The Physical Chemistry of Pesticides in Soil and Water
Abstract
:1. Introduction
2. What Has Been Done
3. What Must Be Done Next
4. What Is Still Going Wrong
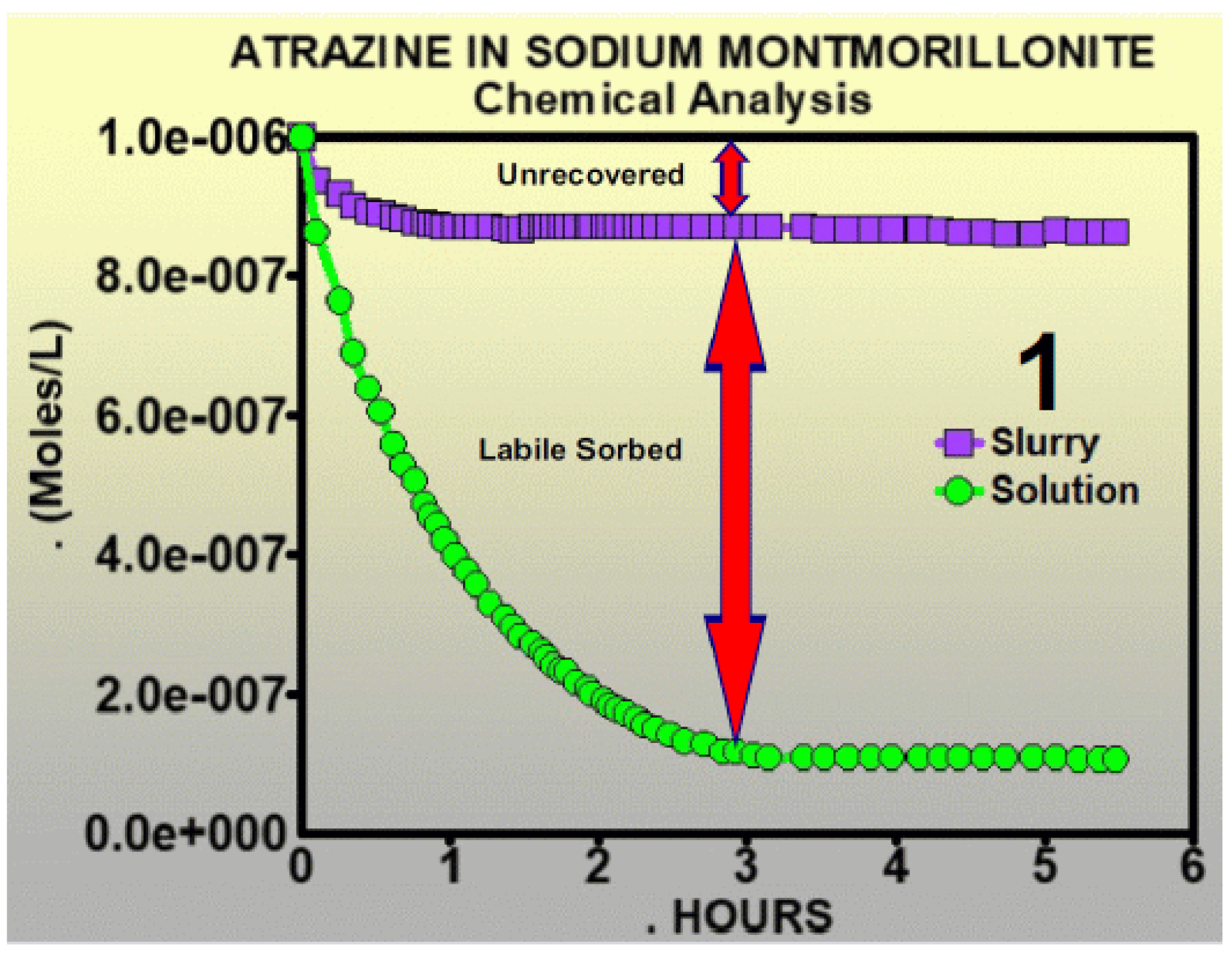
- 1
- Chemical kinetics order is defined for individual processes or reactions, not groups of them lumped together as these authors have done [17].
- 2
- For each pesticide soil process, all of the reactants and products have to be accounted for. That includes empty and filled sorption sites. Stoichiometry was ignored in this chapter.
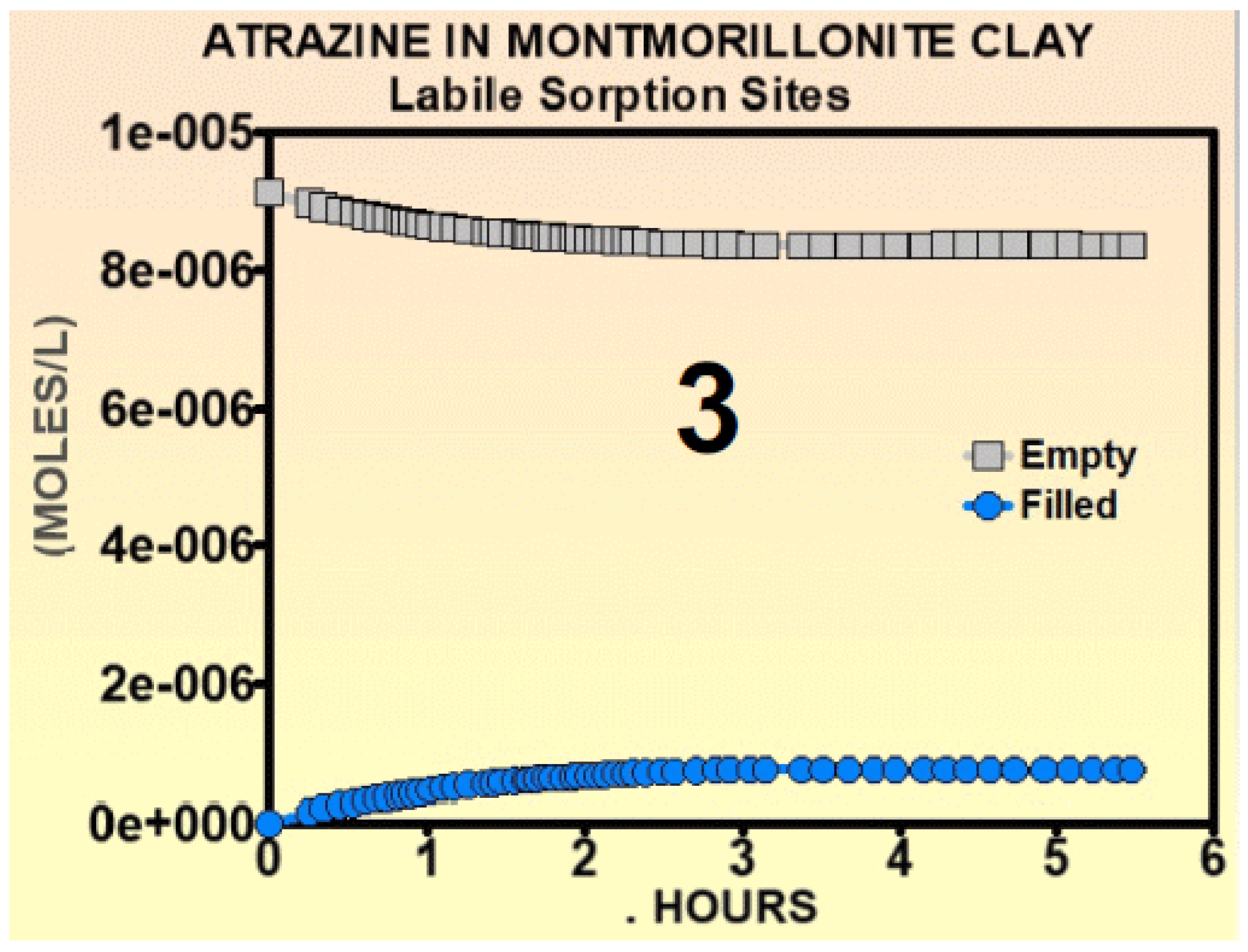
- 3
- Contrary to experimental evidence [17], the authors assumed that the kinetic rate coefficients were constants.
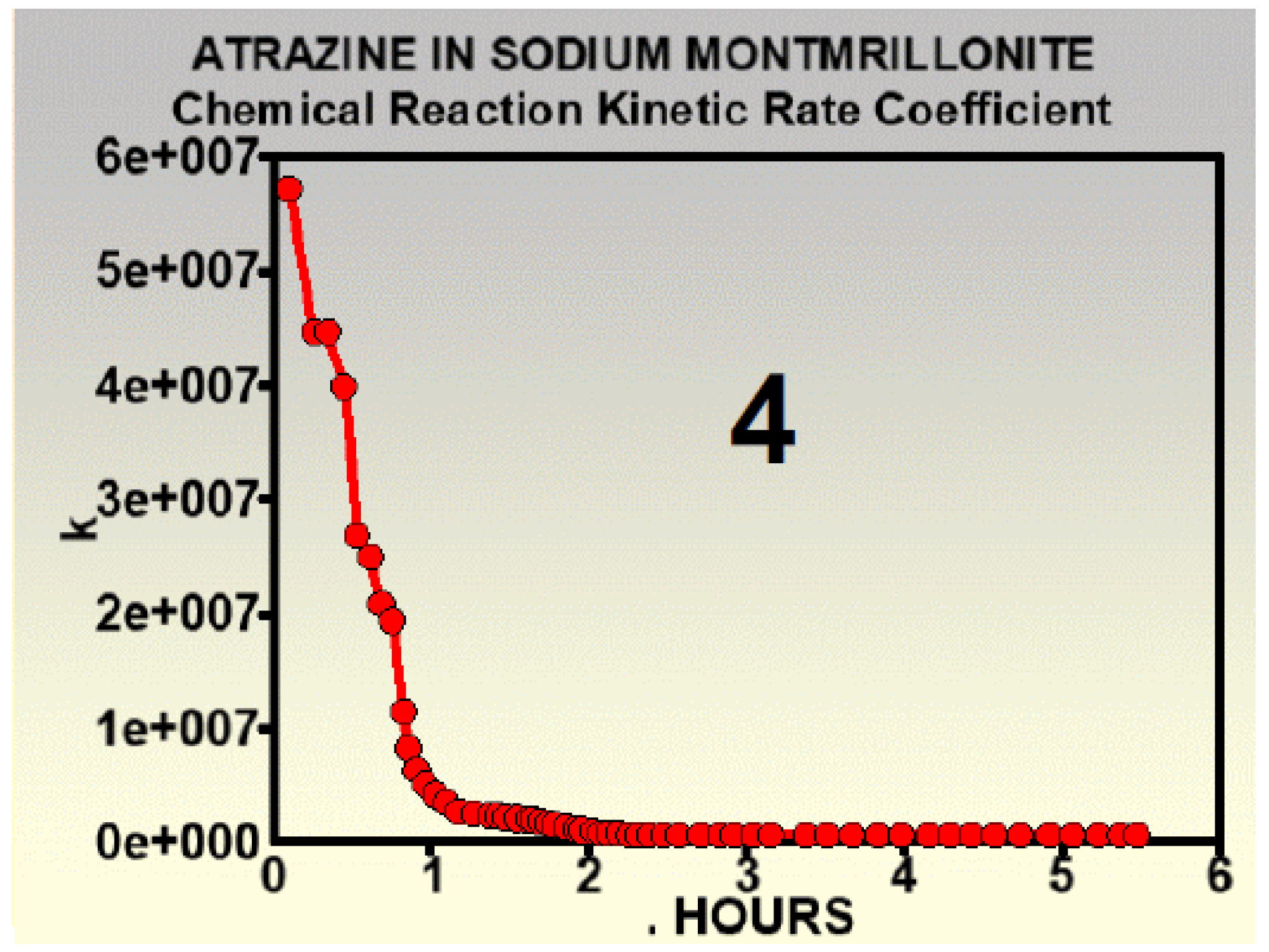
- 4
- Constant half-lives are not obtainable from variable kinetic rate coefficients [17]. The authors ignored that.
- 5
- 6
- Rate determining kinetic processes could not be identified, because the processes were not separately described.
5. Conclusions
Conflicts of Interest
References
- Gamble, D.S. Discoveries Leading to Conventional Chemical Kinetics for Pesticides in Soils: A Review. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier: Atlanta, GA, USA, 2013; Volume 120, pp. 381–4162. [Google Scholar]
- Gamble, D.S.; Webster, G.R.B.; Lamoureux, M. Pesticide reaction mechanism in soil: An interactive spreadsheet model based on conventional chemical kinetics. J. Environ. Monit. 2012, 14, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Mak, M.K.S.; Langford, C.H. A kinetic study of the interaction of hydrous aluminum oxide colloids with a well-characterized soil fulvic acid. Can. J. Chem. 1982, 60, 2023–2028. [Google Scholar] [CrossRef]
- Shuman, M.S.; Colins, B.J.; Fitzgerald, P.J.; Olson, D.L. Distribution of stability constantsand dissociation rate constants among binding sites on estuarine copper-organic complexes: Rotated disk electrode studies and an affinity spectrum analysis of ion-selective electrode and potentiometric data. In Aquatic and Terrestrial Humic Materials; Christman, R.F., Gjessing, E.T., Eds.; Ann Arbor Science: Ann Arbor, MI, USA, 1983; p. 4816. [Google Scholar]
- Li, J.; Langford, C.H.; Gamble, D.S. Atrazine sorption by a mineral soil: Processes of labile and nonlabile uptake. J. Agric. Food Chem. 1996, 44, 3672–3679. [Google Scholar] [CrossRef]
- Gamble, D.S.; Khan, S.U. Atrazine in organic soil: Chemical speciation during heterogeneous catalysis. J. Agric. Food Chem. 1990, 38, 297–308. [Google Scholar] [CrossRef]
- Gamble, D.S.; Ismaily, L.A. Atrazine in mineral soil: The analytical chemistry of speciation. Can. J. Chem. 1992, 70, 1590–1596. [Google Scholar] [CrossRef]
- Gamble, D.S.; Khan, S.U. Atrazine in mineral soil: Chemical species and catalysed hydrolysis. Can. J. Chem. 1992, 70, 1597–1603. [Google Scholar] [CrossRef]
- Sha’ato, R.; Buncel, E.; Gamble, D.S.; van Loon, G.W. Kinetics and equilibria of Metribuzin sorption on model soil components. Can. J. Soil Sci. 2000, 80, 301–307. [Google Scholar] [CrossRef]
- Langford, C.H.; Gutzman, D.W. Kinetic studies of metal-ion speciation. Anal. Chim. Acta 1992, 256, 183–201. [Google Scholar] [CrossRef]
- Gutzman, D.W.; Langford, C.H. Kinetic studies of a hydrous metal oxide. Environ. Sci. Technol. 1992, 27, 1388–1393. [Google Scholar] [CrossRef]
- Gilchrist, F.R.G.; Gamble, D.S.; Kodama, H.; Khan, S.U. Atrazine interactions with clay minerals: Kinetics and equilibria of sorption. J. Agric. Food Chem. 1993, 41, 1748–1755. [Google Scholar] [CrossRef]
- Gamble, D.S. Physical Chemistry Parameters that Control Pesticide Persistence and Leaching in Watershed Soils; Final Report Submitted; Great Lakes Water Quality Program Committee: Guelph, ON, Canada, 1994. [Google Scholar]
- Lew, S.; Lew, M.; Szarek, J.; Mieszczyñski, T. Effect of Pesticides on Soil and Aquatic Environmental Microorganisms—A Short Review. Fresenius Environ. Bull. 2009, 18, 1390–1395. [Google Scholar]
- Albright, V.C., III; Murphy, I.J.; Anderson, J.A.; Coats, J.R. Fate of atrazine in switchgrass-soil column system. Chemosphere 2013, 90, 1847–1853. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Prasher, S.O.; Clemente, R.S.; Reynolds, W.D.; Smith, W.N.; Gamble, D.S.; Topp, E.; Langford, C.H.; Salehi, F. Modeling the sorption behavior of atrazine on intact soil columns. J. Irrig. Drain. Eng. 1999, 125, 212–222. [Google Scholar] [CrossRef]
- Gamble, D.S.; Bruccoleri, A.G. Pesticide regulations for agriculture: Chemically flawed regulatory practice. J. Environ. Sci. Health Part B. 2016, 51, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Sabljic, A.; Cryer, S.A.; Kookana, R.S. (Eds.) Non-First Order Degradation and Time-Dependent Sorption of Organic Chemicals in Soil; American Chemical Society, Oxford University Press: Washington, DC, USA, 2014; SBN/ISSN 978-0-84-122978-5/0-84-122978-3, OCLC 931691805. [Google Scholar]

|
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamble, D.S. The Physical Chemistry of Pesticides in Soil and Water. Agriculture 2017, 7, 91. https://doi.org/10.3390/agriculture7110091
Gamble DS. The Physical Chemistry of Pesticides in Soil and Water. Agriculture. 2017; 7(11):91. https://doi.org/10.3390/agriculture7110091
Chicago/Turabian StyleGamble, Donald S. 2017. "The Physical Chemistry of Pesticides in Soil and Water" Agriculture 7, no. 11: 91. https://doi.org/10.3390/agriculture7110091




