Crop and Soil Responses to Using Corn Stover as a Bioenergy Feedstock: Observations from the Northern US Corn Belt
Abstract
:1. Introduction
2. Material and Methods
2.1. Characterization and History of Study Fields
2.2. Corn Stover Return Treatments
2.3. Agronomic Management
2.4. Soil Parameters
2.5. Statistical Analysis
3. Results
3.1. Return Rates and Crop Production
| a Average annual residue returned | b Average corn stover returned | |
|---|---|---|
| Return Rate | Mg ha−1 | |
| Chisel | ||
| Full | 5.99 ± 0.24 | 7.97 ± 0.25 |
| Moderate | 4.42 ± 0.10 | 4.10 ± 0.15 |
| Low | 3.00 ± 0.20 | 1.72 ± 0.16 |
| NT2005 | ||
| Full | 6.15 ± 0.13 | 8.05 ± 0.33 |
| Moderate | 3.99 ± 0.13 | 3.67 ± 0.21 |
| Low | 2.96 ± 0.13 | 1.20 ± 0.13 |
| NT1995 | ||
| Full | 5.73 ± 0.21 | 7.33 ± 0.28 |
| Moderate | 4.06 ± 0.24 | 3.65 ± 0.25 |
| Low | 2.82 ± 0.29 | 1.57 ± 0.22 |
| Corn grain | Dry Stover | Soybean | Dry straw | |
|---|---|---|---|---|
| Return Rate | Mg ha−1 | |||
| Chisel | ||||
| Full | 9.81 | 7.73 | 3.21 | 4.30 |
| Moderate | 10.2 | 7.81 | 3.25 | 4.07 |
| Low | 10.3 | 8.12 | 3.37 | 4.72 |
| NT2005 | ||||
| Full | 9.49 | 8.41 | 3.39 | 4.79 |
| Moderate | 9.75 | 7.97 | 3.43 | 4.28 |
| Low | 9.56 | 8.42 | 3.47 | 5.27 |
| NT1995 | ||||
| Full | 8.19 | 7.29 | 3.13aa | 4.50 |
| Moderate | 8.40 | 7.02 | 3.13a | 4.41 |
| Low | 7.69 | 7.09 | 2.88b | 4.91 |
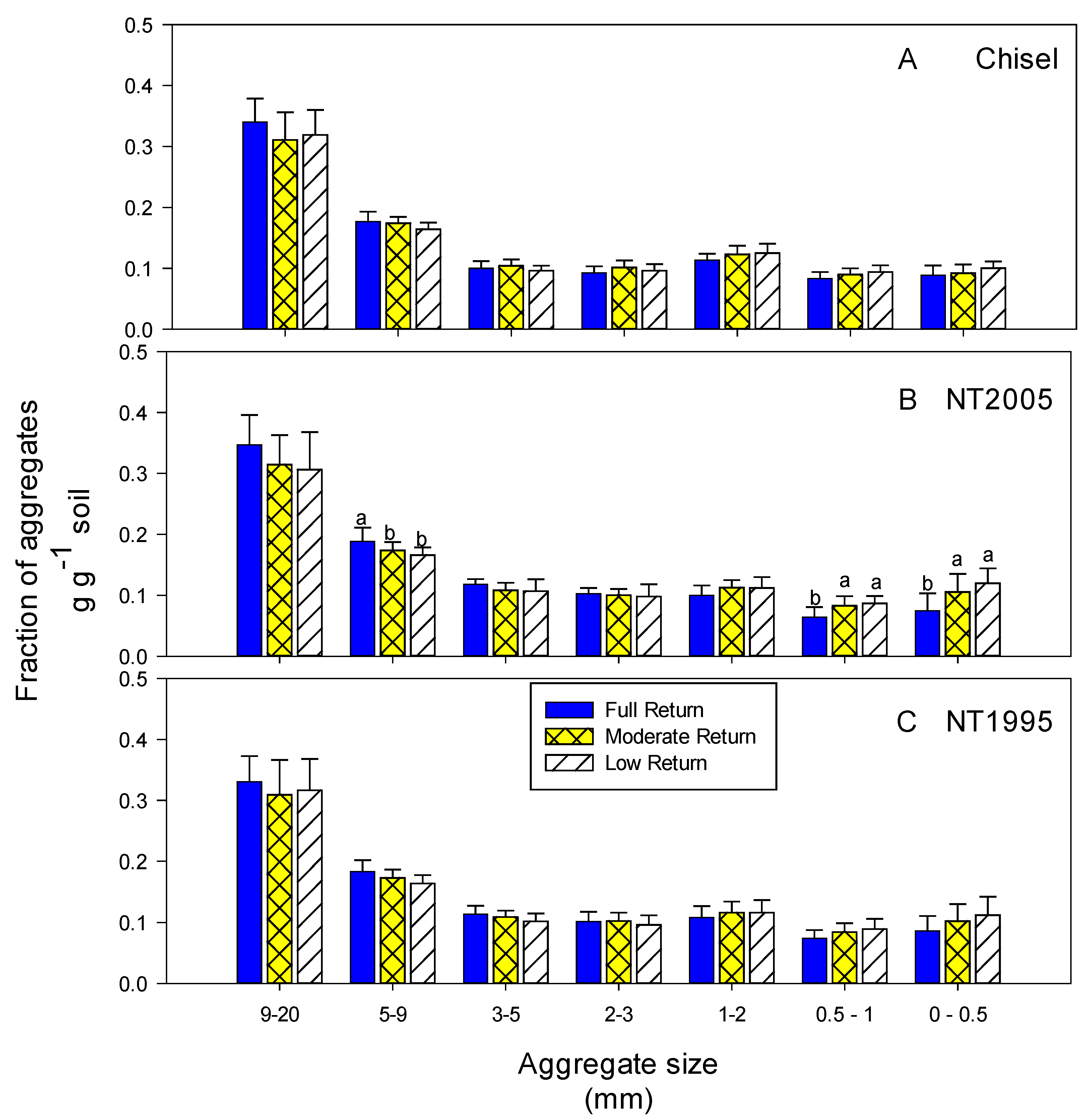
3.2. Soil Parameters
| Depth | Sand | Clay | pH CaCl2 | Bulk density | Total C | Organic C | Total N | POM a | Pext | Kext |
|---|---|---|---|---|---|---|---|---|---|---|
| cm | g kg−1 | g cm−3 | g kg−1 | |||||||
| Chisel | ||||||||||
| 0–5 | 360 | 280 | 6.79 | 1.26 | 25.8 | 24.8 | 2.14 | 8.2 | 20.7 | 178 |
| 5–10 | 350 | 280 | 6.77 | 1.29 | 23.3 | 22.3 | 1.94 | 5.9 | 16.7 | 153 |
| NT2005 | ||||||||||
| 0–5 | 370 | 270 | 6.04 | 1.24 | 25.5 | 25.4 | 2.28 | 9.5 | 26.3 | 250 |
| 5–10 | 370 | 260 | 5.98 | 1.38 | 22.1 | 22.0 | 2.02 | 7.4 | 23.0 | 155 |
| NT 1995 | ||||||||||
| 0–5 | 430 | 230 | 6.06 | 1.37 | 27.9 | 27.5 | 2.43 | 14.7 | 34.9 | 178 |
| 5–10 | 420 | 240 | 6.27 | 1.41 | 20.9 | 20.4 | 1.87 | 6.3 | 17.2 | 132 |
| Stover return | Depth | POM | SOC | Total N |
| cm | g kg−1 soil | |||
| Chisel | ||||
| Full | 0–5 | 7.42 | 25.9 | 2.19 |
| Moderate | 7.23 | 24.1 | 2.11 | |
| Low | 7.18 | 26.3 | 2.23 | |
| Full | 5–10 | 5.78 | 23.9 | 2.07 |
| Moderate | 5.82 | 22.5 | 2.02 | |
| Low | 5.66 | 25.0 | 2.18 | |
| NT 2005 | ||||
| Full | 0–5 | 10.42 | 26.7 | 2.27 |
| Moderate | 9.60 | 26.5 | 2.25 | |
| Low | 10.78 | 26.9 | 2.27 | |
| Full | 5–10 | 5.25 | 23.7 | 2.19 |
| Moderate | 5.53 | 23.0 | 2.09 | |
| Low | 5.40 | 23.5 | 2.14 | |
| NT1995 | ||||
| Full | 0–5 | 14.5a | 28.8 | 2.46 |
| Moderate | 14.0a | 27.0 | 2.36 | |
| Low | 11.3b | 26.2 | 2.27 | |
| Full | 5–10 | 5.56 | 23.1 | 2.14 |
| Moderate | 5.48 | 22.7 | 2.10 | |
| Low | 4.76 | 22.9 | 2.10 | |
| Microbial Biomass | Enzyme Activities | Bacterial fatty acid | Fungal fatty acids | ||||||
| StoverReturn Rate | Depth | C | N | Acid Phosphatase | β-Glucos aminidase | β-Gluco sidase | i17:0 | 16:1ω5c | 18:3ω6c |
| cm | mg g−1 soil | mg PN kg−1 soil h−1 | Nano-mol g−1 soil | ||||||
| Chisel | |||||||||
| Full | 0–5 | 729 | 35.5 | 269 | 23.3 | 138 | 0.74 | 7.31aa | 0.86 |
| Low | 744 | 32.0 | 289 | 23.0 | 137 | 0.83 | 5.97b | 0.84 | |
| Full | 5–10 | 669 | 29.7 | 293 | 21.5 | 121 | 0.72b | 9.14 | 0.83b |
| Low | 704 | 28.0 | 288 | 20.5 | 131 | 0.79a | 7.72 | 0.97a | |
| NT2005 | |||||||||
| Full | 0–5 | 823 | 34.0 | 464 a | 35.3 | 178 | 0.84 | 7.54 | 1.22 |
| Low | 804 | 33.8 | 366 b | 31.9 | 180 | 1.00 | 7.55 | 1.22 | |
| Full | 5–10 | 705 | 23.7 | 313 | 23.3 | 129 | 0.92 | 9.41 | 1.16 |
| Low | 788 | 27.0 | 312 | 23.5 | 133 | 0.85 | 10.11 | 1.15 | |
| NT1995 | |||||||||
| Full | 0–5 | 1010 | 55.0 | 405 | 44.5 | 258 | 0.56 | 7.50 | 0.75 |
| Low | 937 | 41.6 | 388 | 37.8 | 222 | 0.64 | 6.76 | 0.70 | |
| Full | 5–10 | 809 | 27.0 | 241 | 19.6 | 118 | 0.74 | 12.8 | 1.11 |
| Low | 774 | 21.5 | 244 | 16.9 | 103 | 0.81 | 9.45 | 1.11 | |
4. Discussion
4.1. Return Rates and Crop Production
4.2. Soil Parameters
5. Conclusions
Acknowledgments
Conflict of Interest
References
- USDA National Agriculture Statistics Service. Available online: http://quickstats.nass.usda.gov/ (accessed on 18 December 2012).
- US DOE U.S. Billion-ton update: Biomass supply for a bioenergy and bioproducts industry. R.D. Perlack and b.J. Stokes (leads), ornl/tm-2011/224. Available online: http://www1.eere.energy.gov/biomass/pdfs/billion_ton_update.pdf (accessed on 9 August 2012).
- Perlack, R.D.; Wright, L.L.; Turhollow, A.; Graham, R.L.; Stokes, B.; Erbach, D.C. Biomass as feedstock for a bioenergy and bioproducts industry: The technical feasibility of a billion-ton annual supply. Available online: http://www.eere.energy.gov/biomass/pdfs/final_billionton_vision_report2.pdf (accessed on 6 August 2012).
- BRDB Increasing feedstock production for biofuels: Economic drivers, environmental implications, and the role of research. Available online: http://www.usbiomassboard.gov/pdfs/increasing_feedstock_revised.pdf (accessed on 6 August 2012).
- Johnson, J.M.F.; Papiernik, S.K.; Mikha, M.M.; Spokas, K.A.; Tomer, M.D.; Weyers, S.L. Soil processes and residue harvest management. In Carbon Management, Fuels, and Soil Quality; Lal, R., Stewart, B.A., Eds.; Taylor and Francis, LLC: New York, NY, USA, 2010; pp. 1–44. [Google Scholar]
- Wilhelm, W.W.; Johnson, J.M.F.; Hatfield, J.L.; Voorhees, W.B.; Linden, D.R. Crop and soil productivity response to corn residue removal: A literature review. Agron. J. 2004, 96, 1–17. [Google Scholar] [CrossRef]
- Karlen, D.L.; Birell, S.J.; Hess, J.R. A five-year assessment of corn stover harvest in central iowa, USA. Soil Tillage Res. 2011, 115–116, 47–55. [Google Scholar] [CrossRef]
- Lindstrom, M.J. Effects of residue harvesting on water runoff, soil erosion and nutrient loss. Agric. Ecosyst. Environ. 1986, 16, 103–112. [Google Scholar] [CrossRef]
- Skidmore, E.L.; Siddoway, F.H. Crop residue requirements to control wind erosion. In Crop Residue Management Systems; Asa Special Publication Number 31; Oschwald, W.R., Stelly, M., Kral, D.M., Nauseef, J.H., Eds.; ASA, CSSA, and SSSA: Madison, WI, USA, 1978; pp. 17–33. [Google Scholar]
- Wilhelm, W.W.; Hess, J.R.; Karlen, D.L.; Johnson, J.M.F.; Muth, D.J.; Baker, J.M.; Gollany, H.T.; Novak, J.M.; Stott, D.E.; Varvel, G.E. Review: Balancing limiting factors and economic drivers for sustainable midwestern us agricultural residue feedstock supplies. Ind. Biotechnol. 2010, 6, 271–287. [Google Scholar]
- Merrill, S.D.; Black, A.L.; Fryrear, D.W.; Saleh, A.; Zobeck, T.M.; Halvorson, A.D.; Tanaka, D.L. Soil wind erosion hazard of spring wheat-fallow as affected by long-term climate and tillage. Soil Sci. Soc. Am. J. 1999, 63, 1768–1777. [Google Scholar]
- Chepil, W.S. Properties of soil which influence wind erosion: 11. Dry aggregate structure as an index of erodibility. Soil Sci. 1950, 69, 403–414. [Google Scholar] [CrossRef]
- Wilhelm, W.W.; Johnson, J.M.F.; Karlen, D.L.; Lightle, D.T. Corn stover to sustain soil organic carbon further constrains biomass supply. Agron. J. 2007, 99, 1665–1667. [Google Scholar]
- Schrumpf, M.; Schulze, E.D.; Kaiser, K.; Schumacher, J. How accurately can soil organic carbon stocks and stock changes be quantified by soil inventories? Biogeosci. Discuss. 2011, 8, 723–769. [Google Scholar] [CrossRef]
- VandenBygaart, A.J.; Bremer, E.; McConkey, B.G.; Ellert, B.H.; Janzen, H.H.; Angers, D.A.; Carter, M.R.; Drury, C.F.; Lafond, G.P.; McKenzie, R.H. Impact of sampling depth on differences in soil carbon stocks in long-term agroecosystem experiments. Soil Sci. Soc. Am. J. 2011, 75, 226–234. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliot, E.T. Particulate soil organic matter changes across a grassland cultivation sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Gajda, A.M.; Doran, J.W.; Wienhold, B.J.; Kettler, T.A. Estimation of particulate and total organic matter by weight loss-on-ignition. In Assessment Methods for Soil Carbon; Lal, R., Kimball, J.M., Follet, R.F., Stewart, B.A., Eds.; Lewis Publishers: Boca Raton, FL, USA, 2001; pp. 349–359. [Google Scholar]
- Hammerbeck, A.L.; Stetson, S.J.; Osborne, S.L.; Schumacher, T.E.; Pikul, J.L., Jr. Corn residue removal impact on soil aggregates in a no-till corn/soybean rotation. Soil Sci. Soc. Am. J. 2012, 4, 1390–1398. [Google Scholar]
- Kushwaha, C.P.; Tripathi, S.K.; Singh, K.P. Variations in soil microbial biomass and n availability due to residue and tillage management in a dryland rice agroecosystem. Soil Tillage Res. 2000, 56, 153–166. [Google Scholar] [CrossRef]
- Karlen, D.L.; Wollenhaupt, N.C.; Erbach, D.C.; Berry, E.C.; Swan, J.B.; Eash, N.S.; Jordahl, J.L. Crop residue effects on soil quality following 10-years of no-till corn. Soil Tillage Res. 1994, 31, 149–167. [Google Scholar] [CrossRef]
- CTIC National crop residue management survey conservation tillage data, 2002. Available online: http://www2.ctic.purdue.edu/CTIC/CRM.html (accessed on 6 August 2012).
- West, T.O.; Marland, G.; King, A.W.; Post, W.M.; Jain, A.K.; Andrasko, K. Carbon management response curves: Estimates of temporal soil carbon dynamics. Environ. Manag. 2004, 33, 507–518. [Google Scholar]
- NOAA-NCDC, Climatography of the United States No. 81: 21 Minnesota; U.S. Department of Commerce National Oceanic and Atmospheric Administration, National Climatic Data Center: Asheville, NC, USA, 2002.
- USDA-SCS, Soil Survey Stevens County, Minnesota; U.S. Department of Agriculture Soil Conservation Service: Washington, DC, USA, 1971.
- Olness, A.E.; Lopez, D.; Archer, D.W.; Cordes, J.; Sweeney, C.; Mattson, N.; Rinke, J.L.; Voorhees, W.B. The ars nitrogen decision aid. Available online: http://www.ars.usda.gov/services/software/download.htm?softwareid=85 (accessed on 6 August 2012).
- Fehr, W.R.; Caviness, C.E.; Burmood, D.T.; Pennington, J.S. Stage of development descriptions of soybeans, glycine max (l.) merrill. Crop Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Donald, C.M.; Hamblin, J. The biological yield and harvest index of cereals as an agronomic and plant breeding criteria. Adv. Agron. 1976, 28, 361–405. [Google Scholar]
- Liebig, M.; Varvel, G.; Honeycutt, W. Chapter 1. Guidelines for site description and soil sampling, processing, analysis, and archiving. In GRACEnet Sampling Protocols; Follett, R., Ed.; USDA-Agricultural Research Service: Washington, DC, USA, 2010; pp. 1–5. [Google Scholar]
- Burt, R. Nrcs Soil Survey Laboratory Methods Manual Report No. 42, Version 4.0, November 2004; USDA-NRCS: Washington, DC, USA, 2004; p. 700. [Google Scholar]
- Day, P.R. Report of the committee on physical analyses, 1954–55. Soil Sci. Soc. Am. J. 1956, 20, 167–169. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis, Part 1. Physical and Mineralogical Methods—Agron. Monogr. No. 9, 2nd ed; ASA: Madison, WI, USA, 1986. [Google Scholar]
- Thomas, G.W. Soil ph and soil acidity. In Methods of Soil Analysis, Part 3 Chemical Methods; SSSA Book Series 5; Bigham, J.M., Bartels, J.M., Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA and ASA: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Wagner, S.W.; Hanson, J.D.; Olness, A.; Voorhees, W.B. A volumetric inorganic carbon analysis system. Soil Sci. Soc. Am. J. 1998, 62, 690–693. [Google Scholar] [CrossRef]
- Bigham, J.M.; Bartels, J.M.; Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Sumner, M.E. Methods of Soil Analysis. Part 3 Chemical Methods; SSSA Book Series No. 5; SSSA and ASA: Madison, WI, USA, 1996. [Google Scholar]
- Gale, W.J.; Cambardella, C.A. Carbon dynamics of surface residue- and root-derived organic matter under simulated no-till. Soil Sci. Soc. Am. J. 2000, 64, 190–195. [Google Scholar] [CrossRef]
- Schulte, E.E. Recommmended soil organic matter tests. In Recommended Chemical Soil Test Procedures for the North Central Region, Ncr publ. No. 221 (revised); Dahnke, W.C., Ed.; Cooperative Extension Service, North Dakota State University: Fargo, ND, USA, 1988; pp. 29–31. [Google Scholar]
- Richards, B.K.; Wafter, M.F.; Muck, R.E. Variation in line transect measurements of crop residue cover. J. Soil Water Conserv. 1984, 39, 60–61. [Google Scholar]
- Laflen, J.M.; Amemiya, M.; Hintz, E.A. Measuring crop residue cover. J. Soil Water Conserv. 1981, 36, 341–343. [Google Scholar]
- Chepil, W.S. A compact rotary sieve and the importance of dry sieving in physical soil analysis. Soil Sci. Soc. Am. J. 1962, 26, 4–6. [Google Scholar] [CrossRef]
- Pikul, J.L., Jr.; Chilom, G.; Rice, J.; Eynard, A.; Schumacher, T.E.; Nichols, K.; Johnson, J.M.F.; Wright, S.; Caesar, T.; Ellsbury, M. Organic matter and water stability of field aggregates affected by tillage in south dakota. Soil Sci. Soc. Am. J. 2009, 73, 197–206. [Google Scholar]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil. Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Wu, J.R.; Jorgensen, G.; Pommerening, B.; Chaussod, R.; Brooke, P.C. Measurement of soil microbial biomass C by fumigation-extraction—An automated procedure. Soil Biol. Biochem. 1990, 25, 1435–1441. [Google Scholar]
- Jenkinson, D.S. Determination of microbial biomass carbon and nitrogen in soil. In Advances in Nitrogen Cycling in Agricultural Ecosystems; Wilson, J.R., Ed.; CAB, Int: Wallingford, UK, 1988; pp. 368–386. [Google Scholar]
- Jenkinson, D.S.; Brookes, P.C.; Powlson, D.S. Measuring soil microbial biomass. Soil Biol. Biochem. 2004, 36, 5–7. [Google Scholar]
- Cavigelli, M.A.; Robertson, G.P.; Klug, M.J. Fatty acid methyl ester (fame) profiles as measures of soil microbial community structure. Plant Soil 1995, 170, 99–113. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Zobeck, T.M.; Allen, V. Soil microbial, chemical and physical properties in continuous cotton and integrated crop-livestock systems. Soil Sci. Soc. Am. J. 2004, 68, 1875–1884. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis. Part 2. Microbiological and Biochemical Properties; SSSA Book Series No. 5; Weaver, R.W., Angle, J.S., Bottomley, P.S., Eds.; SSSA: Madison, WI, USA, 1994; pp. 775–833. [Google Scholar]
- Parham, J.A.; Deng, S.P. Detection, quantification and characterization of b-glucosaminidase activity in soil. Soil Biol. Biochem. 2000, 32, 1183–1190. [Google Scholar] [CrossRef]
- SAS Institute, SAS System for Windows, Release 9.2, SAS Inst.: Cary, NC, USA, 2009.
- R Development Core Team. R: A Language and Environment for Statistical Computing, Version 2.13.1. Available online: http://www.R-project.org/ (accessed on 6 August 2012).
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Henry, M.; Stevens, H.; Wagner, H. Vegan: Community Ecology Package, R Package, Version 1.17–8. Available online: http://CRAN.R-project.org/package=vegan (accessed on 6 August 2012).
- Rehm, G.W.; Randall, G.W.; Lamb, J.; Eliason, R. Fertilizing corn in minnesota, fo-3790-c. Available online: http://www.extension.umn.edu/distribution/cropsystems/components/DC3790.pdf (accessed on 6 August 2012).
- Power, J.F.; Wilhelm, W.W.; Doran, J.W. Crop residue effects on soil environment and dryland maize and soya bean production. Soil Tillage Res. 1986, 8, 101–111. [Google Scholar] [CrossRef]
- Wilts, A.R.; Reicosky, D.C.; Allmaras, R.R.; Clapp, C.E. Long-term corn residue effects: Harvest alternatives, soil carbon turnover, and root-derived carbon. Soil Sci. Soc. Am. J. 2004, 68, 1342–1351. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Lal, R. Crop residue removal impacts on soil productivity and environmental quality. Crit. Rev. Plant Sci. 2009, 28, 139–163. [Google Scholar] [CrossRef]
- Johnson, J.M.F.; Allmaras, R.R.; Reicosky, D.C. Estimating source carbon from crop residues, roots and rhizodeposits using the national grain-yield database. Agron. J. 2006, 98, 622–636. [Google Scholar] [CrossRef]
- Clay, D.E.; Carlson, C.G.; Clay, S.A.; Reese, C.; Liu, Z.; Chang, J.; Ellsbury, M.M. Theoretical derivation of stable and nonisotopic approaches for assessing soil organic carbon turnover. Agron. J. 2006, 98, 443–450. [Google Scholar]
- Huggins, D.R.; Allmaras, R.R.; Clapp, C.E.; Lamb, J.A.; Randall, G.W. Corn-soybean sequence and tillage effects on soil carbon dynamics and storage. Soil Sci. Soc. Am. J. 2007, 71, 145–154. [Google Scholar]
- Cotton, J.; Acosta-Martínez, V.; Moore-Kucera, J.; Burow, G. Early changes due to sorghum biofuel cropping systems in soil microbial communities and metabolic functioning. Biol Fertil Soils 2012, 1–11. [Google Scholar]
- Stetson, S.J.; Osborne, S.L.; Schumacher, T.E.; Eynard, A.; Chilom, G.; Rice, J.; Nichols, K.A.; Pikul, J.L., Jr. Corn residue removal impact on topsoil organic carbon in a corn-soybean rotation. Soil Sci. Soc. Am. J. 2012. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Johnson, J.M.F.; Acosta-Martinez, V.; Cambardella, C.A.; Barbour, N.W. Crop and Soil Responses to Using Corn Stover as a Bioenergy Feedstock: Observations from the Northern US Corn Belt. Agriculture 2013, 3, 72-89. https://doi.org/10.3390/agriculture3010072
Johnson JMF, Acosta-Martinez V, Cambardella CA, Barbour NW. Crop and Soil Responses to Using Corn Stover as a Bioenergy Feedstock: Observations from the Northern US Corn Belt. Agriculture. 2013; 3(1):72-89. https://doi.org/10.3390/agriculture3010072
Chicago/Turabian StyleJohnson, Jane M. F., Veronica Acosta-Martinez, Cynthia A. Cambardella, and Nancy W. Barbour. 2013. "Crop and Soil Responses to Using Corn Stover as a Bioenergy Feedstock: Observations from the Northern US Corn Belt" Agriculture 3, no. 1: 72-89. https://doi.org/10.3390/agriculture3010072





