Selecting Appropriate Sarcopenia Screening Methods for Asian Populations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Studied Populations
2.2.1. Healthy Control Population
2.2.2. Urban Population
2.2.3. Community-Based Population
2.3. Measurements
2.3.1. Anthropometric Measurements
2.3.2. DXA Examinations
2.3.3. Handgrip Strength Assessment
2.4. Screening for Sarcopenia
2.5. Statistics
3. Results
3.1. Healthy Controls
3.1.1. Physiological TBC Changes
3.1.2. Acquiring the Cut-off Value
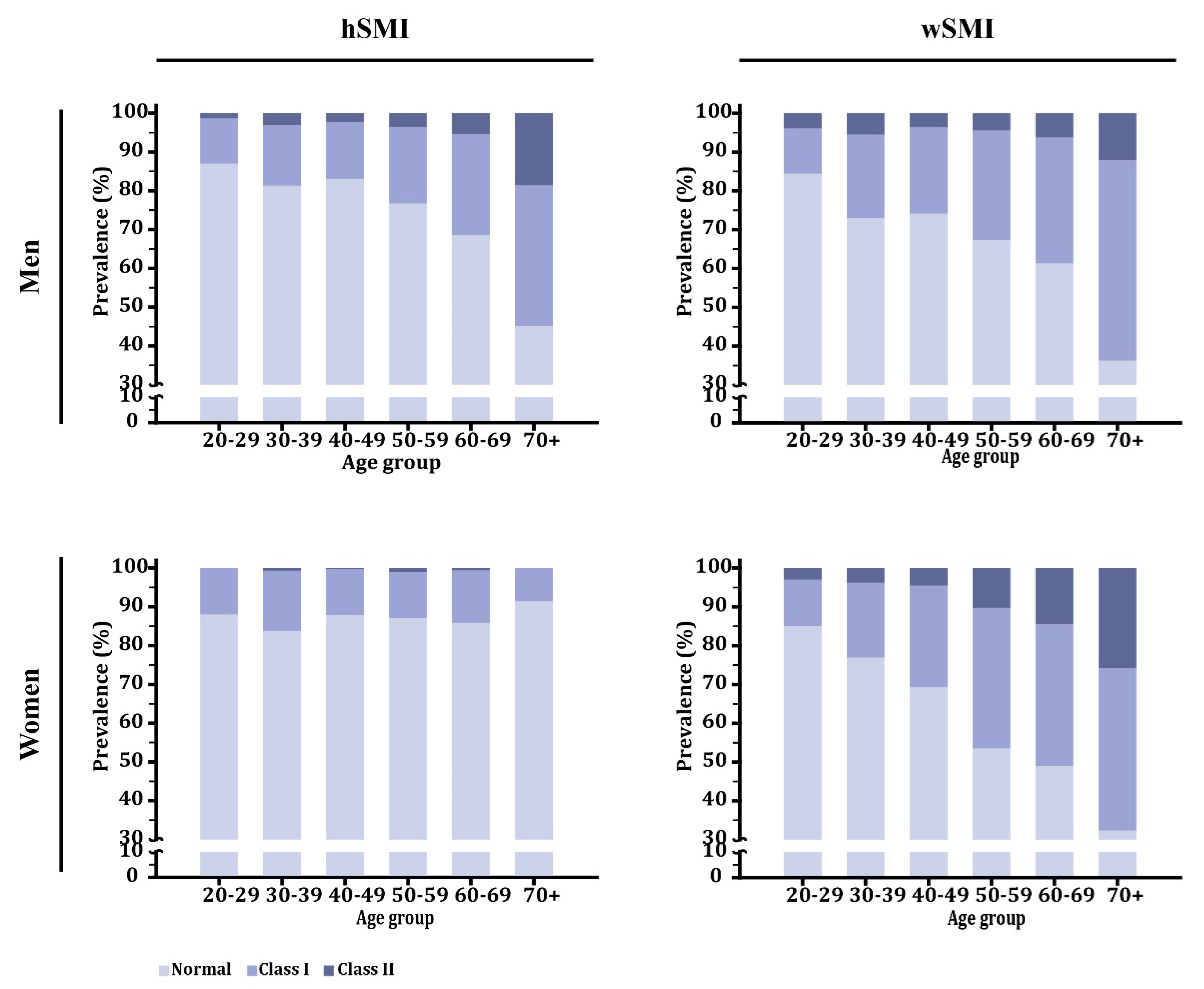
3.1.3. Prevalence of Low Skeletal Mass According to Age Decade
3.2. Urban and Community-Based Elderly Populations
3.3. Handgrip Strength
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pagotto, V.; Silveira, E.A. Methods, diagnostic criteria, cutoff points, and prevalence of sarcopenia among older people. Sci. World J. 2014, 2014, 231312. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Hai, S.; Cao, L.; Zhou, J.; Liu, P.; Dong, B.R. Estimation of prevalence of sarcopenia by using a new bioelectrical impedance analysis in Chinese community-dwelling elderly people. BMC Geriatr. 2016, 16, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, Y.C.; Wahlqvist, M.L.; Huang, Y.C.; Chuang, S.Y.; Wang, C.F.; Lee, M.S. Medical costs of a low skeletal muscle mass are modulated by dietary diversity and physical activity in community-dwelling older Taiwanese: A longitudinal study. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 31. [Google Scholar] [CrossRef] [Green Version]
- Rier, H.N.; Jager, A.; Sleijfer, S.; Maier, A.B.; Levin, M.-D. The Prevalence and Prognostic Value of Low Muscle Mass in Cancer Patients: A Review of the Literature. Oncologist 2016, 21, 1396–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothney, M.P.; Martin, F.P.; Xia, Y.; Beaumont, M.; Davis, C.; Ergun, D.; Fay, L.; Ginty, F.; Kochhar, S.; Wacker, W.; et al. Precision of GE Lunar iDXA for the measurement of total and regional body composition in nonobese adults. J. Clin. Densitom. 2012, 15, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Laskey, M.A. Dual-energy X-ray absorptiometry and body composition. Nutrition 1996, 12, 45–51. [Google Scholar] [CrossRef]
- Kim, Y.P.; Joh, J.Y.; Kim, S.; Hwang, H.S.; Shin, I.S. The application of different appendicular skeletal muscle cutoff points and research definitions associated with health-related quality of life in Korean older people: Data from KNHANES 2008–2011. BMC Geriatr. 2014, 14, 144. [Google Scholar] [CrossRef] [Green Version]
- Pongchaiyakul, C.; Limpawattana, P.; Kotruchin, P.; Rajatanavin, R. Prevalence of sarcopenia and associated factors among Thai population. J. Bone Miner. Metab. 2013, 31, 346–350. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.N.; Yang, S.J.; Yoo, H.J.; Lim, K.I.; Kang, H.J.; Song, W.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: The Korean sarcopenic obesity study. Int. J. Obes. 2009, 33, 885–892. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Meng, N.H.; Li, C.I.; Liu, C.S.; Lin, C.H.; Lin, W.Y.; Chang, C.K.; Li, T.C.; Lin, C.C. Comparison of height- and weight-adjusted sarcopenia in a Taiwanese metropolitan older population. Geriatr. Gerontol. Int. 2015, 15, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, R.; Matsui, Y.; Tange, C.; Nishita, Y.; Tomida, M.; Ando, F.; Shimokata, H.; Arai, H. What is the best adjustment of appendicular lean mass for predicting mortality or disability among Japanese community dwellers? BMC Geriatr. 2018, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Hangartner, T.N.; Warner, S.; Braillon, P.; Jankowski, L.; Shepherd, J. The Official Positions of the International Society for Clinical Densitometry: Acquisition of dual-energy X-ray absorptiometry body composition and considerations regarding analysis and repeatability of measures. J. Clin. Densitom. 2013, 16, 520–536. [Google Scholar] [CrossRef]
- Liu, L.K.; Lee, W.J.; Liu, C.L.; Chen, L.Y.; Lin, M.H.; Peng, L.N.; Chen, L.K. Age-related skeletal muscle mass loss and physical performance in Taiwan: Implications to diagnostic strategy of sarcopenia in Asia. Geriatr. Gerontol. Int. 2013, 13, 964–971. [Google Scholar] [CrossRef]
- Jackson, A.S.; Janssen, I.; Sui, X.; Church, T.S.; Blair, S.N. Longitudinal changes in body composition associated with healthy ageing: Men, aged 20–96 years. Br. J. Nutr. 2012, 107, 1085–1091. [Google Scholar] [CrossRef] [Green Version]
- Speakman, J.R.; Westerterp, K.R. Associations between energy demands, physical activity, and body composition in adult humans between 18 and 96 y of age. Am. J. Clin. Nutr. 2010, 92, 826–834. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Moriguch, Y.; Mitani, T.; Aoyama, T.; Arai, H. Age-dependent changes in skeletal muscle mass and visceral fat area in Japanese adults from 40 to 79 years-of-age. Geriatr. Gerontol. Int. 2014, 14 (Suppl. 1), 8–14. [Google Scholar] [CrossRef] [Green Version]
- Hunter, G.R.; Gower, B.A.; Kane, B.L. Age Related Shift in Visceral Fat. Int. J. Body Compos. Res. 2010, 8, 103–108. [Google Scholar]
- Cheng, Q.; Zhu, X.; Zhang, X.; Li, H.; Du, Y.; Hong, W.; Xue, S.; Zhu, H. A cross-sectional study of loss of muscle mass corresponding to sarcopenia in healthy Chinese men and women: Reference values, prevalence, and association with bone mass. J. Bone Miner. Metab. 2014, 32, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Ambikairajah, A.; Walsh, E.; Tabatabaei-Jafari, H.; Cherbuin, N. Fat mass changes during menopause: A metaanalysis. Am. J. Obstet. Gynecol. 2019, 221, 393–409.e350. [Google Scholar] [CrossRef]
- Greendale, G.A.; Sternfeld, B.; Huang, M.; Han, W.; Karvonen-Gutierrez, C.; Ruppert, K.; Cauley, J.A.; Finkelstein, J.S.; Jiang, S.F.; Karlamangla, A.S. Changes in body composition and weight during the menopause transition. JCI Insight 2019, 4, e124865. [Google Scholar] [CrossRef] [PubMed]
- Furushima, T.; Miyachi, M.; Iemitsu, M.; Murakami, H.; Kawano, H.; Gando, Y.; Kawakami, R.; Sanada, K. Comparison between clinical significance of height-adjusted and weight-adjusted appendicular skeletal muscle mass. J. Physiol. Anthropol. 2017, 36, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.K.; Lee, W.J.; Peng, L.N.; Liu, L.K.; Arai, H.; Akishita, M.; Asian Working Group for, S. Recent Advances in Sarcopenia Research in Asia: 2016 Update From the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2016, 17, 767.e1–767.e7. [Google Scholar] [CrossRef] [PubMed]
- Meng, P.; Hu, Y.X.; Fan, L.; Zhang, Y.; Zhang, M.X.; Sun, J.; Liu, Y.; Li, M.; Yang, Y.; Wang, L.H.; et al. Sarcopenia and sarcopenic obesity among men aged 80 years and older in Beijing: Prevalence and its association with functional performance. Geriatr. Gerontol. Int. 2014, 14 (Suppl. 1), 29–35. [Google Scholar] [CrossRef] [Green Version]
- Coelho-Junior, H.J.; Milano-Teixeira, L.; Rodrigues, B.; Bacurau, R.; Marzetti, E.; Uchida, M. Relative Protein Intake and Physical Function in Older Adults: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2018, 10, 1330. [Google Scholar] [CrossRef] [Green Version]
- Yuki, A.; Ando, F.; Otsuka, R.; Shimokata, H. Sarcopenia based on the Asian Working Group for Sarcopenia criteria and all-cause mortality risk in older Japanese adults. Geriatr. Gerontol. Int. 2017, 17, 1642–1647. [Google Scholar] [CrossRef]
- Auyeung, T.W.; Lee, S.W.; Leung, J.; Kwok, T.; Woo, J. Age-associated decline of muscle mass, grip strength and gait speed: A 4-year longitudinal study of 3018 community-dwelling older Chinese. Geriatr. Gerontol. Int. 2014, 14 (Suppl. 1), 76–84. [Google Scholar] [CrossRef]


| All Healthy Individuals | Young Referents | |||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| n | 3028 | 2853 | 77 | 134 |
| Age (y) | 50.16 (11.27) | 48.20 (11.52) | 26.52 (2.42) | 26.66 (2.33) |
| Height (m) | 1.70 (0.06) | 1.58 (0.06) | 1.74 (0.04) | 1.61 (0.05) |
| Weight (kg) | 72.37 (10.78) | 57.06 (9.03) | 73.08 (11.20) | 55.70 (10.78) |
| BMI (kg/m2) | 25.00 (3.22) | 22.85 (3.59) | 24.19 (3.71) | 21.60 (4.08) |
| TBM (kg) | 2.84 (0.42) | 2.19 (0.34) | 3.07 (0.43) | 2.30 (0.29) |
| TFM (kg) | 19.10 (6.50) | 19.96 (6.30) | 17.89 (7.74) | 18.24 (7.20) |
| TLM (kg) | 50.43 (5.69) | 34.61 (3.91) | 52.17 (5.03) | 35.02 (4.58) |
| ASM (kg) | 22.63 (3.04) | 14.56 (2.00) | 24.32 (2.68) | 15.28 (2.33) |
| hSMI (kg/m2) | 7.81 (0.84) | 5.82 (0.69) | 8.04 (0.82) | 5.92 (0.80) |
| wSMI (%) | 31.47 (3.10) | 25.76 (2.95) | 33.65 (3.57) | 27.73 (2.92) |
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Urban | Community | p-Value | Urban | Community | p-Value | |
| n | 51 | 117 | 50 | 232 | ||
| Age (y) | 71.04 (9.68) | 74.56 (7.10) | 0.022 | 76.90 (11.00) | 72.76 (7.89) | 0.014 |
| Weight (kg) | 68.01 (11.79) | 62.37 (10.57) | 0.002 | 55.88 (8.81) | 54.76 (9.11) | 0.428 |
| Height (m) | 1.65 (0.07) | 1.62 (0.06) | 0.003 | 1.52 (0.06) | 1.52 (0.06) | 0.740 |
| BMI (kg/m2) | 24.87 (3.90) | 23.87 (3.82) | 0.122 | 24.18 (3.45) | 23.76 (3.60) | 0.450 |
| TBM (kg) | 2.42 (0.41) | 2.32 (0.37) | 0.112 | 1.59 (0.24) | 1.67 (0.27) | 0.056 |
| TFM (kg) | 19.92 (6.03) | 17.88 (6.43) | 0.057 | 20.17 (5.83) | 20.16 (5.90) | 0.990 |
| TLM (kg) | 45.54 (6.94) | 41.74 (5.39) | <0.001 | 34.33 (5.03) | 32.42 (3.90) | 0.014 |
| ASM (kg) | 19.79 (4.07) | 18.14 (2.90) | 0.003 | 14.23 (2.69) | 13.64 (2.08) | 0.148 |
| hSMI (kg/m2) | 7.25 (1.29) | 6.93 (0.97) | 0.079 | 6.13 (0.99) | 5.92 (0.79) | 0.100 |
| wSMI (%) | 29.13 (3.22) | 29.25 (2.95) | 0.812 | 25.53 (3.30) | 25.06 (2.25) | 0.343 |
| HS (kg) | 25.10 (11.40) | 24.22 (7.89) | 0.617 | 14.63 (7.50) | 17.87 (4.90) | 0.005 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-C.; Lu, Y.-C.; Chen, F.-P.; Lin, Y.C.; Cheung, Y.-C.; Chan, W.P. Selecting Appropriate Sarcopenia Screening Methods for Asian Populations. J. Clin. Med. 2020, 9, 2333. https://doi.org/10.3390/jcm9082333
Lin Y-C, Lu Y-C, Chen F-P, Lin YC, Cheung Y-C, Chan WP. Selecting Appropriate Sarcopenia Screening Methods for Asian Populations. Journal of Clinical Medicine. 2020; 9(8):2333. https://doi.org/10.3390/jcm9082333
Chicago/Turabian StyleLin, Yu-Ching, Yi-Chien Lu, Fang-Ping Chen, Ying Chin Lin, Yun-Chung Cheung, and Wing P. Chan. 2020. "Selecting Appropriate Sarcopenia Screening Methods for Asian Populations" Journal of Clinical Medicine 9, no. 8: 2333. https://doi.org/10.3390/jcm9082333






