Changes in the Polyphenolic Profile, Carotenoids and Antioxidant Potential of Apricot (Prunus armeniaca L.) Leaves during Maturation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Collection and Preparation
2.3. Sample Preparation
2.4. Chromatography of Phenolic Compounds
2.5. Chromatography of Carotenoids
2.6. Determination of Pigments Contents
2.7. Determination of Total Phenolic Contents
2.8. Radical Scavenging Activity
2.9. Data Analyses
3. Results
3.1. Phenolic Compounds in Apricot Leaves
3.2. Changes in the Phenolic Compounds
3.3. Carotenoid Contents
3.4. Changes in Carotenoid Contents
3.5. Changes in Pigment Contents
3.6. Changes in TPC & Radical Scavenging Activity
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HPLC-DAD | High performance liquid chromatography with diode array detection |
| TPC | Total phenolic contents |
| RSA | Radical scavenging activity |
| GAE | Gallic acid equivalent |
| DPPH | Diphenylpicryl hydrazine radical |
| ANOVA | Analysis of variance |
References
- Ali, S.; Masud, T.; Abbasi, K.S. Physico-chemical characteristics of apricot (Prunus armeniaca L.) grown in northern areas of Pakistan. Sci. Hortic. 2011, 130, 386–392. [Google Scholar] [CrossRef]
- Ali, S.; Masud, T.; Abbasi, K.S.; Mahmood, T.; Ali, A. Some physico-chemical and functional attributes of six indigenous apricot genotypes from Gilgit-Baltistan, Pakistan. Int. J. Biosci. 2014, 4, 221–231. [Google Scholar]
- Erdogan-Orhan, I.; Kartal, M. Insights into research on phytochemistry and biological activities of Prunus armeniaca L. (apricot). Food Res. Int. 2011, 44, 1238–1243. [Google Scholar] [CrossRef]
- Zaghdoudi, K.; Pontvianne, S.; Framboisier, X.; Achard, M.; Kudaibergenova, R.; Ayadi-Trabelsi, M.; Kalthoum-cherif, J.; Vanderesse, R.; Frochot, C.; Guiavarc’h, Y. Accelerated solvent extraction of carotenoids from: Tunisian kaki (Diospyros kaki L.), peach (Prunus persica L.) and apricot (Prunus armeniaca L.). Food Chem. 2015, 184, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Campbell, O.E.; Merwin, I.A.; Padilla-Zakour, O.I. Characterization and the effect of maturity at harvest on the phenolic and carotenoid content of northeast USA apricot (Prunus armeniaca) varieties. J. Agric. Food Chem. 2013, 61, 12700–12710. [Google Scholar] [CrossRef] [PubMed]
- Dragovic-Uzelac, V.; Levaj, B.; Mrkic, V.; Bursac, D.; Boras, M. The content of polyphenols and carotenoids in three apricot cultivars depending on stage of maturity and geographical region. Food Chem. 2007, 102, 966–975. [Google Scholar] [CrossRef]
- Roussos, P.A.; Sefferou, V.; Denaxa, N.-K.; Tsantili, E.; Stathis, V. Apricot (Prunus armeniaca L.) fruit quality attributes and phytochemicals under different crop load. Sci. Hortic. 2011, 129, 472–478. [Google Scholar] [CrossRef]
- Wang, H.; Petri, C.; Burgos, L.; Alburquerque, N. Efficient in vitro shoot regeneration from mature apricot (Prunus armeniaca L.) cotyledons. Sci. Hortic. 2013, 160, 300–305. [Google Scholar] [CrossRef]
- Azevedo-Meleiro, C.H.; Rodriguez-Amaya, D.B. Qualitative and quantitative differences in carotenoid composition among Cucurbita moschata, Cucurbita maxima, and Cucurbita pepo. J. Agric. Food Chem. 2007, 55, 4027–4033. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. A reversed phase HPLC-DAD method for the determination of phenolic compounds in plant leaves. Anal. Methods 2015, 7, 7753–7757. [Google Scholar] [CrossRef]
- Zeb, A. A simple, sensitive HPLC-DAD method for simultaneous determination of carotenoids, chlorophylls and α-tocopherol leafy vegetables. J. Food Meas. Charact. 2016. [Google Scholar] [CrossRef]
- De Rosso, V.V.; Mercadante, A.Z. Identification and quantification of carotenoids, by HPLC-PDA-MS/MS, from Amazonian fruits. J. Agric. Food Chem. 2007, 55, 5062–5072. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Carvalho, A.M.; Morais, J.S.; Ferreira, I.C.F.R. Strawberry-tree, blackthorn and rose fruits: Detailed characterisation in nutrients and phytochemicals with antioxidant properties. Food Chem. 2010, 120, 247–254. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Ullah, S. Sea buckthorn seed oil protects against the oxidative stress produced by thermally oxidized lipids. Food Chem. 2015, 186, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Oliveira, M.B.; Ibanez, E.; Herrero, M. Phenolic profile evolution of different ready-to-eat baby-leaf vegetables during storage. J. Chromatogr. A 2014, 1327, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Weisz, G.M.; Kammerer, D.R.; Carle, R. Identification and quantification of phenolic compounds from sunflower (Helianthus annuus L.) kernels and shells by HPLC-DAD/ESI-MSN. Food Chem. 2009, 115, 758–765. [Google Scholar] [CrossRef]
- Aaby, K.; Ekeberg, D.; Skrede, G. Characterization of phenolic compounds in strawberry (Fragaria × ananassa) fruits by different HPLC detectors and contribution of individual compounds to total antioxidant capacity. J. Agric. Food Chem. 2007, 55, 4395–4406. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, D.; Egea, J.; Gil, M.I.; Tomas-Barberan, F.A. Characterization and quantitation of phenolic compounds in new apricot (Prunus armeniaca L.) varieties. J. Agric. Food Chem. 2005, 53, 9544–9552. [Google Scholar] [CrossRef] [PubMed]
- Campbell, O.E.; Padilla-Zakour, O.I. Phenolic and carotenoid composition of canned peaches (Prunus persica) and apricots (Prunus armeniaca) as affected by variety and peeling. Food Res. Int. 2013, 54, 448–455. [Google Scholar] [CrossRef]
- Dragovic-Uzelac, V.; Delonga, K.; Levaj, B.; Djakovic, S.; Pospisil, J. Phenolic profiles of raw apricots, pumpkins, and their purees in the evaluation of apricot nectar and jam authenticity. J. Agric. Food Chem. 2005, 53, 4836–4842. [Google Scholar] [CrossRef] [PubMed]
- Madrau, M.; Piscopo, A.; Sanguinetti, A.; Del Caro, A.; Poiana, M.; Romeo, F.; Piga, A. Effect of drying temperature on polyphenolic content and antioxidant activity of apricots. Eur. Res. Technol. 2009, 228, 441–448. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F. Flavonols (kaempeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem. 2008, 108, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.; Zahri, S.; Zarrini, G.; Nazemiyeh, H.; Mohammadi, S. Biological activity of quercetin-3-O-glucoside, a known plant flavonoid. Russian J. Bioorgan. Chem. 2009, 35, 376–378. [Google Scholar] [CrossRef]
- Morand, C.; Manach, C.; Crespy, V.; Remesy, C. Quercetin 3-O-β-glucoside is better absorbed than other quercetin forms and is not present in rat plasma. Free Radic. Res. 2000, 33, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Katayama, S.; Ogawa, H.; Nakamura, S. Apricot carotenoids possess potent anti-amyloidogenic activity in vitro. J. Agric. Food Chem. 2011, 59, 12691–12696. [Google Scholar] [CrossRef] [PubMed]
- Katayama, T.; Nakayama, T.O.M.; Lee, T.H.; Chichester, C.O. Carotenoid transformations in ripening apricots and peaches. J. Food Sci. 1971, 36, 804–806. [Google Scholar] [CrossRef]
- Sabir, A.; Ekbic, H.; Erdem, H.; Tangolar, S. Response of four grapevine (Vitis spp.) genotypes to direct or bicarbonate-induced iron deficiency. Span. J. Agric. Res. 2010, 8, 823–829. [Google Scholar] [CrossRef]
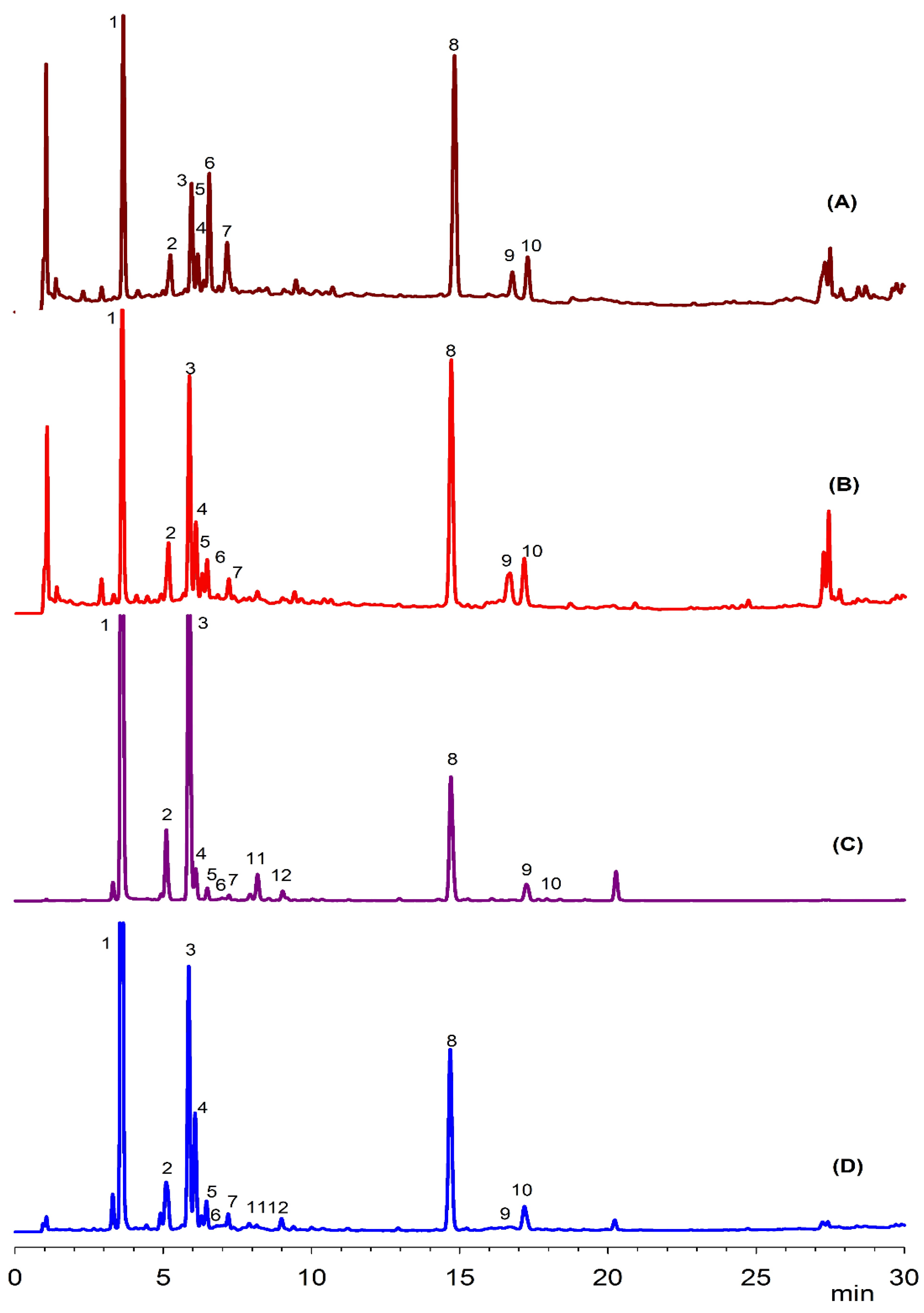
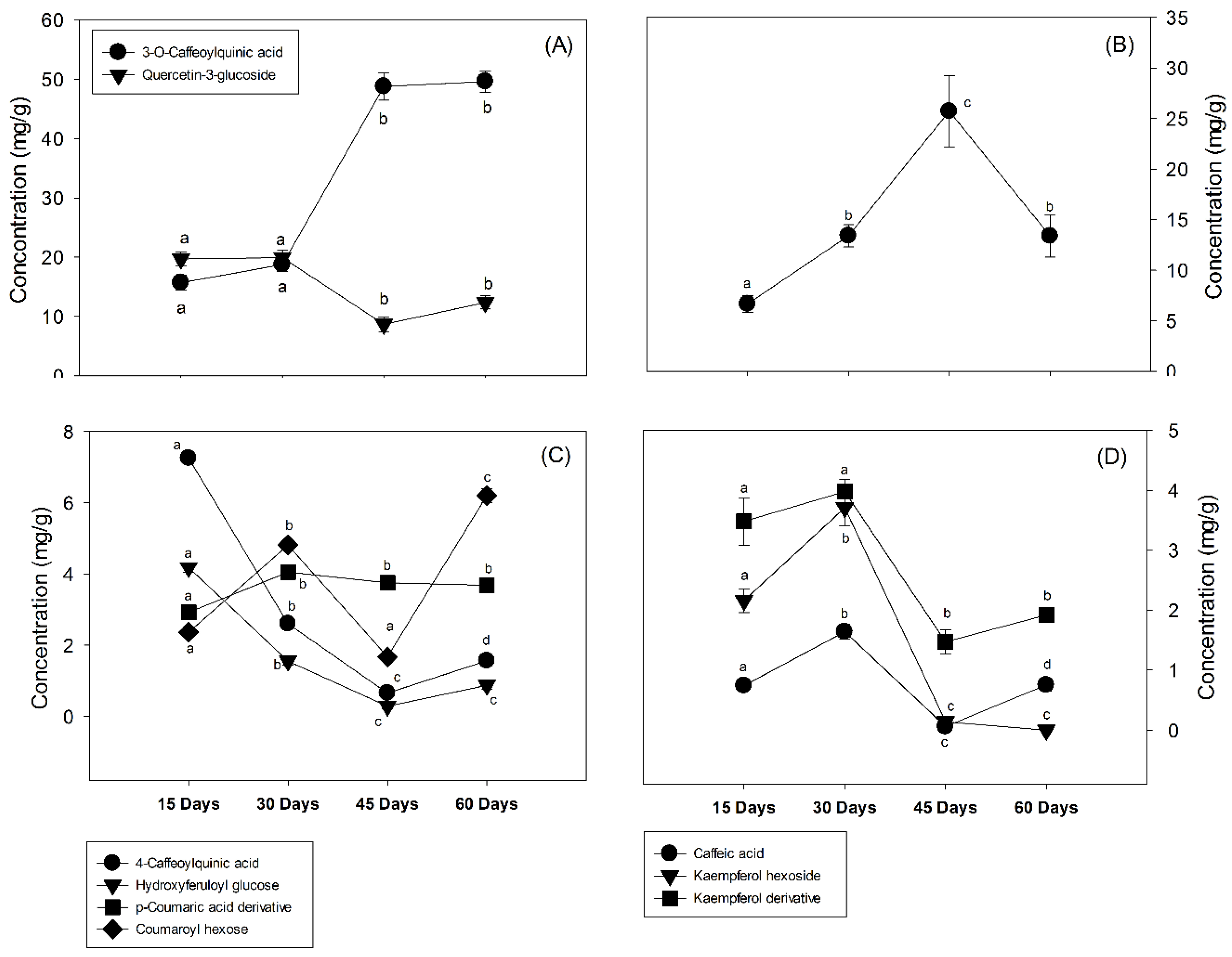

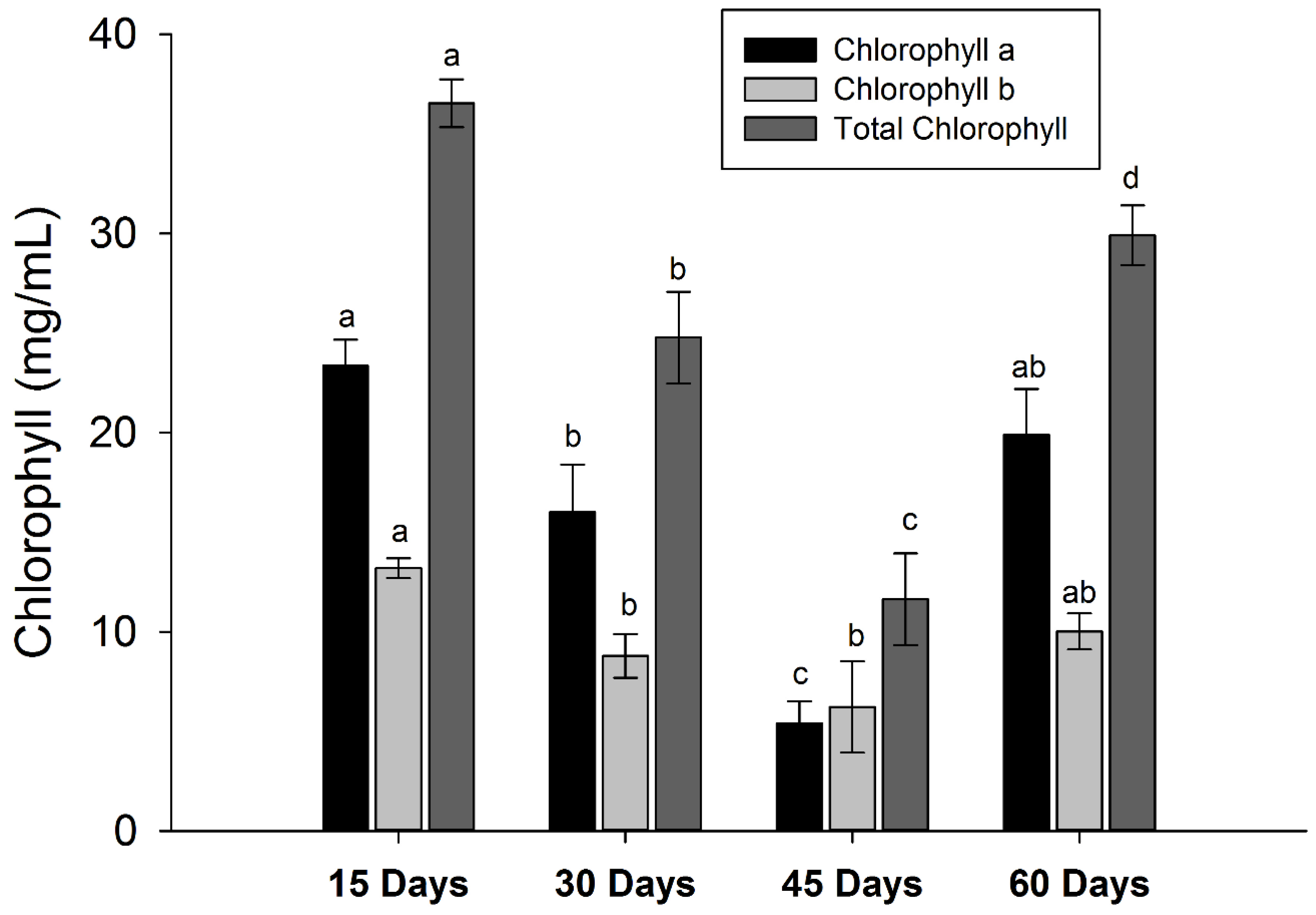

| Peak | Rt (min) | Tentative Identification | HPLC-DAD λmax (nm) | Identification |
|---|---|---|---|---|
| 1 | 3.6 | 3-O-Caffeoylquinic acid | 234, 298sh, 325 | Standard |
| 2 | 5.2 | p-Coumaric acid derivative | 283, 288, 314 | [16] |
| 3 | 5.9 | 5-O-Caffeoylquinic acid | 236, 298sh, 326 | Standard |
| 4 | 6.1 | p-Coumaroyl hexose | 285, 316 | [16] |
| 5 | 6.3 | Caffeic acid | 232, 302, 326 | Standard |
| 6 | 6.6 | 4-Caffeoylquinic acid | 232, 303, 326 | Standard |
| 7 | 7.1 | Hydroxy feruloyl glucose | 240, 305, 326 | [15] |
| 8 | 14.8 | Quercetin-3-glucoside | 256, 354 | Standard |
| 9 | 16.7 | Kaempferol hexoside | 285, 325, 346 | [17] |
| 10 | 17.2 | Kaempferol derivative | 285, 324, 345 | [17] |
| 11 | 8.1 | Coumaric acid | 230, 312 | Standard |
| 12 | 9.2 | Caftaric acid | 245, 298sh, 328 | Standard |
| Peak | Rt (min) | Tentative Identification | HPLC-DAD λmax (nm) | Carotenoids (µg/g) | |||
|---|---|---|---|---|---|---|---|
| 15 | 30 | 45 | 60 | ||||
| 1 | 9.1 | All-trans-neoxanthin | 468, 444, 420 | 2.01 ± 0.1 a | 4.79 ± 0.3 b | 0.66 ± 0.1 c | 4.01 ± 0.3 b |
| 2 | 9.8 | 9-Cis-neoxanthin | 464, 436, 418 | 2.46 ± 0.2 a | 3.6 ± 0.14 b | 1.01 ± 0.2 c | 2.34 ± 0.3 a |
| 3 | 10.1 | Cis-γ-carotene | 464, 438, 422 | 1.18 ± 0.1 a | 1.55 ± 0.1 a | 1.33 ± 0.1 a | 2.34 ± 0.1 b |
| 4 | 12.3 | All-trans-violaxanthin | 472, 444, 420, 330 | 0.54 ± 0.02 a | 0.42 ± 0.01 a | 0.27 ± 0.1 b | 0.26 ± 0.1 b |
| 5 | 15.0 | All-trans-lutein | 474, 446, 422 | 65.7 ± 1.4 a | 61.1 ± 0.9 b | 58.2 ± 0.4 c | 56.7 ± 0.8 d |
| 6 | 17.2 | 5,6-epoxy-α-carotene | 468, 440, 420 | 7.78 ± 0.5 a | 7.9 ± 0.3 a | 5.89 ± 0.2 b | 6.41 ± 0.7 c |
| 7 | 17.9 | 15-Cis-α-carotene | 466, 440, 420, 330 | 4.84 ± 0.3 a | 4.69 ± 0.2 a | 2.0 ± 0.2 b | 4.14 ± 0.1 a |
| 8 | 24.7 | 13-Cis-α-carotene | 466, 440, 420 | 0.39 ± 0.02 a | 0.32 ± 0.02 a | 0.62 ± 0.06 b | 0.21 ± 0.03 c |
| 9 | 32.8 | β-Carotene | 476, 450 | 12.3 ± 0.1 a | 13.6 ± 0.2 a | 26.9 ± 1.1 b | 18.5 ± 2.1 c |
| 10 | 33.2 | 9-Cis-β-carotene | 468, 446, 335 | 0.81 ± 0.04 a | 1.21 ± 0.06 b | 2.2 ± 0.1 c | 1.13 ± 0.2 b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeb, A.; Khadim, N.; Ali, W. Changes in the Polyphenolic Profile, Carotenoids and Antioxidant Potential of Apricot (Prunus armeniaca L.) Leaves during Maturation. Agriculture 2017, 7, 9. https://doi.org/10.3390/agriculture7020009
Zeb A, Khadim N, Ali W. Changes in the Polyphenolic Profile, Carotenoids and Antioxidant Potential of Apricot (Prunus armeniaca L.) Leaves during Maturation. Agriculture. 2017; 7(2):9. https://doi.org/10.3390/agriculture7020009
Chicago/Turabian StyleZeb, Alam, Nadia Khadim, and Waqar Ali. 2017. "Changes in the Polyphenolic Profile, Carotenoids and Antioxidant Potential of Apricot (Prunus armeniaca L.) Leaves during Maturation" Agriculture 7, no. 2: 9. https://doi.org/10.3390/agriculture7020009






