 155, 403–411. [Google Scholar] [CrossRef]
- Yeatts, K.; Davis, K.J.; Sotir, M.; Herget, C.; Shy, C. Who gets diagnosed with asthma? Frequent wheeze among adolescents with and without a diagnosis of asthma. Pediatrics 2003, 111, 1046–1054. [Google Scholar] [CrossRef]
- Kurukulaaratchy, R.J.; Zhang, H.; Raza, A.; Patil, V.; Karmaus, W.; Ewart, S.; Arshad, S.H. The Diversity of Young Adult Wheeze; a Cluster Analysis in a Longitudinal Birth Cohort. Clin. Exp. Allergy 2014, 44, 724–735. [Google Scholar] [CrossRef] [Green Version]
- Thomson, N.C. Asthma and smoking-induced airway disease without spirometric COPD. Eur. Respir. J. 2017, 49, 1602061. [Google Scholar] [CrossRef]
- Chatkin, J.M.; Dullius, C.R. The management of asthmatic smokers. Asthma Res. Pract. 2016, 2, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simoni, M.; Baldacci, S.; Puntoni, R.; Pistelli, F.; Farchi, S.; Lo Presti, E.; Pistelli, R.; Corbo, G.; Agabiti, N.; Basso, S.; et al. Respiratory symptoms/diseases and environmental tobacco smoke (ETS) in never smoker Italian women. Respir. Med. 2007, 101, 531–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hersoug, L.G.; Husemoen, L.L.; Sigsgaard, T.; Madsen, F.; Linneberg, A. Indoor exposure to environmental cigarette smoke, but not other inhaled particulates associates with respiratory symptoms and diminished lung function in adults. Respirology 2010, 15, 993–1000. [Google Scholar] [CrossRef]
- Pesce, G.; Locatelli, F.; Cerveri, I.; Bugiani, M.; Pirina, P.; Johannessen, A.; Accordini, S.; Zanolin, M.E.; Verlato, G.; de Marco, R. Seventy Years of Asthma in Italy: Age, Period and Cohort Effects on Incidence and Remission of Self-Reported Asthma from 1940 to 2010. PLoS ONE 2015, 10, e0138570. [Google Scholar] [CrossRef] [PubMed]
- Pistelli, F.; Maio, S. Questionnaires and lung function. In Respiratory Epidemiology; Annesi-Maesano, I., Lundback, B., Viegi, G., Eds.; European Respiratory Society Publications: Sheffield, UK, 2014; Volume 65, pp. 257–272. [Google Scholar]
- Bougas, N.; Just, J.; Beydon, N.; De Blic, J.; Gabet, S.; Lezmi, G.; Amat, F.; Rancière, F.; Momas, I. Unsupervised trajectories of respiratory/allergic symptoms throughout childhood in the PARIS cohort. Pediatr. Allergy Immunol. 2019, 30, 315–324. [Google Scholar] [CrossRef] [PubMed]

| Gender (%): | |
| Males | 47.3 |
| Females | 52.7 |
| Age (mean ± SD 1) (years) | 56.7 ± 15.5 |
| Age range (min-max) | 17–91 |
| BMI 2 (kg/m2) groups 3 (%): | |
| obese (BMI ≥ 30 kg/m2) | 15.7 |
| overweight (BMI 25.0–29.9 kg/m2) | 35.3 |
| normal weight (BMI 18.5–24.9 kg/m2) | 46.6 |
| underweight (BMI < 18.5 kg/m2) | 2.4 |
| Educational level (%): | |
| elementary/junior high school | 45.2 |
| high school | 35.9 |
| university | 18.9 |
| Host Risk Factors | |
| Reported SPT 1 positivity (%) | 74.9 |
| Family history of asthma (%) | 40.7 |
| Allergic rhinitis (%) | 40.8 |
| GERD 2 (%) | 29.6 |
| Sleep apnea (%) | 14.7 |
| Recurrent respiratory infections (%) | 13.5 |
| COPD 3 (%) | 12.5 |
| Nasal polyps (%) | 3.4 |
| Environmental Risk Factors | |
| Traffic exposure at home address (%) | 58.4 |
| Smoking habits (%): | |
| current smokers | 19.4 |
| ex-smokers | 40.3 |
| Pack-years in current smokers (mean ± SD 4), n | 23.6 ± 17.7 |
| Pack-years in ex-smokers (mean ± SD 4), n | 27.2 ± 27.9 |
| Secondhand smoke exposure (%) | 17.4 |
| Baseline | Follow-Up | |||||
|---|---|---|---|---|---|---|
| Asthma Diagnosis and Current Asthma Symptoms (28.5%) | Current Asthma Symptoms (45.6%) | Previous Asthma Diagnosis (25.9%) | Asthma Diagnosis and Current Asthma Symptoms (27.0%) | Current Asthma Symptoms (44.2%) | Previous Asthma Diagnosis (28.8%) | |
| Current wheeze | 64.8 | 30.2 | 12 | 73.8 | 32 | 9.2 |
| Current asthma attacks | 57.4 | 10.2 | 0 | 50.4 | 13.5 | 3.1 |
| Current asthma diagnosis | 85.3 | 0 | 0 | 86.1 | 1.5 | 0.8 |
| Previous asthma diagnosis | 14.7 | 0 | 100 | 11.5 | 0 | 99.2 |
| Persistent Asthma Diagnosis with Persistent/Incident Asthma Symptoms (n = 123) | Persistent Asthma Diagnosis with Remittent Asthma Symptoms (n = 21) | Persistent Asthma Symptoms without Asthma Diagnosis (n = 199) | Ex-Asthma (n = 109) | p-Value | |
|---|---|---|---|---|---|
| Sex (%): | 0.108 | ||||
| males | 39.0 | 52.4 | 52.8 | 45.9 | |
| females | 61.0 | 47.6 | 47.2 | 54.1 | |
| Age (years) (mean ± SD 1) | 55.6 ± 16.8 | 47.9 ± 14.8 | 61.6 ± 14.1 | 50.6 ± 13.7 | 0.000 |
| BMI 2 groups (%): | 0.000 | ||||
| obese | 15.4 | 4.86 | 19.1 | 12.0 | |
| overweight | 31.7 | 47.6 | 43.2 | 22.2 | |
| underweight/normal weight | 52.8 | 47.6 | 37.7 | 65.7 | |
| Educational level (%): | 0.000 | ||||
| elementary/junior high school | 43.3 | 23.8 | 55.8 | 32.1 | |
| high school | 37.5 | 33.3 | 30.7 | 44.0 | |
| university | 19.2 | 42.9 | 13.6 | 23.9 |
| Persistent Asthma Diagnosis with Persistent/Incident Asthma Symptoms (n = 123) | Persistent Asthma Diagnosis with Remittent Asthma Symptoms (n = 21) | Persistent Asthma Symptoms without Asthma Diagnosis (n = 199) | Ex-Asthma (n = 109) | p-Value | |
|---|---|---|---|---|---|
| Age at asthma diagnosis (mean ± SD 1) 2 | 27.1 ± 20.6 | 9.9 ± 11.6 | --- | 13.8 ± 12.7 | 0.000 |
| Age at first asthma symptoms (mean ± SD 1) 2 | 25.3 ± 20.3 | 8.0 ± 7.6 | --- | 13.1 ± 12.6 | 0.000 |
| Family history of asthma (%) | 56.1 | 52.4 | 32.6 | 35.2 | 0.000 |
| Last 12-month asthma exacerbations 3 (%) | 15.6 | 5.9 | 4.9 | 0.0 | 0.002 |
| Last 12-month asthma hospitalizations 3 (%) | 5.0 | 5.9 | 0.0 | 1.6 | 0.144 |
| Last 12-month asthma ED 4 visits 3 (%) | 4.2 | 5.9 | 1.2 | 1.6 | 0.473 |
| Reported SPT 5 positivity (%) | 83.5 | 80.0 | 65.5 | 70.5 | 0.081 |
| Persistent Asthma Diagnosis with Persistent/Incident Asthma Symptoms (n = 123) | Persistent Asthma Diagnosis with Remittent Asthma Symptoms (n = 21) | Persistent Asthma Symptoms without Asthma Diagnosis (n = 199) | Ex-Asthma (n = 109) | p-Value | |
|---|---|---|---|---|---|
| Asthma comorbidities | |||||
| Allergic rhinitis (%) | 61.0 | 66.7 | 28.3 | 35.8 | 0.000 |
| GERD 1 (%) | 30.1 | 23.8 | 34.8 | 20.6 | 0.067 |
| Sleep apnea (%) | 20.3 | 10.0 | 18.3 | 2.8 | 0.000 |
| Recurrent respiratory infections (%) | 23.1 | 4.8 | 11.3 | 8.3 | 0.003 |
| COPD 2 (%) | 21.1 | 9.5 | 11.9 | 4.6 | 0.002 |
| Nasal polyps | 5.7 | 0.0 | 4.1 | 0.0 | 0.074 |
| Environmental risk factors | |||||
| Traffic exposure at home address (%) | 69.1 | 38.1 | 56.3 | 54.1 | 0.015 |
| Smoking habits (%): | 0.000 | ||||
| smokers | 15.4 | 14.3 | 27.1 | 11.0 | |
| ex-smokers | 34.1 | 28.6 | 47.2 | 36.7 | |
| nonsmokers | 50.4 | 57.1 | 25.6 | 52.3 | |
| Secondhand smoke exposure (%) | 18.9 | 9.5 | 21.4 | 10.2 | 0.066 |
| Persistent Asthma Diagnosis with Persistent/Incident Asthma Symptoms | Persistent Asthma Diagnosis with Remittent Asthma Symptoms | Persistent Asthma Symptoms without Asthma Diagnosis | |
|---|---|---|---|
| Host factors | |||
| Allergic rhinitis | 3.12 (1.72–5.69) | 3.38 (1.17–9.76) | 0.99 (0.56–1.78) |
| (ref: no) | 1.00 | 1.00 | 1.00 |
| COPD 1 | 4.76 (1.60–4.16) | 3.48 (0.55–21.94) | 1.32 (0.44–3.97) |
| (ref: no) | 1.00 | 1.00 | 1.00 |
| Sleep apnea | 5.99 (1.67–21.39) | 3.49 (0.50–24.44) | 5.32 (1.52–18.58) |
| (ref: no) | 1.00 | 1.00 | 1.00 |
| Environmental factors | |||
| Traffic exposure at | |||
| home address | 1.86 (1.02–3.38) | 0.48 (0.17–1.35) | 1.31 (0.75–2.28) |
| (ref: no) | 1.00 | 1.00 | 1.00 |
| Smoking habits: | |||
| smokers | 1.59 (0.64–3.96) | 1.19 (0.26–5.47) | 6.24 (2.68–14.51) |
| ex-smokers | 0.71 (0.36–1.40) | 0.45 (0.13–1.60) | 1.71 (0.90–3.25) |
| (ref: non smokers) | 1.00 | 1.00 | 1.00 |
| Secondhand smoke exposure | 2.64 (1.10–6.33) | 0.93 (0.17–4.94) | 3.28 (1.41–7.66) |
| (ref: no) | 1.00 | 1.00 | 1.00 |
| Adjustment factors | |||
| Age (unit increase) | 1.02 (1.00–1.04) 1.00 | 0.98 (0.94–1.02) 1.00 | 1.05 (1.03–1.07) 1.00 |
| Males | 0.84 (0.44–1.59) | 1.49 (0.47–4.70) | 0.84 (0.47–1.53) |
| (ref: females) | 1.00 | 1.00 | 1.00 |
| BMI categories: | |||
| obese | 1.46 (0.60–3.51) | 0.63 (0.07–5.85) | 1.79 (0.80–4.03) |
| overweight | 1.77 (0.84–3.72) | 4.58 (1.33–15.72) | 2.37 (1.20–4.68) |
| (ref: underweight/normal weight) | 1.00 | 1.00 | 1.00 |
| Family history of asthma | 2.15 (1.18–3.92) | 2.60 (0.91–7.45) | 0.91 (0.51–1.62) |
| (ref: no) | 1.00 | 1.00 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maio, S.; Baldacci, S.; Simoni, M.; Angino, A.; La Grutta, S.; Muggeo, V.; Fasola, S.; Viegi, G.; on behalf of the AGAVE Pisa Group. Longitudinal Asthma Patterns in Italian Adult General Population Samples: Host and Environmental Risk Factors. J. Clin. Med. 2020, 9, 3632. https://doi.org/10.3390/jcm9113632
Maio S, Baldacci S, Simoni M, Angino A, La Grutta S, Muggeo V, Fasola S, Viegi G, on behalf of the AGAVE Pisa Group. Longitudinal Asthma Patterns in Italian Adult General Population Samples: Host and Environmental Risk Factors. Journal of Clinical Medicine. 2020; 9(11):3632. https://doi.org/10.3390/jcm9113632
Chicago/Turabian StyleMaio, Sara, Sandra Baldacci, Marzia Simoni, Anna Angino, Stefania La Grutta, Vito Muggeo, Salvatore Fasola, Giovanni Viegi, and on behalf of the AGAVE Pisa Group. 2020. "Longitudinal Asthma Patterns in Italian Adult General Population Samples: Host and Environmental Risk Factors" Journal of Clinical Medicine 9, no. 11: 3632. https://doi.org/10.3390/jcm9113632





