Nephroprotection by SGLT2 Inhibition: Back to the Future?
Abstract
:1. Introduction
2. The New Era of Nephroprotection in Diabetic Patients: From Efficacy to Effectiveness of SGLT2i
3. Nephroprotection by SGLT2i in Diabetic Patients: From Bench to Bedside
4. SGLT2i Nephroprotection Independent of Diabetic Status
5. The “Check-Mark Sign” (√) in Patients Starting SGLT2i Therapy
6. The Check-Mark Sign: Not Only SGLT2i
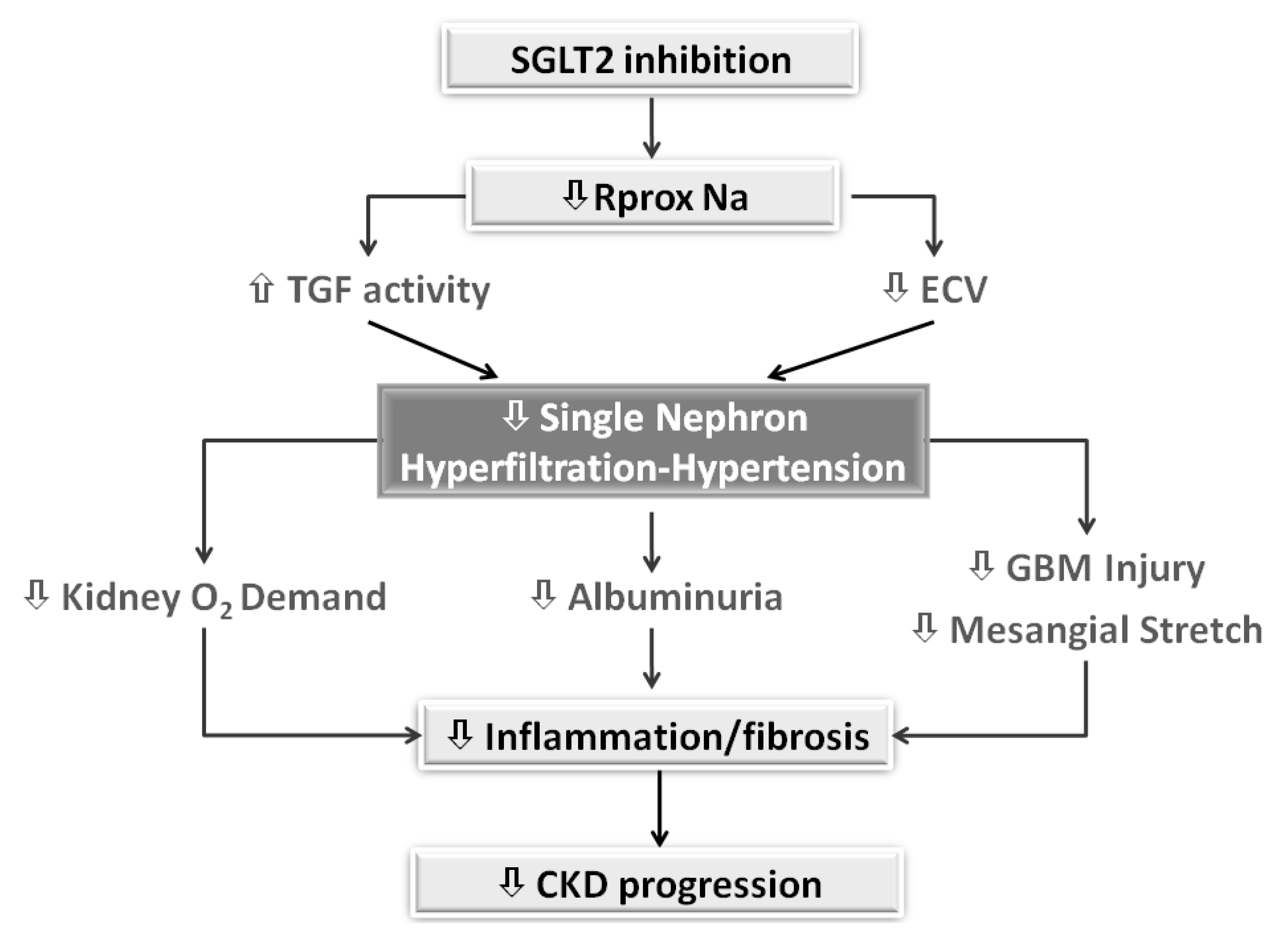
7. The Checkmark Sign: Pathophysiological Insights
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019, 96, 1048–1050. [Google Scholar] [CrossRef] [PubMed]
- Smart, N.A.; Titus, T.T. Outcomes of Early versus Late Nephrology Referral in Chronic Kidney Disease: A Systematic Review. Am. J. Med. 2011, 124, 1073–1080.e2. [Google Scholar] [CrossRef] [PubMed]
- Lundström, U.H.; Gasparini, A.; Bellocco, R.; Qureshi, A.R.; Carrero, J.-J.; Evans, M. Low renal replacement therapy incidence among slowly progressing elderly chronic kidney disease patients referred to nephrology care: An observational study. BMC Nephrol. 2017, 18, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brück, K.; Jager, K.J.; Zoccali, C.; Bello, A.K.; Minutolo, R.; Ioannou, K.; Verbeke, F.; Völzke, H.; Ärnlöv, J.; Leonardis, D.; et al. Different rates of progression and mortality in patients with chronic kidney disease at outpatient nephrology clinics across Europe. Kidney Int. 2018, 93, 1432–1441. [Google Scholar] [CrossRef]
- Pacilio, M.; Minutolo, R.; Garofalo, C.; Liberti, M.E.; Conte, G.; de Nicola, L. Stage 5-CKD under nephrology care: To dialyze or not to dialyze, that is the question. J. Nephrol. 2015, 29, 153–161. [Google Scholar] [CrossRef]
- Kliger, A.S.; Brosius, F.C. Diabetic Kidney Disease Task Force of the American Society of Nephrology. Preserving Kidney Function Instead of Replacing It. Clin. J. Am. Soc. Nephrol. 2020, 15, 129–131. [Google Scholar] [CrossRef] [Green Version]
- Chan, G.C.W.; Tang, S.C.W. Diabetic nephropathy: Landmark clinical trials and tribulations. Nephrol. Dial. Transplant. 2015, 31, 359–368. [Google Scholar] [CrossRef] [Green Version]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B. EMPA-REG OUTCOME Investigators. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef]
- Perkovic, V.; de Zeeuw, D.; Mahaffey, K.W.; Fulcher, G.; Erondu, N.; Shaw, W.; Barrett, T.D.; Weidner-Wells, M.; Deng, H.; Matthews, D.R.; et al. Canagliflozin and renal outcomes in type 2 diabetes: Results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018, 6, 691–704. [Google Scholar] [CrossRef]
- Mosenzon, O.; Wiviott, S.D.; Cahn, A.; Rozenberg, A.; Yanuv, I.; Goodrich, E.; Murphy, S.A.; Heerspink, H.J.L.; Zelniker, T.A.; Dwyer, J.P.; et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: An analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019, 7, 606–617. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. New Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giugliano, D.; Ceriello, A.; De Nicola, L.; Perrone-Filardi, P.; Cosentino, F.; Esposito, K. Primary versus secondary cardiorenal prevention in type 2 diabetes: Which newer anti-hyperglycaemic drug matters? Diabetes Obes. Metab. 2020, 22, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; de Nicola, L.; Maiorino, M.I.; Bellastella, G.; Garofalo, C.; Chiodini, P.; Ceriello, A.; Esposito, K. Preventing major adverse cardiovascular events by SGLT-2 inhibition in patients with type 2 diabetes: The role of kidney. Cardiovasc. Diabetol. 2020, 19, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wetmore, J.B.; Yan, H.; Horne, L.; Peng, Y.; Gilbertson, D.T. Risk of hyperkalemia from renin–angiotensin–aldosterone system inhibitors and factors associated with treatment discontinuities in a real-world population. Nephrol. Dial. Transplant. 2019. [Google Scholar] [CrossRef]
- Qiao, Y.; Shin, J.-I.; Chen, T.K.; Inker, L.A.; Coresh, J.; Alexander, G.C.; Jackson, J.W.; Chang, A.R.; Grams, M.E. Association Between Renin-Angiotensin System Blockade Discontinuation and All-Cause Mortality Among Persons With Low Estimated Glomerular Filtration Rate. JAMA Intern. Med. 2020, 180, 718. [Google Scholar] [CrossRef]
- Dave, C.V.; Schneeweiss, S.; Patorno, E. Comparative risk of genital infections associated with sodium-glucose co-transporter-2 inhibitors. Diabetes Obes. Metab. 2018, 21, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; la Verde, A.; Carfora, G.; Garofalo, C.; Provenzano, M.; Sasso, F.C.; de Nicola, L. Nephrology Consultation for Severe SGLT2 Inhibitor-Induced Ketoacidosis in Type 2 Diabetes: Case Report. Medicina 2019, 55, 462. [Google Scholar] [CrossRef] [Green Version]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Juurlink, D.N.; Mamdani, M.M.; Lee, D.S.; Kopp, A.; Austin, P.C.; Laupacis, A.; Redelmeier, D.A. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N. Engl. J. Med. 2004, 351, 543–551. [Google Scholar] [CrossRef]
- Birkeland, K.I.; Jørgensen, M.E.; Carstensen, B.; Persson, F.; Gulseth, H.L.; Thuresson, M.; Fenici, P.; Nathanson, D.; Nyström, T.; Eriksson, J.W.; et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): A multinational observational analysis. Lancet Diabetes Endocrinol. 2017, 5, 709–717. [Google Scholar] [CrossRef]
- Kosiborod, M.; Lam, C.S.; Kohsaka, S.; Kim, D.J.; Karasik, A.; Shaw, J.; Tangri, N.; Goh, S.-Y.; Thuresson, M.; Chen, H.; et al. Cardiovascular Events Associated With SGLT-2 Inhibitors Versus Other Glucose-Lowering Drugs. J. Am. Coll. Cardiol. 2018, 71, 2628–2639. [Google Scholar] [CrossRef] [PubMed]
- Cavender, M.A.; Norhammar, A.; Birkeland, K.I.; Jørgensen, M.E.; Wilding, J.P.; Khunti, K.; Fu, A.Z.; Bodegård, J.; Blak, B.T.; Wittbrodt, E.; et al. CVD-REAL Investigators and Study Group. SGLT-2 Inhibitors and Cardiovascular Risk: An Analysis of CVD-REAL. J. Am. Coll. Cardiol. 2018, 71, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.; Karasik, A.; Thuresson, M.; Melzer-Cohen, C.; Chodick, G.; Khunti, K.; Wilding, J.P.H.; Rodriguez, L.A.G.; Cea-Soriano, L.; Kohsaka, S.; et al. Kidney outcomes associated with use of SGLT2 inhibitors in real-world clinical practice (CVD-REAL 3): A multinational observational cohort study. Lancet Diabetes Endocrinol. 2020, 8, 27–35. [Google Scholar] [CrossRef]
- Pasternak, B.; Wintzell, V.; Melbye, M.; Eliasson, B.; Svensson, A.-M.; Franzén, S.; Gudbjörnsdottir, S.; Hveem, K.; Jonasson, C.; Svanström, H.; et al. Use of sodium-glucose co-transporter 2 inhibitors and risk of serious renal events: Scandinavian cohort study. BMJ 2020, 369, m1186. [Google Scholar] [CrossRef] [PubMed]
- Birkeland, K.I.; Bodegard, J.; Norhammar, A.; Kuiper, J.G.; Georgiado, E.; Beekman-Hendriks, W.L.; Thuresson, M.; Pignot, M.; Herings, R.M.C.; Kooy, A. How representative of a general type 2 diabetes population are patients included in cardiovascular outcome trials with SGLT2 inhibitors? A large European observational study. Diabetes Obes. Metab. 2019, 21, 968–974. [Google Scholar] [CrossRef] [Green Version]
- Lytvyn, Y.; Bjornstad, P.; van Raalte, D.H.; Heerspink, H.L.; Cherney, D.Z. The New Biology of Diabetic Kidney Disease—Mechanisms and Therapeutic Implications. Endocr. Rev. 2019, 41, 202–231. [Google Scholar] [CrossRef] [PubMed]
- De Nicola, L.; Gabbai, F.B.; Liberti, M.E.; Sagliocca, A.; Conte, G.; Minutolo, R. Sodium/Glucose Cotransporter 2 Inhibitors and Prevention of Diabetic Nephropathy: Targeting the Renal Tubule in Diabetes. Am. J. Kidney Dis. 2014, 64, 16–24. [Google Scholar] [CrossRef]
- Heerspink, H.J.; Kosiborod, M.; Inzucchi, S.E.; Cherney, D.Z.I. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int. 2018, 94, 26–39. [Google Scholar] [CrossRef]
- Cherney, D.Z.I.; Kanbay, M.; Lovshin, J.A. Renal physiology of glucose handling and therapeutic implications. Nephrol. Dial. Transplant. 2020, 35, i3–i12. [Google Scholar] [CrossRef] [Green Version]
- Packer, M. Role of Impaired Nutrient and Oxygen Deprivation Signaling and Deficient Autophagic Flux in Diabetic CKD Development: Implications for Understanding the Effects of Sodium-Glucose Cotransporter 2-Inhibitors. J. Am. Soc. Nephrol. 2020, 31, 907–919. [Google Scholar] [CrossRef]
- Pirklbauer, M.; Schupart, R.; Fuchs, L.C.; Staudinger, P.; Corazza, U.; Sallaberger, S.; Leierer, J.; Mayer, G.; Schramek, H. Unraveling reno-protective effects of SGLT2 inhibition in human proximal tubular cells. Am. J. Physiol-Ren. Physiol. 2019, 316, F449–F462. [Google Scholar] [CrossRef] [PubMed]
- Tonneijck, L.; Muskiet, M.H.; Smits, M.M.; van Bommel, E.J.; Heerspink, H.J.; van Raalte, D.H.; Joles, J.A. Glomerular Hyperfiltration in Diabetes: Mechanisms, Clinical Significance, and Treatment. J. Am. Soc. Nephrol. 2017, 28, 1023–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallon, V.; Thomson, S.C. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat. Rev. Nephrol. 2020, 16, 317–336. [Google Scholar] [CrossRef]
- Schnermann, J.; Briggs, J.P. The macula densa is worth its salt. J. Clin. Investig. 1999, 104, 1007–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Wei, J.; Jiang, S.; Xu, L.; Wang, L.; Cheng, F.; Buggs, J.; Koepsell, H.; Vallon, V.; Liu, R. Macula Densa SGLT1-NOS1-Tubuloglomerular Feedback Pathway, a New Mechanism for Glomerular Hyperfiltration during Hyperglycemia. J. Am. Soc. Nephrol. 2019, 30, 578–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pessoa, T.D.; Campos, L.C.; Carraro-Lacroix, L.; Girardi, A.C.; Malnic, G. Functional role of glucose metabolism, osmotic stress, and sodium-glucose cotransporters isoform-mediated transport on Na+/H+ exchanger isoform 3 activity in the renal proximal tubule. J. Am. Soc. Nephrol. 2014, 25, 2028–2039. [Google Scholar] [CrossRef] [Green Version]
- Kidokoro, K.; Cherney, D.Z.I.; Bozovic, A.; Nagasu, H.; Satoh, M.; Kanda, E.; Sasaki, T.; Kashihara, N. Evaluation of Glomerular Hemodynamic Function by Empagliflozin in Diabetic Mice Using In Vivo Imaging. Circulation 2019, 140, 303–315. [Google Scholar] [CrossRef]
- Hommel, E.; Bruun, N.E.; Arnold-Larsen, S.; Parving, H.-H. Effects of acetazolamide on kidney function in Type 1 (insulin-dependent) diabetic patients with diabetic nephropathy. Diabetologia 1988, 31, 806–810. [Google Scholar] [CrossRef] [Green Version]
- Hodrea, J.; Balogh, D.B.; Hosszu, A.; Lenart, L.; Besztercei, B.; Koszegi, S.; Sparding, N.; Genovese, F.; Wagner, L.J.; Szabo, A.J.; et al. Reduced O-GlcNAcylation and tubular hypoxia contribute to the antifibrotic effect of SGLT2 inhibitor dapagliflozin in the diabetic kidney. Am. J. Physiol. Physiol. 2020, 318, F1017–F1029. [Google Scholar] [CrossRef] [PubMed]
- Hesp, A.C.; Schaub, J.A.; Prasad, P.V.; Vallon, V.; Laverman, G.D.; Bjornstad, P.; Van Raalte, D.H. The role of renal hypoxia in the pathogenesis of diabetic kidney disease: A promising target for newer renoprotective agents including SGLT2 inhibitors? Kidney Int. 2020. [Google Scholar] [CrossRef]
- Cannon, C.P.; Perkovic, V.; Agarwal, R.; Baldassarre, J.; Bakris, G.; Charytan, D.M.; De Zeeuw, D.; Edwards, R.; Greene, T.; Heerspink, H.J.; et al. Evaluating the Effects of Canagliflozin on Cardiovascular and Renal Events in Patients With Type 2 Diabetes Mellitus and Chronic Kidney Disease According to Baseline HbA1c, Including Those With HbA1c <7%: Results From the CREDENCE Trial. Circulation 2020, 141, 407–410. [Google Scholar] [CrossRef]
- Petrie, M.C.; Verma, S.; Docherty, K.F.; Inzucchi, S.E.; Anand, I.; Belohlávek, J.; Böhm, M.; Chiang, C.-E.; Chopra, V.K.; De Boer, R.A.; et al. Effect of Dapagliflozin on Worsening Heart Failure and Cardiovascular Death in Patients With Heart Failure With and Without Diabetes. JAMA 2020, 323, 1353. [Google Scholar] [CrossRef] [PubMed]
- FARXIGA Phase III DAPA-CKD Trial Will Be Stopped Early After Overwhelming Efficacy in Patients With Chronic Kidney Disease. Available online: https://www.astrazeneca.com/media-centre/press-releases/2020/farxiga-phase-iii-dapa-ckd-trial-will-be-stopped-early-after-overwhelming-efficacy-in-patients-with-chronic-kidney-disease.html (accessed on 25 April 2020).
- Cherney, D.Z.I.; Heerspink, H.J.L.; Frederich, R.; Maldonado, M.; Liu, J.; Pong, A.; Xu, Z.J.; Patel, S.; Hickman, A.; Mancuso, J.P.; et al. Effects of ertugliflozin on renal function over 104 weeks of treatment: A post hoc analysis of two randomised controlled trials. Diabetologia 2020, 63, 1128–1140. [Google Scholar] [CrossRef] [Green Version]
- Jardine, M.J.; Zhou, Z.; Mahaffey, K.W.; Oshima, M.; Agarwal, R.; Bakris, G.; Bajaj, H.S.; Bull, S.; Cannon, C.P.; Charytan, D.M.; et al. Renal, Cardiovascular, and Safety Outcomes of Canagliflozin by Baseline Kidney Function: A Secondary Analysis of the CREDENCE Randomized Trial. J. Am. Soc. Nephrol. 2020, 31, 1128–1139. [Google Scholar] [CrossRef]
- Cherney, D.Z.I.; Zinman, B.; Inzucchi, S.E.; Koitka-Weber, A.; Mattheus, M.; Von Eynatten, M.; Wanner, C. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: An exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 610–621. [Google Scholar] [CrossRef]
- Mayer, G.J.; Wanner, C.; Weir, M.R.; Inzucchi, S.E.; Koitka-Weber, A.; Hantel, S.; Von Eynatten, M.; Zinman, B.; Cherney, D.Z.I. Analysis from the EMPA-REG OUTCOME® trial indicates empagliflozin may assist in preventing the progression of chronic kidney disease in patients with type 2 diabetes irrespective of medications that alter intrarenal hemodynamics. Kidney Int. 2019, 96, 489–504. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42 (Suppl. 1), S124–S138. [Google Scholar] [CrossRef] [Green Version]
- Sarafidis, P.; Ferro, C.J.; Morales, E.; Ortiz, A.; Malyszko, J.; Hojs, R.; Khazim, K.; Ekart, R.; Valdivielso, J.M.; Fouque, D.; et al. SGLT-2 inhibitors and GLP-1 receptor agonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease. A consensus statement by the EURECA-m and the DIABESITY working groups of the ERA-EDTA. Nephrol. Dial. Transplant. 2019, 34, 208–230. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. ESC Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [Green Version]
- Björck, S.; Mulec, H.; Johnsen, S.A.; Nordén, G.; Aurell, M. Renal protective effect of enalapril in diabetic nephropathy. BMJ 1992, 304, 339–343. [Google Scholar] [CrossRef] [Green Version]
- Holtkamp, F.A.; de Zeeuw, D.; Thomas, M.; Cooper, M.E.; de Graeff, P.A.; Hillege, H.J.; Parving, H.-H.; Brenner, B.M.; Shahinfar, S.; Heerspink, H.J.L. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011, 80, 282–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weil, E.J.; Fufaa, G.; Jones, L.I.; Lovato, T.; Lemley, K.V.; Hanson, R.L.; Knowler, W.C.; Bennett, P.H.; Yee, B.; Myers, B.D.; et al. Effect of Losartan on Prevention and Progression of Early Diabetic Nephropathy in American Indians With Type 2 Diabetes. Diabetes 2013, 62, 3224–3231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modification of Diet in Renal Disease Study Group. Short-term effects of protein intake, blood pressure, and antihypertensive therapy on glomerular filtration rate in the Modification of Diet in Renal Disease Study. J. Am. Soc. Nephrol. 1996, 7, 2097–2109. [Google Scholar]
- Nielsen, F.S.; Rossing, P.; Gall, M.A.; Skøtt, P.; Smidt, U.M.; Parving, H.H. Long-term effect of lisinopril and atenolol on kidney function in hypertensive NIDDM subjects with diabetic nephropathy. Diabetes 1997, 46, 1182–1188. [Google Scholar] [CrossRef]
- Peralta, C.A.; McClure, L.A.; Scherzer, R.; Odden, M.C.; White, C.L.; Shlipak, M.; Benavente, O.; Pergola, P. Effect of Intensive Versus Usual Blood Pressure Control on Kidney Function Among Individuals With Prior Lacunar Stroke: A Post Hoc Analysis of the Secondary Prevention of Small Subcortical Strokes (SPS3) Randomized Trial. Circulation 2016, 133, 584–591. [Google Scholar] [CrossRef] [Green Version]
- Cheung, A.K.; Rahman, M.; Reboussin, D.M.; Craven, T.E.; Greene, T.; Kimmel, P.L.; Cushman, W.C.; Hawfield, A.T.; Johnson, K.C.; Lewis, C.E.; et al. Effects of Intensive BP Control in CKD. J. Am. Soc. Nephrol. 2017, 28, 2812–2823. [Google Scholar] [CrossRef]
- Apperloo, A.J.; de Zeeuw, D.; de Jong, P.E. A short-term antihypertensive treatment-induced fall in glomerular filtration rate predicts long-term stability of renal function. Kidney Int. 1997, 51, 793–797. [Google Scholar] [CrossRef] [Green Version]
- Maschio, G.; Mann, J.F.; Zucchelli, P.; Locatelli, F.; Ponticelli, C.; Alberti, D.; Janin, G.; Motolese, M.; Ritz, E. Effect of the Angiotensin-Converting–Enzyme Inhibitor Benazepril on the Progression of Chronic Renal Insufficiency. N. Engl. J. Med. 1996, 334, 939–945. [Google Scholar] [CrossRef]
- Cianciaruso, B.; Bellizzi, V.; Capuano, A.; Bovi, G.; Nastasi, A.; Conte, G.; De Nicola, L. Short-term effects of low protein-normal sodium diet on renal function in chronic renal failure. Kidney Int. 1994, 45, 852–860. [Google Scholar] [CrossRef] [Green Version]
- Cianciaruso, B.; Bellizzi, V.; Minutolo, R.; Colucci, G.; Bisesti, V.; Russo, D.; Conte, G.; de Nicola, L. Renal adaptation to dietary sodium restriction in moderate renal failure resulting from chronic glomerular disease. J. Am. Soc. Nephrol. 1996, 7, 306–313. [Google Scholar]
- Friedman, A.N.; Quinney, S.K.; Inman, M.; Mattar, S.G.; Shihabi, Z.; Moe, S. Influence of dietary protein on glomerular filtration before and after bariatric surgery: A cohort study. Am. J. Kidney Dis. 2013, 63, 598–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minutolo, R.; de Nicola, L.; Gallo, C.; Chiodini, P.; Provenzano, M.; Conte, G.; Garofalo, C.; Borrelli, S.; Collaborative Study Group on the Conservative Treatment of CKD of the Italian Society of Nephrology. Generalizability of SPRINT-CKD cohort to CKD patients referred to renal clinics. J. Nephrol. 2019, 32, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Bricker, N.S. On the pathogenesis of the uremic state--an exposition of the trade-off hypothesis. Nihon Jinzo Gakkai Shi 1974, 16, 327. [Google Scholar]
- Brenner, P.D.B.M.; Brenner, B.M. Hemodynamically mediated glomerular injury and the progressive nature of kidney disease. Kidney Int. 1983, 23, 647–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
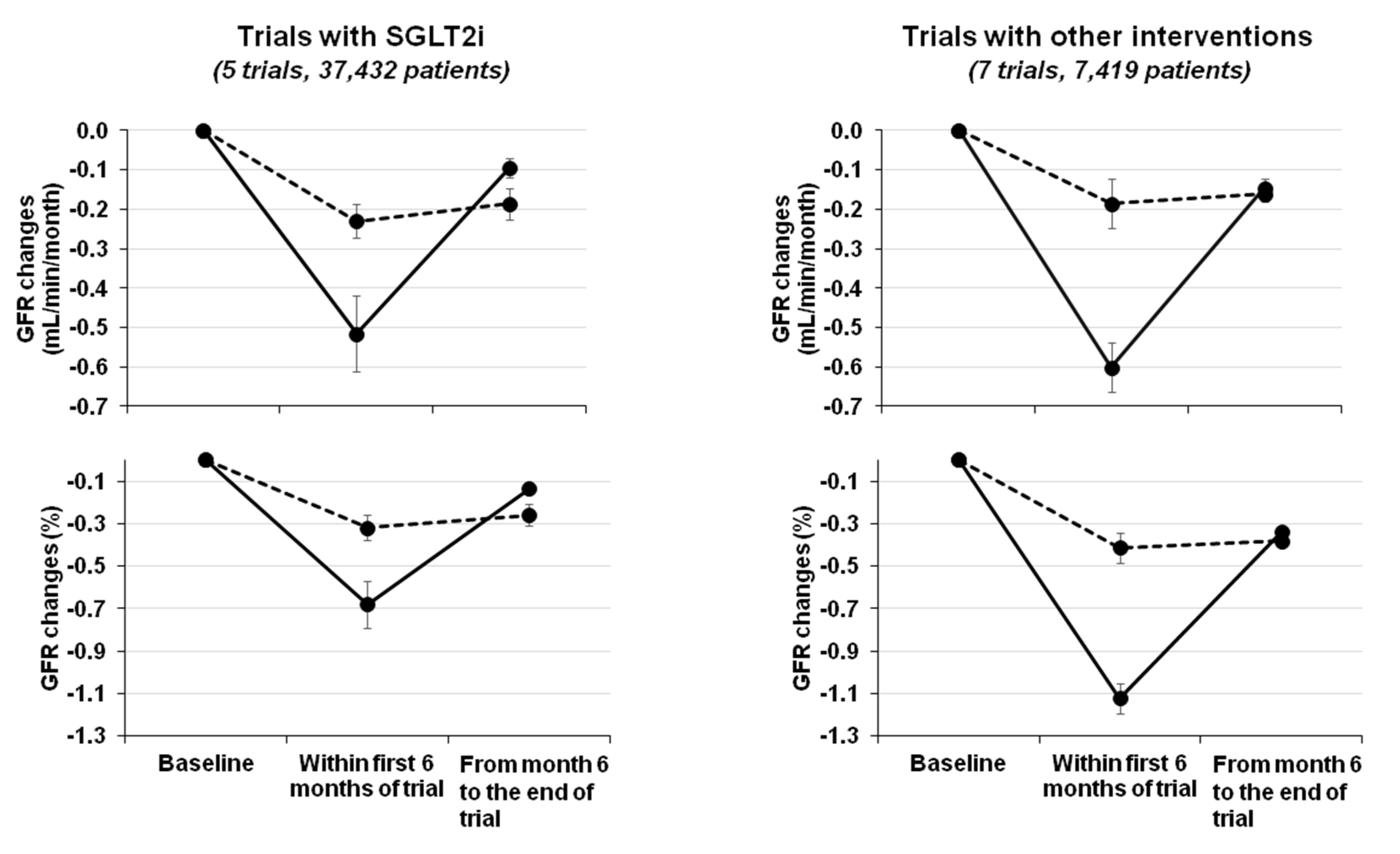
| Trial ref | Active Arm | Control Arm | N (Active/Control) † | DKD | GFR/Scr | Time (Years) | eGFR Changes (mL/min/month) | P for Overall eGFR Decline ° | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Active Arm | Control Arm | ||||||||||
| Within 6 Months | 6 Months-End Trial | Within 6 Months | 6 Months-End Trial | ||||||||
| SGLT2 Inhibition | |||||||||||
| EMPAREG ^, [8] | Empaglifozin | Placebo | 2322/2323 | Yes | 30–59 | 4.0 | −0.21 * | −0.04 | −0.03 | −0.15 | <0.001 |
| CANVAS [9] | Canaglifozin | Placebo | 5711/4276 | Yes | >30 | 4.0 | −0.50 * | −0.04 | −0.18 | −0.08 | <0.001 |
| DECLARE [10] | Dapaglifozin | Placebo | 8581/8578 | Yes | 85 ± 16 | 4.0 | −0.55 * | −0.13 | −0.22 | −0.21 | <0.001 |
| CREDENCE [11] | Canaglifozin | Placebo | 2179/2178 | Yes | 30–90 | 3.5 | −0.80 * | −0.21 | −0.60 | −0.36 | <0.001 |
| VERTIS § [44] | Ertuglifozin | Placebo | 640/644 | Yes | ≥55 | 2.2 | −0.33 * | 0.08 | −0.20 | −0.06 | <0.05 |
| Other Interventions | |||||||||||
| Bjorck [51] | Enalapril | Metoprolol | 20/16 | Yes | 46 ± 14 | 3.0 | −1.00 * | −0.01 | −0.60 | −0.093 | 0.021 |
| RENAAL [52] | Losartan | Placebo | 719/716 | Yes | 1.3–3.0 | 3.4 | −0.77 * | −0.35 | −0.53 | −0.42 | 0.01 |
| PIMA (ACR 30–299) [53] | Losartan | Placebo | 39/39 | Yes | 167 ± 43 | 6.0 | −5.20 * | −0.25 | 0.99 | −0.45 | 0.42 |
| MDRD study A [54] | LPD | Usual diet | 287/291 | No | 25–55 | 3.0 | −0.93 * | −0.21 | −0.47 | −0.32 | 0.30 |
| MAP < 92 mmHg | MAP < 107 mmHg | 296/282 | No | 25–55 | 3.0 | −0.90 * | −0.20 | −0.50 | −0.31 | 0.18 | |
| Nielsen [55] | Lisinopril | Atenolol | 17/19 | Yes | 75 ± 6 | 3.5 | −1.25 | −0.59 | −0.81 | −0.54 | 0.63 |
| SPS3 [56] | SBP < 130 mmHg | SBP 130–149 mmHg | 1301/1309 | Mixed | 80 ± 19 | 5.0 | −0.58 | −0.10 | −0.35 | −0.07 | <0.001 |
| SPRINT-CKD [57] | SBP < 120 mmHg | SBP < 140 mmHg | 1330/1316 | No | 20–59 | 3.3 | −0.25 * | −0.04 | 0.28 | −0.03 | 0.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Nicola, L.; Gabbai, F.B.; Garofalo, C.; Conte, G.; Minutolo, R. Nephroprotection by SGLT2 Inhibition: Back to the Future? J. Clin. Med. 2020, 9, 2243. https://doi.org/10.3390/jcm9072243
De Nicola L, Gabbai FB, Garofalo C, Conte G, Minutolo R. Nephroprotection by SGLT2 Inhibition: Back to the Future? Journal of Clinical Medicine. 2020; 9(7):2243. https://doi.org/10.3390/jcm9072243
Chicago/Turabian StyleDe Nicola, Luca, Francis B. Gabbai, Carlo Garofalo, Giuseppe Conte, and Roberto Minutolo. 2020. "Nephroprotection by SGLT2 Inhibition: Back to the Future?" Journal of Clinical Medicine 9, no. 7: 2243. https://doi.org/10.3390/jcm9072243





