A Minimal PKPD Interaction Model for Evaluating Synergy Effects of Combined NSCLC Therapies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antiangiogenesis Therapy
2.2. Immunotherapy
2.3. Stereotactic Body Radiotherapy (SBRT)
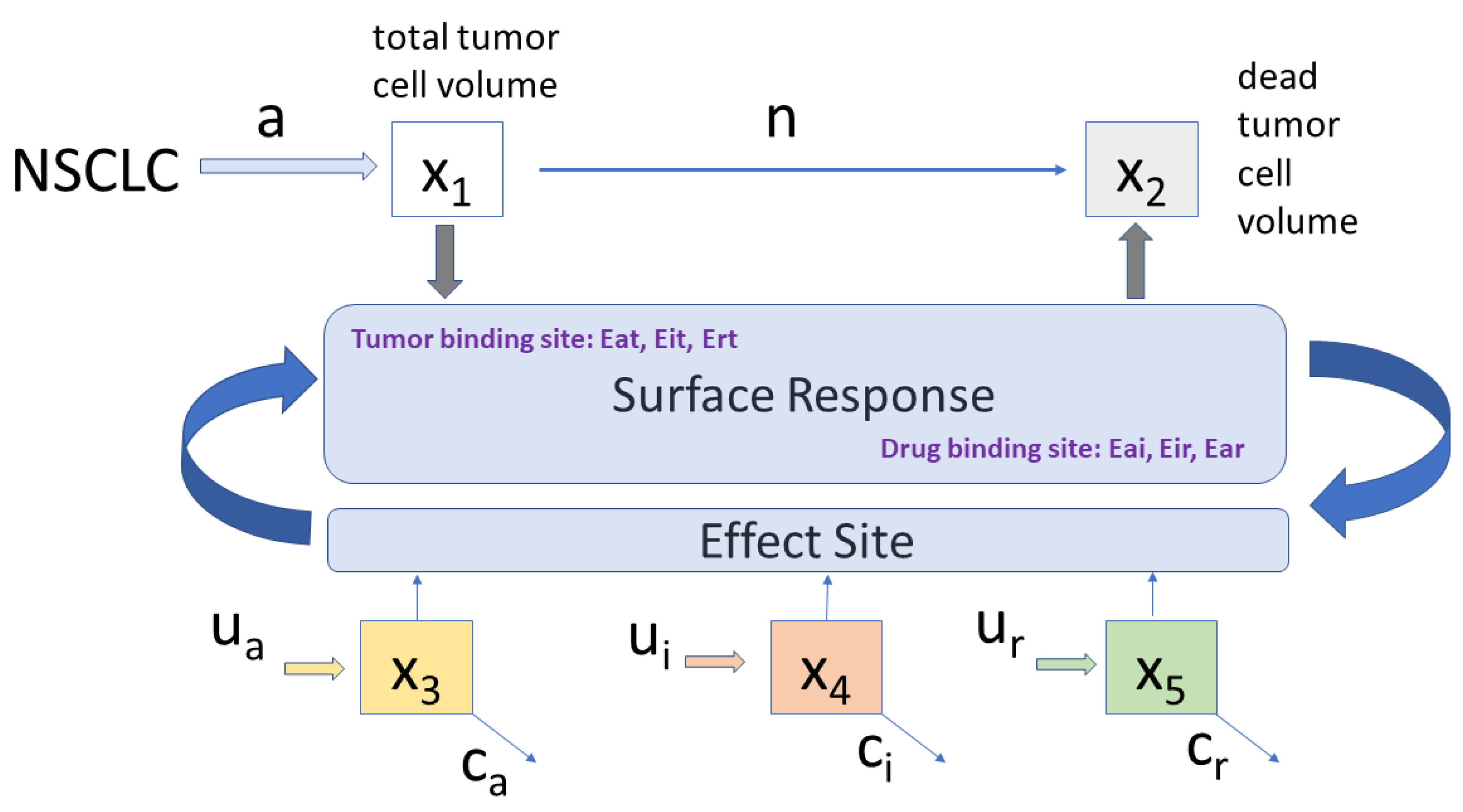
2.4. Proposed Combined Therapy Minimal Model
2.5. Protocols
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AG | Antiangiogenic therapy |
| COPD | Chronic Obstructive Pulmonary Disease |
| IM | Immunotherapy |
| LQ | Linear-quadratic |
| NSCLC | Non small cell lung cancer |
| PD | Pharmacodynamic |
| PK | Pharmacokinetic |
| RP | Radiation Pneumonitis |
| RT | Radiotherapy |
| SBRT | Stereotactic Body Radiotherapy |
Appendix A
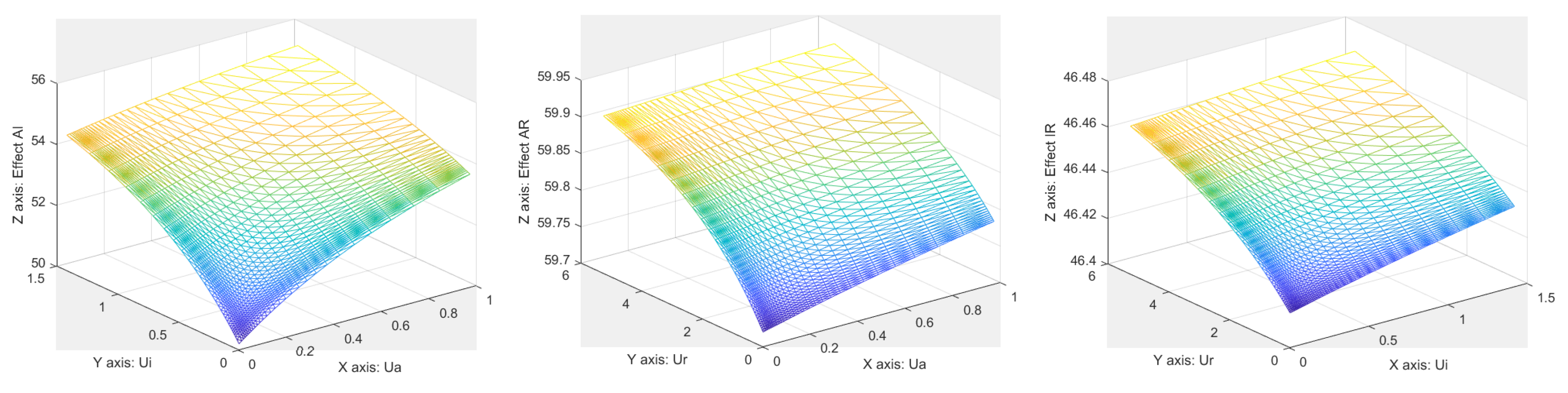
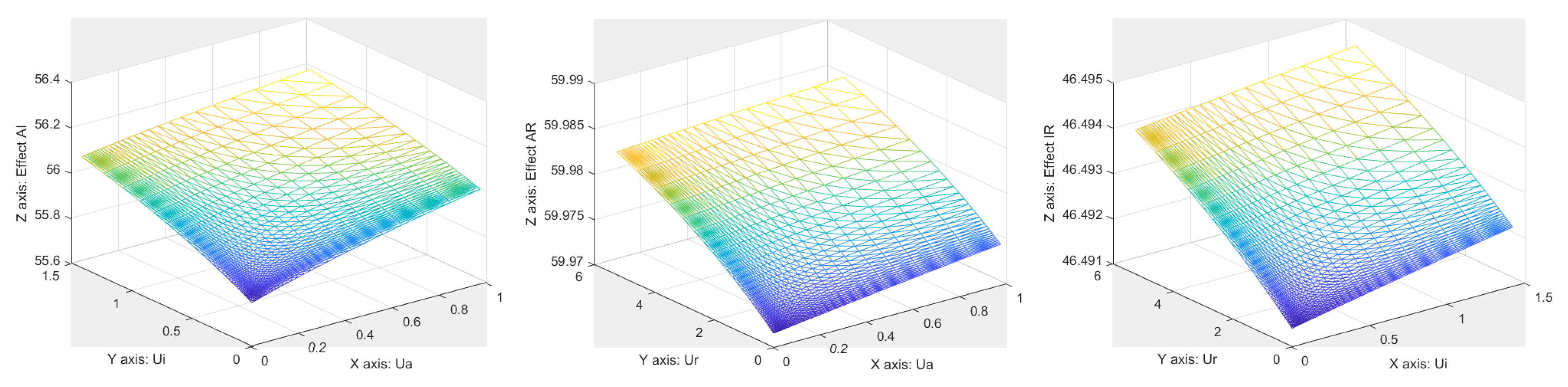



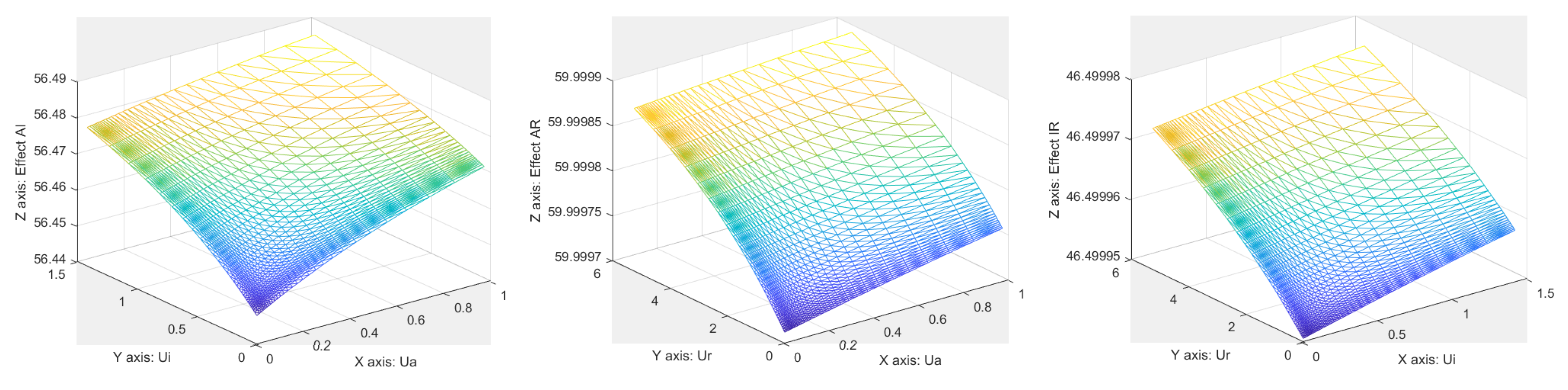
References
- Mennitto, A.; Huber, V.; Ratta, R.; Sepe, P.; de Braud, F.; Procopio, G.; Guadalupi, V.; Claps, M.; Stellato, M.; Daveri, E.; et al. Angiogenesis and immunity in renal carcinoma: Can we turn an unhappy relationship into a happy marriage? J. Clin. Med. 2020, 9, 930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iafrate, M.; Fruhwirth, G.O. How non-invasive in vivo cell tracking supports the development and translation of cancer immunotherapies. Front. Physiol. 2020, 11, 154. [Google Scholar] [CrossRef] [PubMed]
- Liauw, S.; Connell, P.; Weichselbaum, R. New paradigms and future challenges in radiation oncology: An update of biological targets and technology. Sci. Transl. Med. 2013, 5, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roesch, J.; Andratschke, N.; Guckenberger, M. SBRT in operable early stage lung cancer patients. Transl. Lung Cancer Res. 2014, 3, 212–224. [Google Scholar] [PubMed]
- de Jong, E.; Guckenberger, M.; Andratschke, N.; Dieckmann, K.; Milder, M.; Moller, D.S.; Nyeng, T.B.; Tanadini-Lang, S.; Lartigau, E.; Lacornerie, T.; et al. Variation in current prescription practice of stereotactic body radiotherapy for peripherally located early stage non-small cell lung cancer: Recommendations for prescribing and recording according to the ACROP guideline and ICRU report 91. Radiother. Oncol. 2020, 142, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Gonze, D.; Abou-Jaoude, W. The Goodwin model: Behind the Hill function. PLoS ONE 2013, 8, e69573. [Google Scholar] [CrossRef] [PubMed]
- Goutelle, S.; Maurin, M.; Rougier, F.; Barbaut, X.; Bourguignon, L.; Ducher, M.; Maire, P. The Hill equation: A review of its capabilities in pharmacological modelling. Fundam. Clin. Pharmacol. 2008, 22, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Sopasakis, P.; Sarimveis, H.; Macheras, P.; Dokoumetzidis, A. Fractional calculus in pharmacokinetics. J. Pharmacokinet. Pharmacodyn. 2018, 45, 107–125. [Google Scholar] [CrossRef]
- Weiss, M. Comparison of distributed and compartmental models of drug disposition: Assessment of tissue uptake kinetics. J. Pharmacokinet. Pharmacodyn. 2016, 43, 505–512. [Google Scholar] [CrossRef]
- Ionescu, C.M.; Copot, D.; De Keyser, R. Modeling doxorubicin effect in various cancer therapies by means of fractional calculus. In Proceedings of the 2016 American Control Conference (ACC), Boston, MA, USA, 6–8 July 2016; pp. 1283–1288. [Google Scholar]
- Copot, D.; Magin, R.; De Keyser, R.; Ionescu, C.M. Data-driven modelling of drug tissue trapping using anomalous kinetics. Chaos Solitons Fractals 2017, 102, 441–446. [Google Scholar] [CrossRef]
- Sapi, J.; Kovacs, L.; Drexler, D.A.; Kocsis, P.; Gajari, D.; Sapi, Z. Tumor volume estimation and quasi-continuous administration for most effective bevacizumab therapy. PLoS ONE 2015, 10, e0142190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacs, L.; Szeles, A.; Sapi, J.; Drexler, D.A.; Rudas, I.; Harmati, I.; Sapi, Z. Model-based angiogenic inhibition of tumor growth using modern robust control method. Comput. Methods Programs Biomed. 2014, 114, e98–e110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cekanova, M.; Rathore, K. Animal models and therapeutic molecular targets of cancer, utility and limitations. Drug Des. Dev. Ther. 2014, 8, 1911–1921. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.W.N.; Huamani, J.; Niermann, K.J.; Lee, H.; Geng, L.; Leavitt, L.L.; Baheza, R.A.; Jones, C.C.; Tumkur, S.; Yankeelov, T.E.; et al. Noninvasive assessment of tumor vasculature response to radiation-mediated, vasculature-targeted therapy using quantified power Doppler Sonography. J. Ultrasound Med. 2006, 25, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.; Buonavoglia, A.; Fasano, R.; Solimando, A.G.; De Re, V.; Cicco, S.; Vacca, A.; Racanelli, V. Insights into the Regulation of Tumor Angiogenesis by Micro-RNAs. J. Clin. Med. 2019, 8, 2030. [Google Scholar] [CrossRef] [Green Version]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Teleanu, D.M. Tumor Angiogenesis and Anti-Angiogenic Strategies for Cancer Treatment. J. Clin. Med. 2020, 9, 84. [Google Scholar] [CrossRef] [Green Version]
- Drexler, D.A.; Sapi, J.; Kovacs, L. Modeling of tumor growth incorporating the effects of necrosis and the effect of bevacizumab. Complexity 2017, 2017, 5985031. [Google Scholar] [CrossRef] [Green Version]
- Drexler, D.A.; Ferenci, T.; Lovrics, A.; Kovacs, L. Modeling of tumor growth incorporating the effect of pegylated liposomal doxorubicin. In Proceedings of the 2019 IEEE 23rd International Conference on Intelligent Engineering Systems (INES), Godollo, Hungary, 25–27 April 2019; pp. 369–374. [Google Scholar]
- Almutairi, A.R.; Alkhatib, N.; Martin, J.; Babiker, H.M.; Garland, L.L.; McBride, A.; Abraham, I. Comparative efficacy and safety of immunotherapies targeting the PD-1/PD-L1 pathway for previously treated advanced non-small cell lung cancer: A Bayesian network meta-analysis. Crit. Rev. Oncol. Hematol. 2019, 142, 16–25. [Google Scholar] [CrossRef]
- Russo, A.R.; McCusker, M.G.; Scilla, K.A.; Arensmeyer, K.E.; Mehra, R.; Adamo, V.; Rolfo, C. Immunotherapy in Lung Cancer: From a Minor God to the Olympus. In Immunotherapy: Advances in Experimental Medicine and Biology; Naing, A., Hajjar, J., Eds.; Springer: Cham, Switzerland, 2020; Volume 1244, pp. 69–92. [Google Scholar]
- Pyfferoen, L.; Brabants, E.; Everaert, C.; De Cabooter, N.; Heyns, K.; Deswarte, K.; Vanheerswynghels, M.; De Prijck, S.; Waegemans, G.; Dullaers, M.; et al. The transcriptome of lung tumor-infiltrating dendritic cells reveals a tumor-supporting phenotype and a microRNA signature with negative impact on clinical outcome. Oncoimmunology 2017, 6, e1253655. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Wang, X.; Bajaj, G.; Agrawal, S.; Bello, A.; Lestini, B.; Finckestein, F.G.; Park, J.-S.; Roy, A. Nivolumab exposure-response analyses of efficacy and safety in previously treated squamous and non-squamous non-small cell lung cancer. Clin. Cancer Res. 2017, 23, 5394–5405. [Google Scholar] [CrossRef] [Green Version]
- Nikanjam, M.; Patel, H.; Kurzrock, R. Dosing immunotherapy combinations analysis of 3526 patients for toxicity and response patterns. Oncoimmunology 2017, 6, e1338997. [Google Scholar] [CrossRef] [Green Version]
- Nikanjam, M.; Liu, S.; Yang, J.; Kurzrock, R. Dosing three-drug combinations that include targeted anti-cancer agents: Analysis of 37763 patients. Oncologist 2017, 22, 576–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renn, A.; Burotto, M.; Rojas, C. Immune checkpoint inhibitor dosing: Can we go lower without compromising clinical efficacy? J. Glob. Oncol. 2019, 5. [Google Scholar] [CrossRef]
- Capasso, A.; Lang, J.; Pitts, T.M.; Jordan, K.R.; Lieu, C.H.; Davis, S.L.; Diamond, J.R.; Kopetz, S.; Barbee, J.; Peterson, J.; et al. Characterization of immune responses to anti-PD-1 mono- and combination therapy in hematopoietic humanized mice implanted with tumor xenographs. J. Immunother. Cancer 2019, 7, 37. [Google Scholar] [CrossRef]
- Lala, M.; Li, T.R.; de Alwis, D.P.; Sinha, V.; Mayawala, K.; Yamamoto, N.; Siu, L.L.; Chartash, E.; Aboshady, H.; Jain, L. A six-weekly dosing schedule for pembrolizumab in patients with cancer based on evaluation using modelling and simulation. Eur. J. Cancer 2020, 131, 68–75. [Google Scholar] [CrossRef]
- Long, G.V.; Tykodi, S.S.; Schneider, J.G.; Garbe, C.; Gravis, G.; Rashford, M.; Agrawal, S.; Grigoryeva, E.; Bello, A.; Roy, A.; et al. Assessment of nivolumab exposure and clinical safety of 480 mg every 4 weeks flat dosing schedule in patients with cancer. Ann. Oncol. 2018, 29, 2208–2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Suryawanshi, S.; Hruska, M.; Feng, Y.; Wang, X.; Shen, J.; Vezina, H.E.; McHenry, M.B.; Waxman, I.M.; Achanta, A.; et al. Assessment of nivolumab benefit–risk profile of a 240-mg flat dose relative to a 3-mg/kg dosing regimen in patients with advanced tumors. Ann. Oncol. 2017, 28, 2002–2008. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, K.; Bulumulle, A.; Brody, H.; Gill, R.; Macherla, S.; Qilleri, A.; McDonald, D.C.; Cherry, C.R.; Shea, M.; Huberman, M.S.; et al. Association of extended dosing intervals or delays in pembrolizumab-based regimens with survival outcomes in advanced non-small cell lung cancer. medRxiv 2020. [Google Scholar] [CrossRef]
- Deloch, L.; Deres, A.; Hartman, J.; Frey, B.; Fietkam, R.; Gaipl, U.S. Modern RT concepts and the impact of radiation on immune activation. Front. Oncol. 2016, 6, 141. [Google Scholar] [CrossRef] [Green Version]
- Abreu, C.E.C.V.; Ferreira, P.P.R.; de Moraes, F.Y.; Neves, W.F.P.; Godia, R.; Carvalho, H.D.A. Stereotactic body radiotherapy in lung cancer: An update. J. Bras. Pneumol. 2015, 41, 376–387. [Google Scholar] [CrossRef]
- Cho, J.; Kodym, R.; Seliounine, S.; Richardson, J.A.; Solberg, T.D.; Story, M.D. High dose-per-fraction irradiation of limited lung volumes using an image-guided, highly focused irradiator: Simulating stereotactic body radiotherapy regimens in a small-animal model. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Lockamy, V.; Zhou, L.; Xue, C.; LeBlanc, J.; Glenn, S.; Shukla, G.; Yu, Y.; Dicker, A.P.; Leeper, D.B.; et al. Stereotactic Body Radiation Therapy delivery in a genetically engineered mouse model of lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 529–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joiner, M.; van der Kogel, A. (Eds.) Basic Clinical Radiobiology, 4th ed.; Hodder Arnold: London, UK, 2009. [Google Scholar]
- Bernstein, M.B.; Krishnan, S.; Hodge, J.W.; Chang, J.Y. Immunotherapy and stereotactic ablative radiotherapy (ISABR): A curative approach? Nat. Rev. Clin. Oncol. 2016, 13, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Theelen, W.S.M.E.; Peulen, H.M.U.; Lalezari, F.; van der Noort, V.; de Vries, J.F.; Aerts, J.G.J.V.; Dumoulin, D.W.; Bahce, I.; Niemeijer, A.-L.N.; de Langen, A.J.; et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.M.; Cai, W.L.; Burkhardt, D.; Gettinger, S.N.; Goldberg, S.B.; Amodio, M.; Kaech, S.; Krishnaswamy, S.; Decker, R.H. Final Results of a Phase II Prospective Trial Evaluating the Combination of Stereotactic Body Radiotherapy (SBRT) with Concurrent Pembrolizumab in Patients with Metastatic Non-Small Cell Lung Cancer (NSCLC). Int. J. Radiat. Oncol. Biol. Phys. 2019, 105 (Suppl. 1), S36–S37. [Google Scholar] [CrossRef]
- Schapira, E.; Hubbeling, H.; Yeap, B.Y.; Mehan, W.A.; Shaw, A.T.; Oh, K.; Gainor, J.F.; Shih, H.A. Improved Overall Survival and Locoregional Disease Control With Concurrent PD-1 Pathway Inhibitors and Stereotactic Radiosurgery for Lung Cancer Patients With Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 624–629. [Google Scholar] [CrossRef]
- Luke, J.J.; Lemons, J.M.L.; Karrison, T.G.; Pitroda, S.P.; Melotek, J.M.; Zha, Y.; Al-Hallaq, H.A.; Arina, A.; Khodarev, N.N.; Janisch, L.; et al. Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors. J. Clin. Oncol. 2018, 36, 1611–1618. [Google Scholar] [CrossRef]
- Hwang, W.L.; Pike, L.R.G.; Royce, T.J.; Mahal, B.A.; Loeffler, J.S. Safety of combining radiotherapy with immune-checkpoint inhibition. Nat. Rev. Clin. Oncol. 2018, 15, 477–494. [Google Scholar] [CrossRef]
- Weichselbaum, R.R.; Liang, H.; Deng, L.; Fu, Y.X. Radiotherapy and immunotherapy: A beneficial liaison? Nat. Rev. Clin. Oncol. 2017, 14, 365–379. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Tsai, T.-H.; Wang, L.-Y.; Hsieh, C.-H. Local radiotherapy affects the drug PK—Exploration of a neglected but significant uncertainty in lung cancer therapy. Technol. Cancer Res. Treat. 2017, 16, 705–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, B.; Rose, J.B.; Liu, Y.; Jaskular-Sztul, R.; Contreras, C.; Beck, A.; Chen, H. Heterogeneity of Vascular Endothelial Cells, De Novo Arteriogenesis and Therapeutic Implications in Pancreatic Neuroendocrine Tumors. J. Clin. Med. 2019, 8, 1980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, S.Z.; Pan, X.N.; Wu, N.; Jin, G.H.; Liu, S.Z. Whole-body low dose irradiation promotes the efficacy of conventional radiotherapy for cancer and possible mechanisms. Dose-Response 2007, 5, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, C.M. A computationally efficient Hill curve adaptation strategy during continuous monitoring of dose-effect relation in anesthesia. Nonlinear Dyn. 2018, 92, 843–852. [Google Scholar] [CrossRef]
- Dhont, J.; Vandemeulebroucke, J.; Burghelea, M.; Poels, K.; Depuydt, T.; Van Den Begin, R.; Jaudet, C.; Collen, C.; Engels, B.; Reynders, T.; et al. The long- and short-term variability of breathing induced tumor motion in lung and liver over the course of a radiotherapy treatment. Radiother. Oncol. 2018, 126, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Vinh-Hung, V.; Leduc, N.; Verellen, D.; Verschraegen, C.; Dipasquale, G.; Nguyen, N.P. The mean absolute dose deviation—A common metric for the evaluation of dose-volume histograms in radiation therapy. Med. Dosim. 2019. [Google Scholar] [CrossRef]
- Selvaraj, J.; Lebesque, J.; Hope, A.; Guckenberger, M.; Werner-Wasik, M.; Peulen, H.; Giuliani, M.; Mantel, F.; Belderbos, J.; Grills, I.; et al. Modelling radiation pneumonitis of pulmonary stereotactic body radiotherapy: The impact of a local dose-effect relationship for lung perfusion loss. Radiother. Oncol. 2019, 132, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Curtis, L.T.; van Berkel, V.H.; Frieboes, H.B. Pharmacokinetic/pharmacodynamic modeling of combination chemotherapy for lung cancer. J. Theor. Biol. 2018, 448, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Drew, P.J.; Abbott, L.F. Models and properties of power-law adaptation in neural systems. J. Neurophysiol. 2006, 96, 826–833. [Google Scholar] [CrossRef]
- Liebovitch, L.S.; Scheurle, D.; Rusek, M.; Zochowski, M. Fractal methods to analyze ion channel kinetics. Methods 2001, 24, 359–375. [Google Scholar] [CrossRef] [Green Version]
- Ionescu, C.M. Phase Constancy in a Ladder Model of Neural Dynamics. IEEE Trans. Syst. Man Cybern. A Syst. Hum. 2012, 42, 1543–1551. [Google Scholar] [CrossRef]
- Holvoet, T.; van Meerbeeck, J.P.; Van de Wiele, C.; Salhi, B.; Derom, E. Quantitative perfusion scintigraphy or anatomic segment method in lung cancer resection. Lung Cancer 2011, 74, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Ghita, M.; Copot, D.; Ghita, M.; Derom, E.; Ionescu, C.M. Low frequency forced oscillation lung function test can distinguish dynamic tissue non-linearity in COPD patients. Front. Physiol. 2019, 10, 1390. [Google Scholar] [CrossRef] [PubMed]
- Copot, D.; De Keyser, R.; Derom, E.; Ionescu, C.M. Structural changes in the COPD lung and related heterogeneity. PLoS ONE 2017, 12, e0177969. [Google Scholar] [CrossRef] [PubMed]
- Baleanu, D.; Fernandez, A. On some new properties of fractional derivatives with Mittag-Leffler kernel. Commun. Nonlinear Sci. Numer. Simul. 2018, 59, 444–462. [Google Scholar] [CrossRef] [Green Version]
- Ionescu, C.; Lopes, A.; Copot, D.; Machado Tenreiro, J.A.; Bates, J.H.T. The role of fractional calculus in modeling biological phenomena: A review. Commun. Nonlinear Sci. Numer. Simul. 2017, 51, 141–159. [Google Scholar] [CrossRef]
- Ionescu, C.; De Keyser, R. Relations between fractional-order model parameters and lung pathology in chronic obstructive pulmonary disease. IEEE Trans. Biomed. Eng. 2009, 56, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, C.; Derom, E.; De Keyser, R. Assessment of respiratory mechanical properties with constant-phase models in healthy and COPD lungs. Comput. Methods Programs Biomed. 2010, 97, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, C.M. The Human Respiratory System: An Analysis of the Interplay between Anatomy, Structure, Breathing and Fractal Dynamics; Springer: London, UK, 2013. [Google Scholar]
- Assadi, I.; Charef, A.; Copot, D.; De Keyser, R.; Bensouici, T.; Ionescu, C.M. Evaluation of respiratory properties by means of fractional order models. Biomed. Signal Process. Control 2017, 34, 206–213. [Google Scholar] [CrossRef]
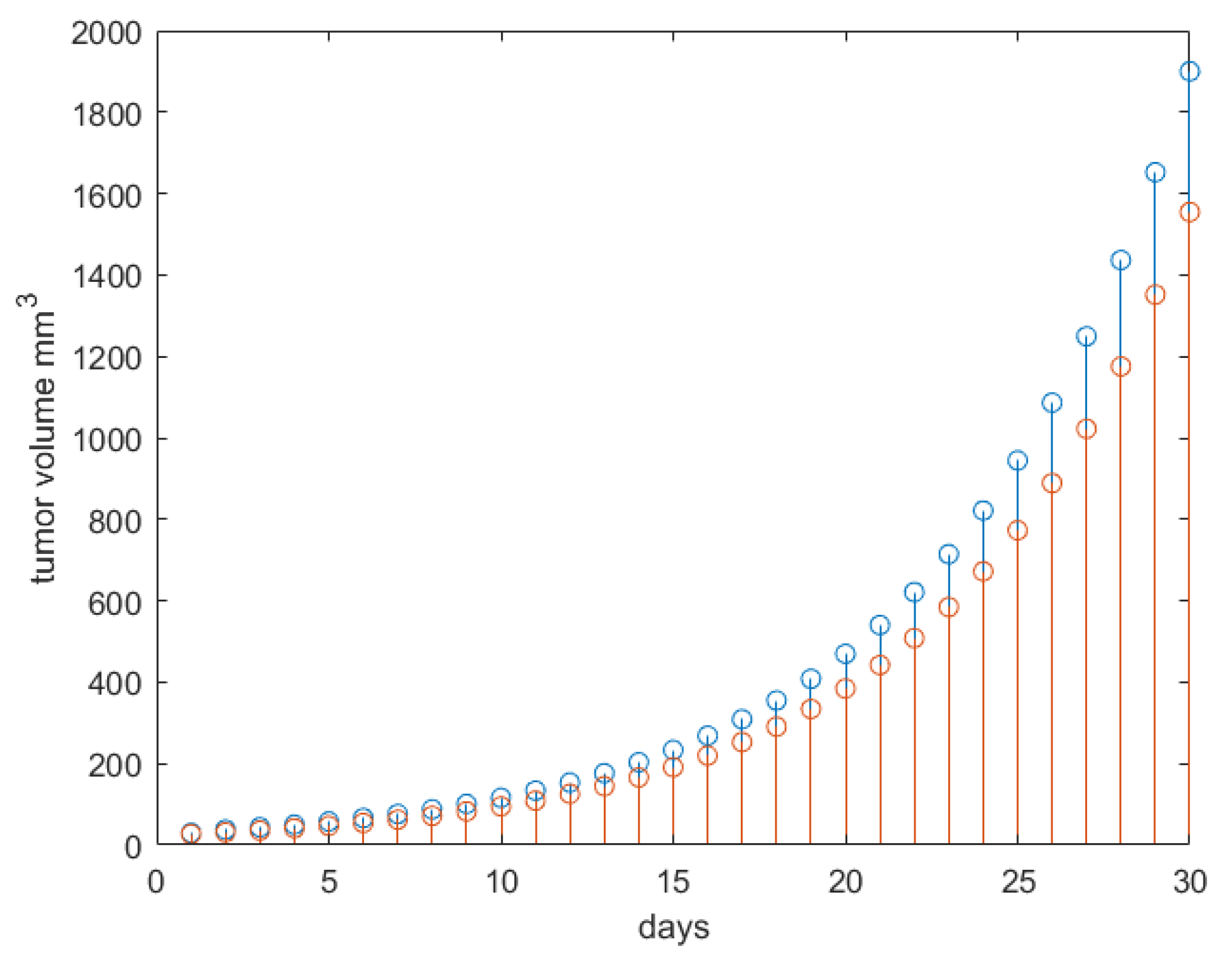

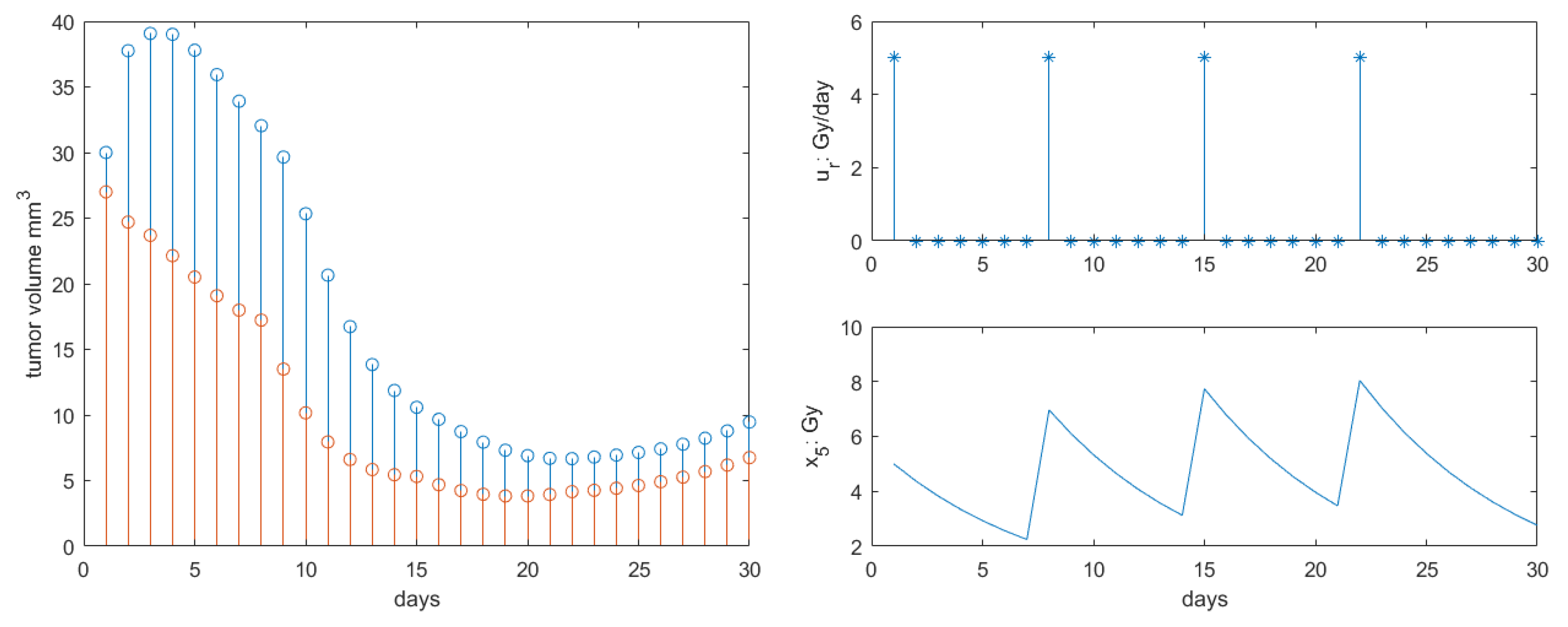


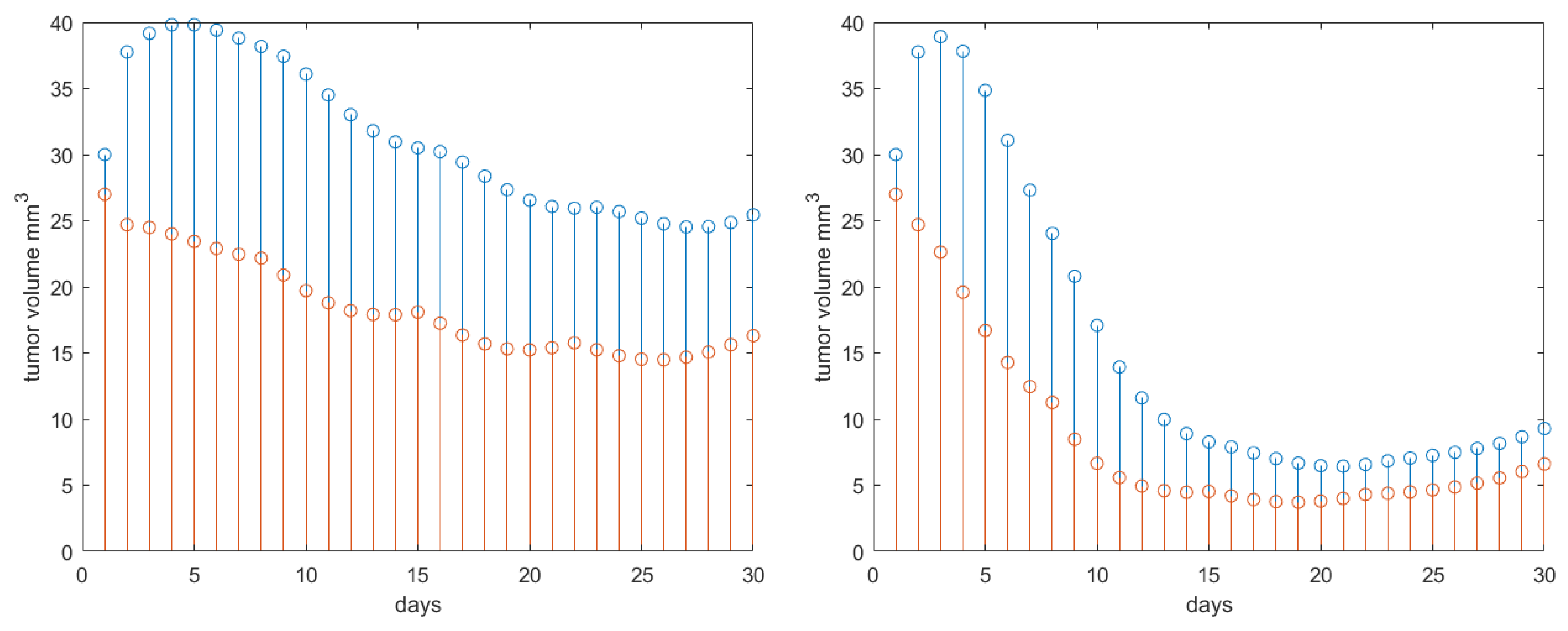
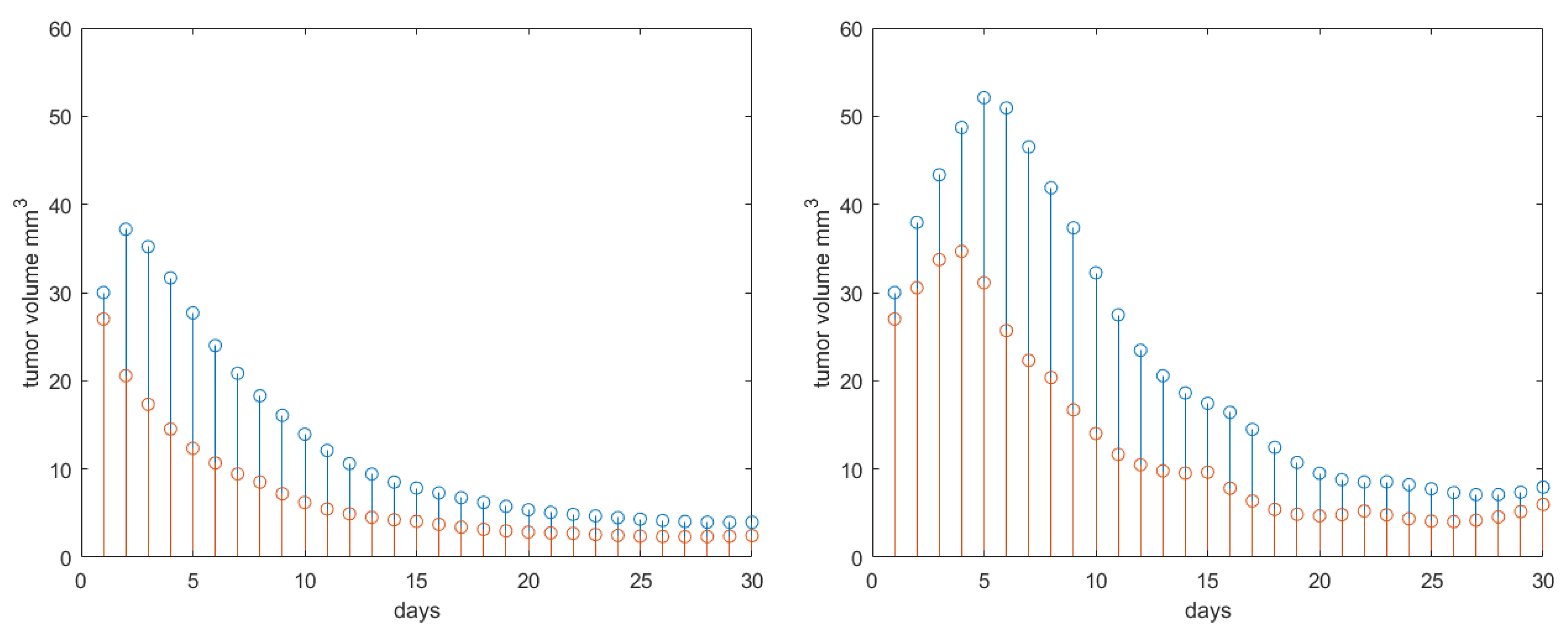
| Parameter | Name | Value | Units |
|---|---|---|---|
| a | tumor growth rate | 0.4579 | 1/day |
| reaction rate | 0.1685 | 1/day | |
| clearance rate | 0.1825 | 1/day | |
| necrosis rate | 0.1030 | 1/day | |
| scaled inhibition rate | 1.0839· | mg/(mL·day) | |
| Michaelis-Menten constant (inhibitor) | 0.4409 | mg/mL | |
| half-effect concentration | 50·10 | mg/mL |
| Parameter | Name | Value | Units | Source |
|---|---|---|---|---|
| a | tumor growth rate | 0.25 | 1/day | [18,46] |
| n | reaction rate | 0.10 | 1/day | [18,46] |
| clearance rate Bevacizumab | 0.1825 | 1/day | [18] | |
| clearance rate Nivolumab | 11.6/24 | mL/day | [23] | |
| clearance rate RT | 3/24 | 1/day | [36] | |
| half-effect concentration Bevacizumab | 0.44 | mg/mL | [18] | |
| half-effect concentration Nivolumab | 32·10 | mg/mL | [23] | |
| half-effect concentration RT | 20 | Gy/day | [36] | |
| half-effect tumor growth | 50 | % mm | [36,46] | |
| max efficacy Bevacizumab | 70 | % | NA | |
| max efficacy Nivolumab | 43 | % | [27] | |
| max effect RT | 50 | % | [27,36] | |
| patient response/resistance to drug | 2.5 | (-) | [47] | |
| drug reaction (synergic) | 4 | (-) | [47] | |
| E | combined effects (all) | calculated | 1/day | NA |
| antiangiogenic drug dose rate | 0.171 | mg/(mL·day) | [18] | |
| immunotherapy drug dose rate | 0.20 | mg/(mL·day) | [23,27] | |
| radiotherapy dose rate | varies | mg/(mL·day) | [46] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionescu, C.M.; Ghita, M.; Copot, D.; Derom, E.; Verellen, D. A Minimal PKPD Interaction Model for Evaluating Synergy Effects of Combined NSCLC Therapies. J. Clin. Med. 2020, 9, 1832. https://doi.org/10.3390/jcm9061832
Ionescu CM, Ghita M, Copot D, Derom E, Verellen D. A Minimal PKPD Interaction Model for Evaluating Synergy Effects of Combined NSCLC Therapies. Journal of Clinical Medicine. 2020; 9(6):1832. https://doi.org/10.3390/jcm9061832
Chicago/Turabian StyleIonescu, Clara Mihaela, Maria Ghita, Dana Copot, Eric Derom, and Dirk Verellen. 2020. "A Minimal PKPD Interaction Model for Evaluating Synergy Effects of Combined NSCLC Therapies" Journal of Clinical Medicine 9, no. 6: 1832. https://doi.org/10.3390/jcm9061832






