Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (FTIR) and Artificial Neural Networks Applied to Investigate Quantitative Changes of Selected Soluble Biomarkers, Correlated with H. pylori Infection in Children and Presumable Consequent Delayed Growth
Abstract
:1. Introduction
2. Material and Methods
2.1. Patients and Controls
2.2. Diagnosis of H. pylori Infection
2.3. The Measurement of Infrared Spectra and Their Processing
2.4. Mathematical Model Development for Patient Differentiation
2.5. Statistical Analysis
3. Results
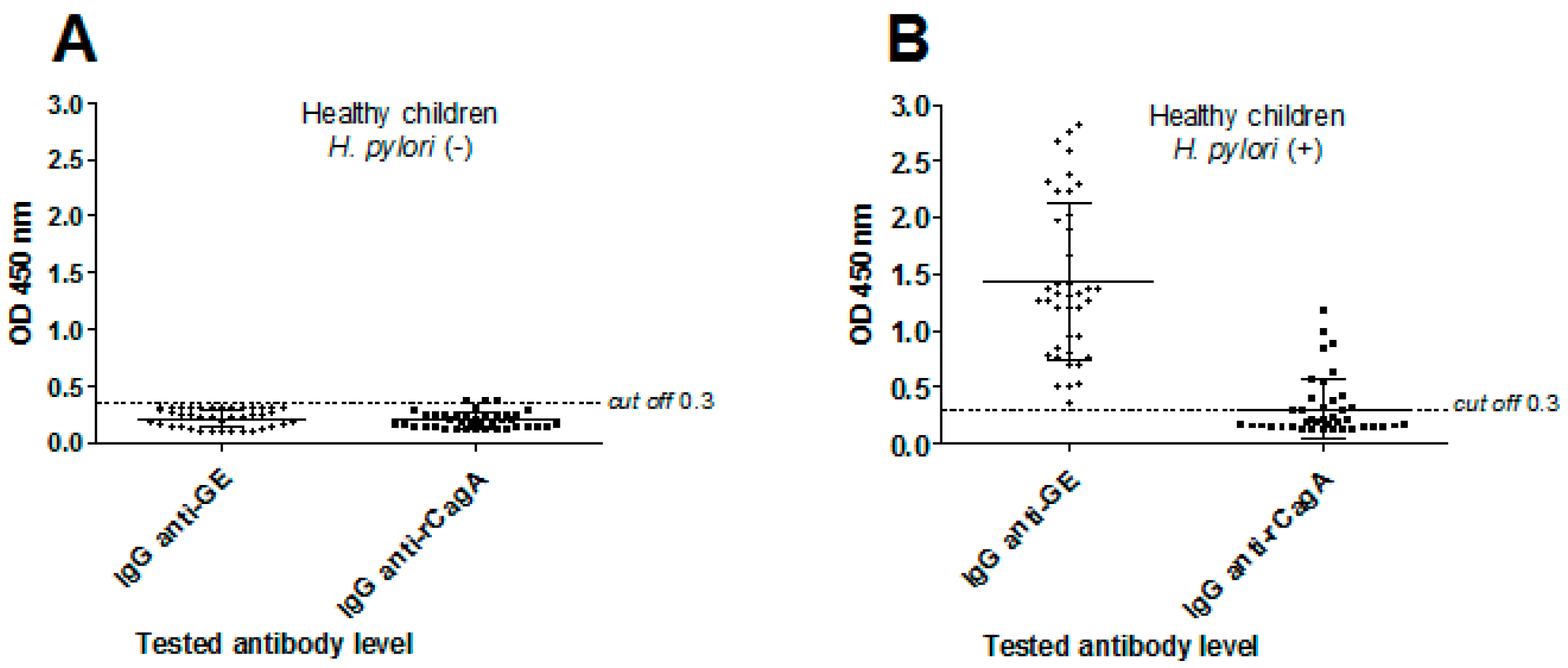
3.1. H. pylori Serological Status in Children
3.2. Analysis of IR Spectra of Human Sera
3.3. Wavenumbers Correlating with H. pylori Infection and Mathematical Models Identifying Sera of Infected Individuals
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Warren, J.R.; Marshall, B.J. Unidentified curved bacilli on the gastric epithelium in active chronic gastritis. Lancet 1983, 1, 1311–1315. [Google Scholar]
- Blaser, M.J.; Atherton, J.C. Helicobacter infection persistence: Biology and disease. J. Clin. Investig. 2004, 113, 321–333. [Google Scholar] [CrossRef] [Green Version]
- Peek, R.M.; Crabtree, J.M. H. pylori infection and gastric neoplasia. J. Pathol. 2006, 208, 233–248. [Google Scholar] [CrossRef]
- Posselt, G.; Backert, S.; Wessler, S. The functional interplay of H. pylori factors with gastric epithelial cells induces a multi-step process in pathogenesis. Cell Commun. Signal. 2013, 11, 77. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N.; Murata-Kamiya, N.; Yanagiya, K.; Suda, W.; Hattori, M.; Kanda, H.; Bingo, A.; Fujii, Y.; Maeda, S.; Koike, K.; et al. Mutual reinforcement of inflammation and carcinogenesis by the H. pylori CagA oncoprotein. Sci. Rep. 2015, 5, 10024. [Google Scholar] [CrossRef] [Green Version]
- Chmiela, M.; Karwowska, Z.; Gonciarz, W.; Allushi, B.; Stączek, P. Host pathogen interactions in Helicobacter pylori related gastric cancer. World J. Gastroenterol. 2017, 23, 1521–1540. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Manoj, G.; Khan, A.A.; Habeeb, A.; Habibullah, C.M. Chronic idiopathic Thrombocytopenia purpura and H. pylori eradication: A case study. Gastroenterology Res. 2009, 2, 57–59. [Google Scholar] [CrossRef] [Green Version]
- Yeh, J.J.; Tsai, S.; Wu, D.C.; Wu, J.Y.; Liu, T.C.; Chen, A. P-selectin-dependent platelet aggregation and apoptosis may explain the decrease in platelet count during H. pylori infection. Blood 2010, 115, 4247–4253. [Google Scholar] [CrossRef] [Green Version]
- Papagiannakis, P.; Michalopoulos, C.; Papalexi, F.; Dalampoura, D.; Diamantidis, M.D. The role of H. pylori infection in haematological disorders. Eur. J. Intern. Med. 2013, 24, 685–690. [Google Scholar] [CrossRef]
- Chao-Hung, K.; Yen-Hsu, C.H.; Khean-Lee, G.; Lin-Li, C. H. pylori and systemic disease. Gastroenterol. Res. Pract. 2014, 358494. [Google Scholar] [CrossRef]
- Tamer, G.S.; Tengiz, I.; Ercan, E.; Duman, C.; Alioglu, E.; Turk, U.O. H. pylori seropositivity in patients with acute coronary syndromes. Did. Dis. Sci. 2009, 54, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.J.; Goh, K.L. Extragastrointestinal manifestations of H. pylori infection: Facts or myth? A critical review. J. Dig. Dis. 2012, 13, 342–349. [Google Scholar] [CrossRef] [PubMed]
- EL-Eshmawy, M.M.; El-Hawary, A.K.; Gawad, S.S.S.; El-Baiomy, A.A. H. pylori infection might be responsible for the interconnection between type 1 diabetes and autoimmune thyroiditis. Diabetol. Matab. Syndr. 2011, 3, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wedi, B.; Raap, U.; Wieczorek, D.; Kaap, A. Urticaria and infections. Allergy Asthma Clin. Immunol. 2009, 5, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, B.; Li, Y.; Zhang, Y.; Bai, L.; Yang, P. H. pylori infection and lung cancer: A review of an emerging hypothesis. Carcinogenesis 2003, 4, 1189–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roper, J.; Francois, F.; Shue, P.L.; Mourad, M.S.; Pei, Z.; Olivares de Perez, A.Z.; Perez-Perez, G.I.; Tseng, C.H.; Blaser, M.J. Leptin and ghrelin in relation to H. pylori in adult males. J. Clin. Endocrinol. Matab. 2008, 93, 2350–2357. [Google Scholar] [CrossRef] [Green Version]
- Paoluzi, O.A.; del Blanco, V.G.; Caruso, R.; Monteleone, G.; Pallone, F. Impairement of ghrelin synthesis in H. pylori-colonized stomach: New clues for the pathogenesis of H. pylori-related gastric inflammation. World J. Gastroenterol. 2014, 20, 639–646. [Google Scholar] [CrossRef]
- Płonka, M.; Bielanski, W.; Konturek, S.J.; Targosz, A.; Sliwowski, Z.; Dobrzanska, M.; Kaminska, A.; Sito, E.; Konturek, P.C.; Brzozowski, T. H.pylori infection and serum gastrin, ghrelin and lepton in children of Polish shepherds. Digest. Liver Dis. 2005, 38, 91–97. [Google Scholar] [CrossRef]
- Pacifico, L.; Osborn, J.F.; Tromba, V.; Romaggioli, S.; Bascetta, S.; Chiesa, C. H. pylori infection and extragastric disorders in children. A critical update. Word J. Gastroenterol. 2014, 20, 1379–1401. [Google Scholar] [CrossRef]
- Stawerska, R.; Czkwianianc, E.; Matusiak, A.; Smyczyńska, J.; Hilczer, M.; Chmiela, M.; Lewiński, A. Prevalence of autoantibodies against some selected growth and appetite-regulating neuropeptides in serum of short children exposed to Candida albicans colonization and/or Helicobacter pylori infection: The molecular mimicry phenomenon. Neuroendocrinol. Lett. 2015, 36, 101–107. [Google Scholar]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.; Hungin, A.P.; Jones, R.; Axon, A.; Graham, D.Y.; Tytgat, G. Current concept in the management of H. pylori infection—The Maastricht 2-2002 consensus report. Aliment. Pharmacol. Therap. 2002, 16, 167–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bąk-Romaniszyn, L.; Małecka-Panas, E. Zakażenie Helicobacter pylori u dzieci—Przebieg kliniczny, postępowanie diagnostyczne i leczenie. Przewodnik Lekarski 2007, 1, 94–102. [Google Scholar]
- Griffiths, P.R.; de Haseth, J.A. Fourier Transform Infrared Spectrometry; John Wiley & Sons: New York, NY, USA, 1986; ISBN 978-0-471-19404-0. [Google Scholar]
- Mantsch, H.H.; Chapman, D. Infrared Ssectroscopy of Bbomolecules; John Wiley & Sons Inc.: New York, NY, USA, 1996; ISBN 978-0-471-02184-1. [Google Scholar]
- Zhou, Y.P.; Xu, L.; Tang, L.J.; Jiang, J.H.; Shen, G.L.; Yu, R.Q.; Ozaki, Y. Gas Chromatography-Inductively Coupled Plasma-Mass Spectrometry for Mercury Speciation in sea food. Anal. Sci. 2007, 23, 793–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deleris, G.; Petibois, C. Applications of FT-IR spectrometry to plasma contents analysis and monitoring. Vib. Spectrosc. 2003, 32, 129. [Google Scholar] [CrossRef]
- Naumann, D.; Helm, D.; Labischinski, H.; Giesbrecht, P. The characterization of microorganisms by Fourier-transform infrared spectroscopy (FT-IR). In Modern Techniques for Rapid Microbiological Analysis; Nelson, W., Ed.; VCH: New York, NY, USA, 1991; Volume 43, p. 96. [Google Scholar]
- Cooper, E.A.; Knutson, K. Fourier transform infrared spectroscopy investigations of protein structure. Pharm. Biotechnol. 1995, 7, 101–143. [Google Scholar] [CrossRef]
- Naumann, D. Infrared Spectroscopy in Microbiology. In Encyclopedia of Analytical Chemistry; Meyers, R., Ed.; Wiley: Chichester, UK, 2000; pp. 102–131. [Google Scholar]
- Banyay, M.; Sarkar, M.; Graslund, A. A library of IR bands of nucleic acids in solution. Biophys. Chem. 2003, 104, 477–488. [Google Scholar] [CrossRef]
- Adamus-Bialek, W.; Lechowicz, Ł.; Kubiak-Szeligowska, A.; Wawszczak, M.; Kamińska, E.; Chrapek, M. A new look at the drug-resistance investigation of uropathogenic E. coli strains. Mol. Biol. Rep. 2017, 44, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Bombalska, A.; Mularczyk-Oliwa, M.; Kwaśny, M.; Włodarski, M.; Kaliszewski, M.; Kopczyński, K.; Szpakowska, M.; Trafny, E.A. Classification of the biological material with use of FTIR spectroscopy and statistical analysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 4, 1221–1226. [Google Scholar] [CrossRef]
- Lechowicz, L.; Urbaniak, M.; Adamus-Bialek, W.; Kaca, W. The use of infrared spectroscopy and artificial neural networks for detection of uropathogenic Escherichia coli strains’ susceptibility to cephalothin. Acta Biochim. Pol. 2013, 60, 713–718. [Google Scholar] [CrossRef]
- Lechowicz, Ł.; Adamus-Bialek, W.; Kaca, W. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy and Artificial Neural Networks Applied to Differentiate Escherichia coli Strains. J. Spectrosc. 2012, 2013, 1–3. [Google Scholar] [CrossRef]
- Bosch, A.; Miñán, A.; Vescina, C.; Degrossi, J.; Gatti, B.; Montanaro, P.; Messina, M.; Franco, M.; Vay, C.; Schmitt, J.; et al. Fourier transform infrared spectroscopy for rapid identification of nonfermenting gram–negative bacteria isolated from sputum samples from cystic fibrosis patients. J. Clin. Microbiol. 2008, 8, 2535–2546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarnowiec, P.; Lechowicz, Ł.; Czerwonka, G.; Kaca, W. Fourier Transform Infrared Spectroscopy (FTIR) as a Tool for the Identification and Differentiation of Pathogenic Bacteria. Curr. Med. Chem. 2015, 22, 1710–1718. [Google Scholar] [CrossRef] [PubMed]
- Bielański, W.; Konturek, S.J. New approach to 13C urea breath test capsule-based modification with low dose of 13C urea in the diagnosis of Helicobacter pylori infection. J. Physiol. Pharmacol. 1996, 47, 545–553. [Google Scholar] [PubMed]
- Rechcinski, T.; Chmiela, M.; Małecka-Panas, E.; Płaneta-Małecka, I.; Rudnicka, W. Serological indicators of Helicobacter pylori infection in adult dyspeptic patients and health blood donors. Microbiol. Immunol. 1997, 41, 387–393. [Google Scholar] [CrossRef]
- Chmiela, M.; Ławnik, M.; Czkwianianc, E.; Rechciński, T.; Płaneta-Małecka, I.; Rudnicka, W. Systemic humoral response to Helicobacter pylori in children and adults. Arch. Immunol. Ther. Exp. 1998, 46, 161–167. [Google Scholar]
- Palczewska, I.; Niedzwiecka, Z. Indices of somatic development of Warsaw children and adolscents. Med. Wieku Rozwojowego 2001, 5, 17–118. [Google Scholar]
- Lechowicz, L.; Chrapek, M.; Gaweda, J.; Urbaniak, M.; Konieczna, I. Use of Fourier-transform infrared spectroscopy in the diagnosis of rheumatoid arthritis: A pilot study. Mol. Biol. Rep. 2016, 43, 1321–1326. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.D.; Rosa, N.F.; Ferreira, A.E.; Boas, L.V.; Maria, R.; Bronze, M.R. Rapid Determination of α-Tocopherol in Vegetable Oils by Fourier Transform Infrared Spectroscopy. Food Analyt. Meth. 2009, 2, 120. [Google Scholar] [CrossRef] [Green Version]
- Linden, A.; Bürgi, B.; Eugster, C.H. Confirmation of the Structures of Lutein and Zeaxanthin. Helv. Chim. Acta 2004, 87, 1254–1269. [Google Scholar] [CrossRef]
- Raouf, A.L.M.; Hammud, K.K.; Mohammed, J.M.; Al-Dulimy, E.M.K. Qualitative and Quantitative Determination of Folic acid in Tablets by FTIR Spectroscopy. Int. J. Adv. Pharmacol. Biol. Chem. 2014, 3, 773–780. [Google Scholar]
- Staes, E.; Absil Lins, L.; Brasseur, R.; Deleu, M.; Lecouturier, N.; Fievez, V.; des Rieux, A.; Mingeot-Leclercq, M.P.; Raussens, V.; Préat, V. Acylated and unacylated ghrelin binding to membranes and to ghrelin receptor: Towards a better understanding of the underlying mechanisms. Biochim. Biophys. Acta (BBA)-Biomembr. 2010, 1798, 2102–2113. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, A.; Pokrajac, D.; Marcano, A.; Melikechi, N. Support vector machine based classification of fast Fourier transform spectroscopy of proteins. Proc. SPIE 2009, 7169, 71690C. [Google Scholar] [CrossRef]
- Aghel, N.; Ramezani, Z.; Amirfakhrian, S. Isolation and quantification of lycopene from tomato cultivated indezfoul. Jundishapur. J. Nat. Pharm. Prod. 2011, 6, 9–15. [Google Scholar]
- Nugrahani, I.; Kartini, C. Determination of thiamine hcl (vitamin B1) and pyridoxine HCL (vitamin B6) Content in tablet by FTIR. Int. J. Pharmacol. Pharmaceut. Sci. 2016, 8, 257–264. [Google Scholar] [CrossRef]
- Sadeghzadeh, S.M.; Zhiani, R.; Emran, S. KCC-1/GMSI/VB12 as a new nano catalyst for the carbonylative Suzuki–Miyaura crosscoupling. RSC Adv. 2017, 7, 32139–32145. [Google Scholar] [CrossRef] [Green Version]
- Panickera, C.Y.; Vargheseb, H.T.; Philipa, D. FT-IR, FT-Raman and SERS spectra of Vitamin C. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 65, 802–804. [Google Scholar] [CrossRef]
- Liu, K.Z.; Schultz, C.P.; Johnston, J.B.; Lee, K.; Mantsch, H. Comparison of infrared spectra of CLL cells with their ex vivo sensitivity (MTT assay) to chlorambucil and cladribine. Leuk. Res. 1997, 21, 1125–1133. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Z.; Sun, S.; Liu, M.; Zhang, H. A rapid method for detecting conformational changes during differentiation and apoptosis of HL60 cells by Fourier transform infrared spectroscopy. Biotechnol. Appl. Biochem. 2001, 33, 127–132. [Google Scholar] [CrossRef]
- Erukhimovitch, V.; Talyshinsky, M.; Souprun, Y.; Huleihel, M. FTIR spectroscopy examination of leukemia patients plasma. Vib. Spectros. 2006, 40, 40–46. [Google Scholar] [CrossRef]
- Shen, Y.C.; Davies, A.G.; Linfield, E.H.; Elsey, T.S.; Taday, P.F.; Arnone, D.D. The use of Fourier-transform infrared spectroscopy for the quantitative determination of glucose concentration in whole blood. Phys. Med. Biol. 2003, 48, 2023–2032. [Google Scholar] [CrossRef]
- Sankari, G.; Krishnamoorthy, E.; Jayakumaran, S.; Gunasekaran, G.; Priya, V.V.; Subramaniam, S.; Subramaniam, S.; Mohan, S.K. Analysis of serum immunoglobulins using Fourier transform infrared spectral measurements. Res. Art. Biol. Med. 2010, 3, 42–48. [Google Scholar]
- Mégraud, F.; Brassens-Rabbé, M.P.; Denis, F.; Belbouri, A.; Hoa, D.Q. Seroepidemiology of Campylobacter pylori infection in various populations. J. Clin. Microbiol. 1989, 27, 1870–1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, J.E.; Dale, A.; Bunn, J.E.; Harding, M.; Coward, W.A.; Cole, T.J.; Weaver, L.T. Early Helicobacter pylori colonisation: The association with growth faltering in The Gambia. Arch. Dis. Child. 2004, 89, 1149–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mera, R.M.; Correa, P.; Fontham, E.E.; Reina, J.C.; Pradilla, A.; Alzate, A.; Bravo, L.E. Effects of a new Helicobacter pylori infection on height and weight in Colombian children. Ann. Epidemiol. 2006, 16, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, M.J.; White, K.L.; Drake, I.M.; Schorah, C.J. Vitamin E and carotenoids in gastric biopsies: The relation to plasma concentrations in patients with and without Helicobacter pylori gastritis. Am. J. Clin. Nutr. 1997, 65, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Capurso, G.; Marignani, M.; Delle, F.G.; Annibale, B. Iron-deficiency anemia in premenopausal women: Why not consider atrophic body gastritis and Helicobacter pylori role? Am. J. Gastroenterol. 1999, 94, 3084–3085. [Google Scholar] [CrossRef]
- Stopeck, A. Links between Helicobacter pylori infection, cobalamin deficiency, and pernicious anemia. Arch Intern Med. 2000, 160, 1229–1230. [Google Scholar] [CrossRef]
- Kaptan, K.; Beyan, C.; Ural, A.U.; Cetin, T.; Avcu, F.; Gülşen, M.; Finci, R.; Yalçín, A. Helicobacter pylori—is it a novel causative agent in Vitamin B12 deficiency? Arch. Intern. Med. 2000, 160, 1349–1353. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Patchett, S.E.; Perrett, D.; Domizio, P.; Farthing, M.J. Gastric alphα-tocopherol and beta-carotene concentrations in association with Helicobacter pylori infection. Eur. J. Gastroenterol. Hepatol. 2000, 12, 497–503. [Google Scholar] [CrossRef]
- Ustündağ, Y.; Boyacioğlu, S.; Haberal, A.; Demirhan, B.; Bilezikçi, B. Plasma and gastric tissue selenium levels in patients with Helicobacter pylori infection. J. Clin. Gastroenterol. 2001, 32, 405–408. [Google Scholar] [CrossRef]
- Akcam, M.; Ozdem, S.; Yilmaz, A.; Gultekin, M.; Artan, R. Serum ferritin, vitamin B(12), folate, and zinc levels in children infected with Helicobacter pylori. Dig. Dis. Sci. 2007, 52, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.H.; Huang, X.L.; Xiong, P.; Zhu, C.Y.; Huang, Y.L.; Lu, L.G.; Sun, X.; Rong, L.; Zhong, L.; Sun, D.Y.; et al. Does Helicobacter pylori infection play a role in iron deficiency anemia? A meta-analysis. World J. Gastroenterol. 2010, 16, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Serin, E.; Gümürdülü, Y.; Ozer, B.; Kayaselçuk, F.; Yilmaz, U.; Koçak, R. Impact of Helicobacter pylori on the development of vitamin B12 deficiency in the absence of gastric atrophy. Helicobacter 2002, 7, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Waring, A.J.; Drake, I.M.; Schorah, C.J.; White, K.L.; Lynch, D.A.; Axon, A.T.; Dixon, M.F. Ascorbic acid and total vitamin C concentrations in plasma, gastric juice, and gastrointestinal mucosa: Effects of gastritis and oral supplementation. Gut 1996, 38, 171–176. [Google Scholar] [CrossRef]
- Woodward, M.; Tunstall-Pedoe, H.; McColl, K. Helicobacter pylori infection reduces systemic availability of dietary vitamin C. Eur. J. Gastroenterol. Hepatol. 2001, 13, 233–237. [Google Scholar] [CrossRef]
- Isomoto, H.; Nishi, Y.; Ohnita, K.; Mizuta, Y.; Kohno, S.; Ueno, H.; Nakazato, M. The Relationship between Plasma and Gastric Ghrelin Levels and Strain Diversity in Helicobacter pylori Virulence. Am. J. Gastroenterol. 2005, 100, 1425–1427. [Google Scholar] [CrossRef]
- Kalra, S.P.; Ueno, N.; Kalra, P.S. Stimulation of appetite by ghrelin is regulated by leptin restraint: Peripheral and central sites of action. J. Nutr. 2005, 135, 1331–1335. [Google Scholar] [CrossRef] [Green Version]
- Liew, P.L.; Lee, W.J.; Lee, Y.C.; Chen, W.Y. Gastric ghrelin expression associated with Helicobacter pylori infection and chronic gastritis in obese patients. Obes. Surg. 2006, 16, 612–619. [Google Scholar] [CrossRef]
- Konturek, P.C.; Cześnikiewicz-Guzik, M.; Bielanski, W.; Konturek, S.J. Involvement of Helicobacter pylori infection in neuro-hormonal control of food intake. J. Physiol. Pharmacol. 2006, 5, 67–81. [Google Scholar]
- Pacifico, L.; Anania, C.; Osborn, J.F.; Ferrara, E.; Schiavo, E.; Bonamico, M.; Chiesa, C. Long-team effects of Helicobacter pylori eradication on circulating ghrelin and leptin, and indulines-like growth factor (IGF)-I concentration in children. Helicobacter 2008, 16, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Checchi, S.; Montanaro, A.; Pasqui, L.; Ciuoli, C.; Cevenini, G.; Sestini, F.; Fioravanti, C.; Pacini, F. Serum ghrelin as a marker of atrophic body gastritis in patients with parietal cell antibodies. J. Clin. Endocrinol. Metab. 2007, 92, 4346–4351. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Molecule | Absorption Band (cm−1) | ID | Description | Component Group | Reference |
|---|---|---|---|---|---|
| α-tocopherol | 1472 1078 | (B1) | CH3/CH2 asymmetrical scissoring C-O symmetric stretching of glucose region | Cyclopropane | [42] |
| β-carotene | 1650 1457 1384 1324 1096 | (B2) | C=O symmetric stretching asymmetric C-H scissoring of -CH3 CH3 deformation O-H bending C-C-C bending | Amide I Amino acid | [43] |
| Folic acid | 3050 1485–1519 1604–1619 1650 1693 | (B3) | N-H asymmetric stretching CO2 asymmetric stretching NH2 scissoring NH2 scissoring NH2 scissoring C=O symmetric stretching | Amino acid Amide I Amide I Amide I Amide I | [44] |
| Ghrelin | 1640 | (B4) | NH2 scissoring | Amides I | [45] |
| Leptin | 1740 1550 | (B5) | C=O stretch N-H in plane bending vibration strongly coupled to C-N stretching vibration protein | Amide II | [46] |
| Lycopene | 3100 2851 1640 1450–1400 1375 | (B6) | N-H asymmetric stretching C-H symmetric stretching of CH2 group NH2 scissoring CH3 asymmetric deformation CH2 wagging | Amino acid Lipids Amide I | [47] |
| Lutein | 1517–1500 | (B7) | CO2 asymmetric stretching | [43] | |
| Vitamin B6 | 1280–1315 | (B8) | C-H/N-H deformation vibration models methyl groups | Amide III | [48] |
| Vitamin B12 | 3120 1670–1665 | (B9) | CH3 asymmetric stretching of CH3 group C=O bands | Fatty acid Amide I | [49] |
| Vitamin C | 1760 1634 1322 | (B10) | C=O stretch NH2 scissoring C-H/N-H deformation vibration models methyl groups | Amide I Amide III | [50] |
| Window | Absorption Band (cm−1) | χ2 Test Value | p-Value (×10−5) | One of Possible Chemical Bonds |
|---|---|---|---|---|
| W4 | 1060 | 15.72 | 7.3 | N-H bending |
| 1087 | 14.33 | 15.4 | C-N stretch | |
| 1124 | 17.15 | 3.4 | C-N stretch | |
| 1138 | 13.03 | 30.6 | C-O stretch | |
| 1139 | 13.03 | 30.6 | C-O stretch | |
| 1180 | 13.36 | 25.7 | C-O stretch | |
| W3 | 1484 | 12.01 | 52.9 | asymmetric C-H scissoring of -CH3 |
| 1493 | 13.36 | 25.7 | CO2 asymmetric stretching | |
| 1495 | 12.01 | 52.9 | CO2 asymmetric stretching | |
| W2 | 1557 | 13.76 | 20.8 | N-H bending |
| No. | Topology | Correct Classifications Percentage | Error Function | Activation Function | ||
|---|---|---|---|---|---|---|
| Training Subset | Validation Subset | Hidden Neurons | Output Neurons | |||
| 1 | 10-3-2 | 100% | 90% | SOS | Exponential | Linear |
| 2 | 8-5-2 | 95% | 90% | SOS | Logistic | Exponential |
| 3 | 9-3-2 | 100% | 88% | SOS | Tanh | Linear |
| 4 | 9-4-2 | 100% | 88% | SOS | Tanh | Linear |
| 5 | 10-4-2 | 100% | 86% | Entropy | Tanh | Softmax |
| 6 | 6-1-2 | 69% | 86% | Entropy | Tanh | Softmax |
| 7 | 8-3-2 | 100% | 86% | SOS | Logistic | Tanh |
| 8 | 5-3-2 | 98% | 83% | Entropy | Exponential | Softmax |
| 9 | 5-2-2 | 98% | 83% | SOS | Tanh | Tanh |
| 10 | 5-1-2 | 83% | 83% | Entropy | Tanh | Softmax |
| 11 | 7-1-2 | 69% | 83% | Entropy | Logistic | Softmax |
| 12 | 7-2-2 | 95% | 83% | Entropy | Logistic | Softmax |
| Type of Measured Indicator | Value | |
|---|---|---|
| 1 | Sensitivity | 0.95 |
| 2 | Miss rate | 0.05 |
| 3 | Specificity | 0.86 |
| 4 | False positive rate | 0.14 |
| 5 | Precision | 0.87 |
| 6 | False discovery rate | 0.13 |
| 7 | False omission rate | 0.05 |
| 8 | Negative predictive value | 0.95 |
| 9 | Positive likelihood ratio | 6.67 |
| 10 | Negative likelihood ratio | 0.06 |
| 11 | Accuracy | 0.90 |
| 12 | Informedness | 0.81 |
| 13 | Markedness | 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonciarz, W.; Lechowicz, Ł.; Urbaniak, M.; Kaca, W.; Chmiela, M. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (FTIR) and Artificial Neural Networks Applied to Investigate Quantitative Changes of Selected Soluble Biomarkers, Correlated with H. pylori Infection in Children and Presumable Consequent Delayed Growth. J. Clin. Med. 2020, 9, 3852. https://doi.org/10.3390/jcm9123852
Gonciarz W, Lechowicz Ł, Urbaniak M, Kaca W, Chmiela M. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (FTIR) and Artificial Neural Networks Applied to Investigate Quantitative Changes of Selected Soluble Biomarkers, Correlated with H. pylori Infection in Children and Presumable Consequent Delayed Growth. Journal of Clinical Medicine. 2020; 9(12):3852. https://doi.org/10.3390/jcm9123852
Chicago/Turabian StyleGonciarz, Weronika, Łukasz Lechowicz, Mariusz Urbaniak, Wiesław Kaca, and Magdalena Chmiela. 2020. "Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (FTIR) and Artificial Neural Networks Applied to Investigate Quantitative Changes of Selected Soluble Biomarkers, Correlated with H. pylori Infection in Children and Presumable Consequent Delayed Growth" Journal of Clinical Medicine 9, no. 12: 3852. https://doi.org/10.3390/jcm9123852





