Coupled Simulation of Hydrate-Bearing and Overburden Sedimentary Layers to Study Hydrate Dissociation and Methane Leakage
Abstract
:1. Introduction
2. Apparatus and Methods
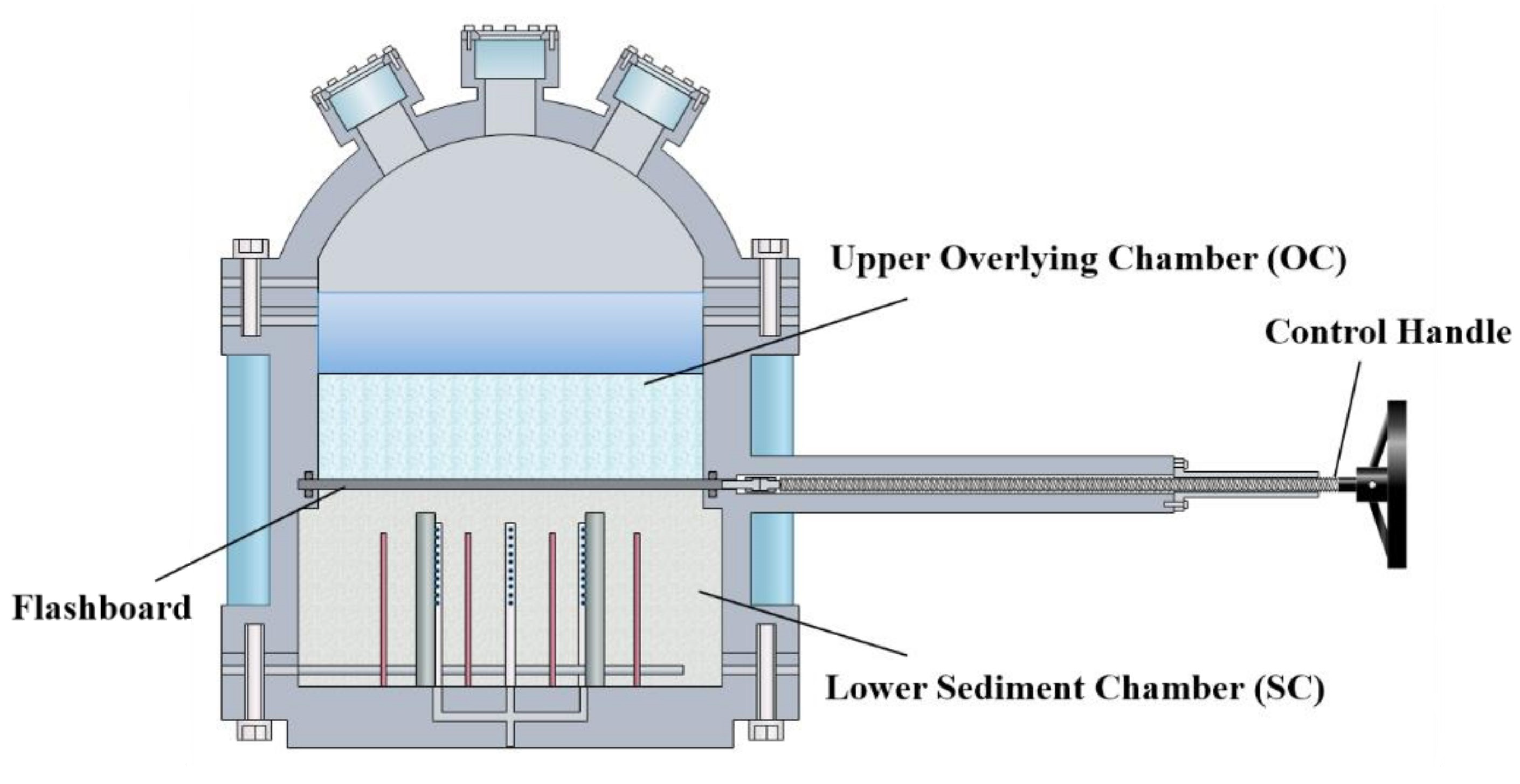
2.1. Apparatus Description
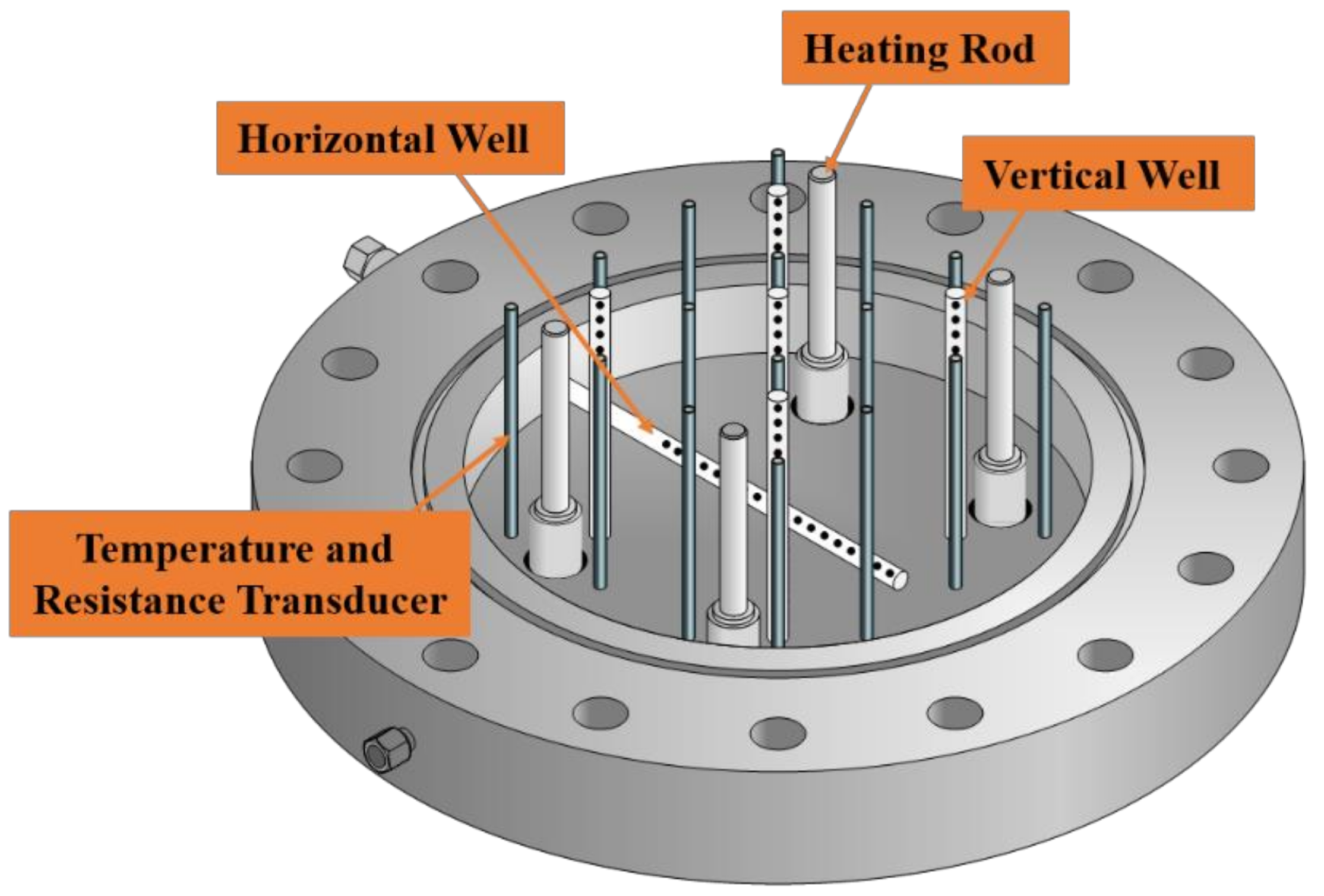
2.1.1. Reaction System
2.1.2. Gas-Liquid Supply System
2.1.3. Gas-Liquid Collection System
2.2. Experimental Methods
3. Experimental Validation for CH4 Hydrate Dissociation
3.1. Evolutions of Pressure and Temperatures
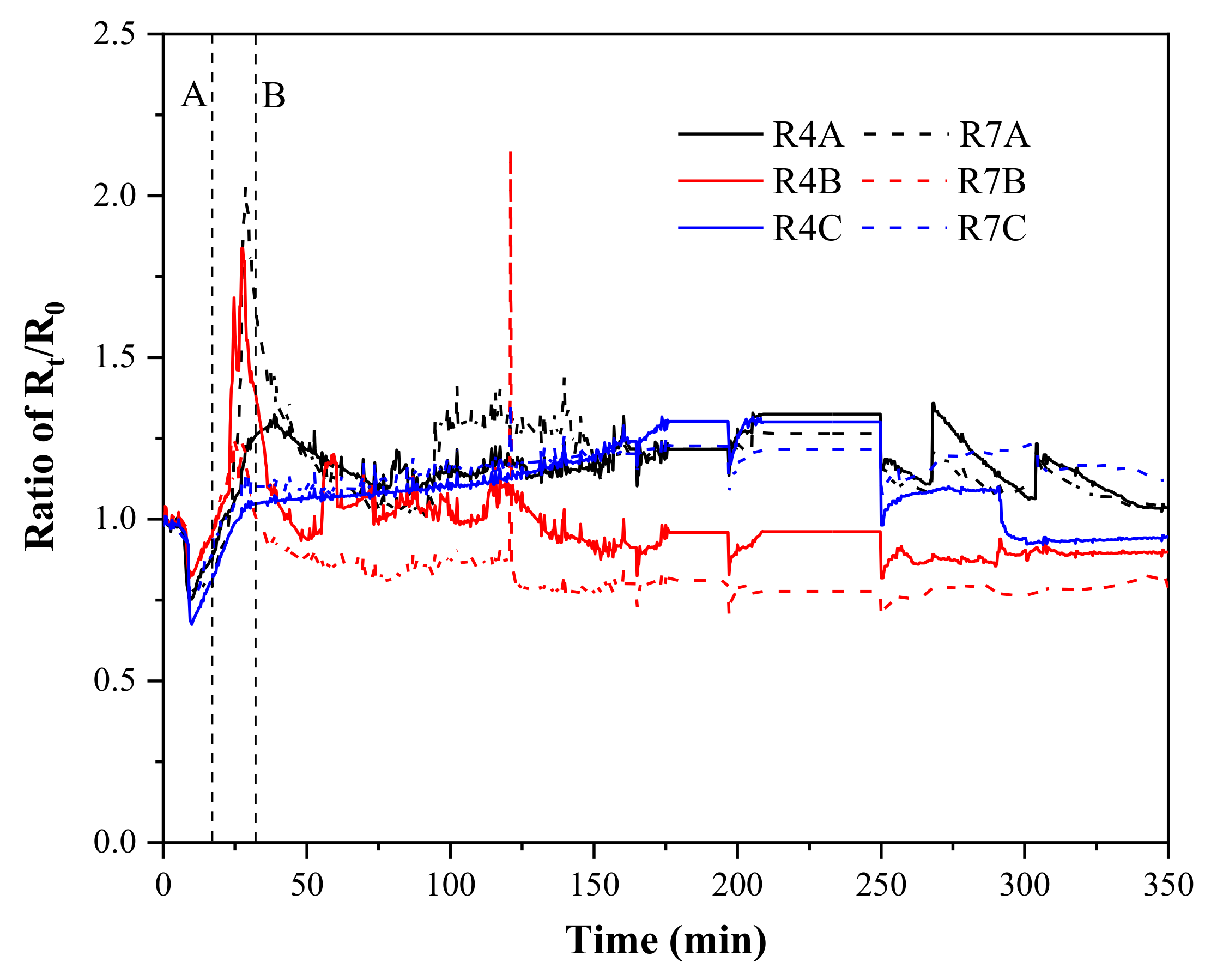
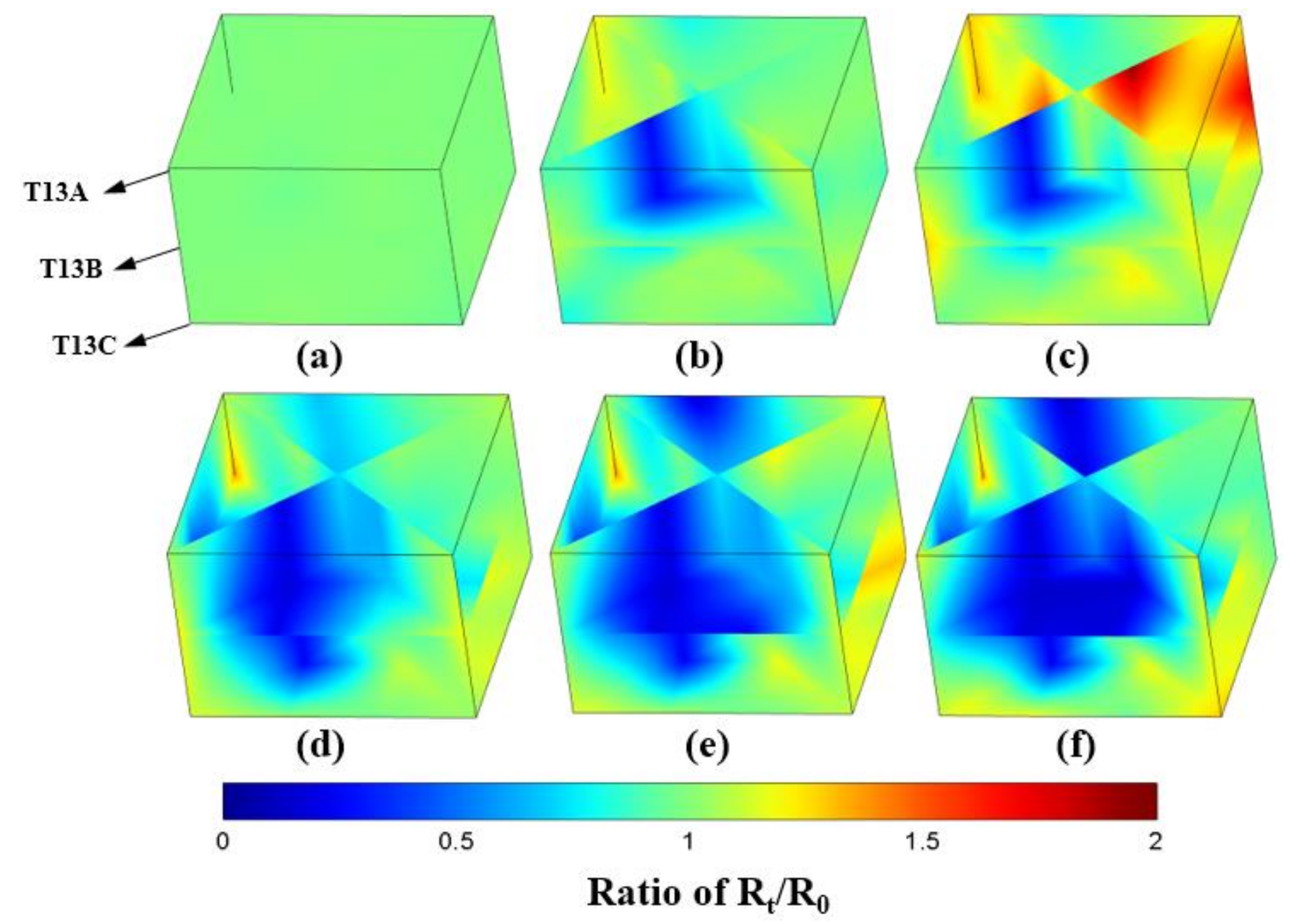
3.2. Electric Resistance Evolution Characteristic
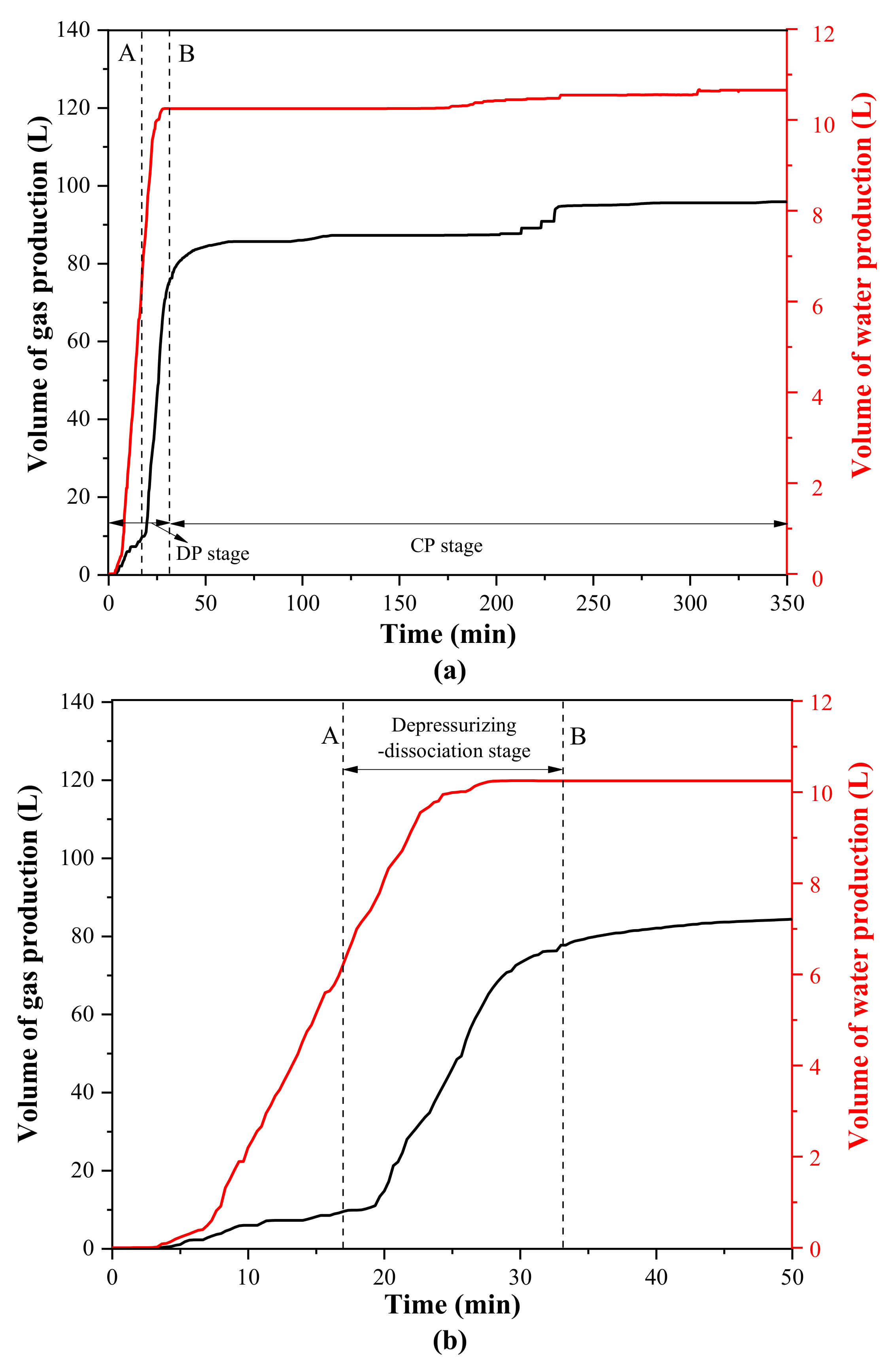
3.3. Production Behaviors
3.4. Morphological Change of Overlying Strata before and after Hydrate Dissociation
4. Conclusions
- In the new device, the in situ natural environment containing hydrate reservoir, over lying strata and overlying seawater could be well simulated by utilizing the elaborately designed mobile separation flashboard. The evolutions of pressure field, temperature field and electric field in the hydrate dissociation process can be well reflected through the integrated temperature, electric resistance and pressure measuring points in the hydrate reservoir systems.
- The real-time recording of overlying strata morphology changes and CH4 bubble leakage can be realized through the overburden monitoring system. In addition, the CH4 concentration in overlying seawater and gas phase space can be measured to further judge whether there is methane leakage.
- The experimental test showed that all functions of the system can be operated normally. According to the experimental result, the downward-flow overlying seawater significantly affected the hydrate dissociation, multi-field evolution and gas production characteristics. The state change of the overlying strata before and after depressurization indicated that there was local collapse of the overlying strata during the hydrate dissociation process.
- Despite the obvious collapse of the overlying strata, CH4 leakage phenomenon did not occur in this test, which may be related to the hydrate distribution or the selection of hydrate exploitation methods in this test. However, it is necessary to avoid the occurrence of overburden fracture channel and seawater downward flow, otherwise it will significantly affect gas production and energy efficiency.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Gambelli, A.M.; Filipponi, M.; Rossi, F. How methane release may affect carbon dioxide storage during replacement processes in natural gas hydrate reservoirs. J. Pet. Sci. Eng. 2021, 205, 108895. [Google Scholar] [CrossRef]
- Boswell, R.; Collett, T.S. Current perspectives on gas hydrate resources. Energy Environ. Sci. 2011, 4, 1206–1215. [Google Scholar] [CrossRef]
- Dallimore, S.R.; Collett, T.S. Scientific results from the Mallik 2002 gas hydrate production research well program, Mackenzie Delta, Northwest Territories, Canada. 2005. Available online: https://www.osti.gov/etdeweb/biblio/20671468 (accessed on 25 April 2022).
- Yamamoto, K. Overview and introduction: Pressure core-sampling and analysis in the 2012–2013 MH21 offshore test of gas production from methane hydrate in teh eastern Nankai Trough. Mar. Petrol. Geol. 2015, 66, 296–309. [Google Scholar] [CrossRef]
- Kvamme, B. Feasibility of simultaneous CO2 storage and CH4 production from natural gas hydrate using mixtures of CO2 and N2. Can. J. Chem. 2015, 93, 897–905. [Google Scholar] [CrossRef]
- Li, J.-F.; Ye, J.-L.; Qin, X.-W.; Qiu, H.-J.; Wu, N.-Y.; Lu, H.-L.; Xie, W.-W.; Lu, J.-A.; Peng, F.; Xu, Z.-Q.; et al. The first offshore natural gas hydrate production test in South China Sea. China Geol. 2018, 1, 5–16. [Google Scholar] [CrossRef]
- Yan, C.; Ren, X.; Cheng, Y.; Song, B.; Li, Y.; Tian, W. Geomechanical issues in the exploitation of natural gas hydrate. Gondwana Res. 2020, 81, 403–422. [Google Scholar] [CrossRef]
- Reeburgh, W.S. Oceanic methane biogeochemistry. Chem. Rev. 2007, 107, 486–513. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Feng, J.; Hu, W.; Zhang, M.; Wang, J.; Peng, B.; Wang, Y.; Zhou, Z.; Wang, Y. Deep-Sea Sediment and Water Simulator for Investigation of Methane Seeping and Hydrate Formation. J. Mar. Sci. Eng. 2022, 10, 514. [Google Scholar] [CrossRef]
- Zhu, C.; Li, Z.; Chen, D.; Li, S.; Song, X.; Shan, H.; Jia, Y. Seafloor breathing helping forecast hydrate-related geohazards. Energy Rep. 2021, 7, 8108–8114. [Google Scholar] [CrossRef]
- Van Dover, C.L.; German, C.R.; Speer, K.G.; Parson, L.M.; Vrijenhoek, R.C. Evolution and Biogeography of Deep-Sea Vent and Seep Invertebrates. Science 2002, 295, 1253–1257. [Google Scholar] [CrossRef] [Green Version]
- Hinrichs, L.R.; Hmelo, L.R.; Sylva, L.R. Molecular fossil record of elevated methane levels in late Pleistocene coastal waters. Science 2003, 299, 1214–1217. [Google Scholar] [CrossRef]
- Kvenvolden, K.A. Natural Gas Hydrate Occurrence and Issues. Ann. N. Y. Acad. Sci. 1994, 715, 232–246. [Google Scholar] [CrossRef]
- Kvenvolden, K.A. Potential effects of gas hydrate on human welfare. Proc. Natl. Acad. Sci. USA 1999, 96, 3420–3426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yudhowijoyo, A.; Rafati, R.; Haddad, A.S.; Raja, M.S.; Hamidi, H. Subsurface methane leakage in unconventional shale gas reservoirs: A review of leakage pathways and current sealing techniques. J. Nat. Gas Sci. Eng. 2018, 54, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.-C.; Li, B.; Li, X.-S.; Wang, Y. Effects of depressurizing rate on methane hydrate dissociation within large-scale experimental simulator. Appl. Energy 2021, 304, 117750. [Google Scholar] [CrossRef]
- Cui, J.-L.; Cheng, L.-W.; Kan, J.-Y.; Pang, W.-X.; Gu, J.-N.; Li, K.; Wang, L.-B.; Sun, C.-Y.; Wang, X.-H.; Chen, G.-J.; et al. Study on the Spatial Differences of Methane Hydrate Dissociation Process by Depressurization Using an L-shape Simulator. Energy 2021, 228, 120635. [Google Scholar] [CrossRef]
- Feng, J.-C.; Wang, Y.; Li, X.; Li, G.; Zhang, Y.; Chen, Z.-Y. Effect of horizontal and vertical well patterns on methane hydrate dissociation behaviors in pilot-scale hydrate simulator. Appl. Energy 2015, 145, 69–79. [Google Scholar] [CrossRef]
- Feng, J.-C.; Wang, Y.; Li, X.-S.; Li, G.; Zhang, Y. Three dimensional experimental and numerical investigations into hydrate dissociation in sandy reservoir with dual horizontal wells. Energy 2015, 90, 836–845. [Google Scholar] [CrossRef]
- Feng, J.-C.; Wang, Y.; Li, X. Dissociation characteristics of water-saturated methane hydrate induced by huff and puff method. Appl. Energy 2018, 211, 1171–1178. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.-C.; Li, X.; Zhang, Y. Experimental investigation of optimization of well spacing for gas recovery from methane hydrate reservoir in sandy sediment by heat stimulation. Appl. Energy 2017, 207, 562–572. [Google Scholar] [CrossRef]
- Feng, J.-C.; Wang, Y.; Li, X. Hydrate dissociation induced by depressurization in conjunction with warm brine stimulation in cubic hydrate simulator with silica sand. Appl. Energy 2016, 174, 181–191. [Google Scholar] [CrossRef]
- Feng, J.-C.; Wang, Y.; Li, X.; Chen, Z.-Y.; Li, G.; Zhang, Y. Investigation into optimization condition of thermal stimulation for hydrate dissociation in the sandy reservoir. Appl. Energy 2015, 154, 995–1003. [Google Scholar] [CrossRef]
- Yuan, Q.; Sun, C.-Y.; Yang, X.; Ma, P.-C.; Ma, Z.-W.; Li, Q.-P.; Chen, G.-J. Gas Production from Methane-Hydrate-Bearing Sands by Ethylene Glycol Injection Using a Three-Dimensional Reactor. Energy Fuels 2011, 25, 3108–3115. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Zhong, J.-R.; Li, W.-Z.; Ma, Y.-M.; Li, R.; Zhu, T.; Ren, L.-L.; Chen, G.-J.; Sun, C.-Y. Methane Recovery from Hydrate-Bearing Sediments by the Combination of Ethylene Glycol Injection and Depressurization. Energy Fuels 2018, 32, 7585–7594. [Google Scholar] [CrossRef]
- Yuan, Q.; Sun, C.-Y.; Wang, X.-H.; Zeng, X.-Y.; Yang, X.; Liu, B.; Ma, Z.-W.; Li, Q.-P.; Feng, L.; Chen, G.-J. Experimental study of gas production from hydrate dissociation with continuous injection mode using a three-dimensional quiescent reactor. Fuel 2013, 106, 417–424. [Google Scholar] [CrossRef]
- Sun, Y.F.; Zhong, J.R.; Li, R.; Zhu, T.; Cao, X.Y.; Chen, G.J.; Wang, X.H.; Yang, L.Y.; Sun, C.Y. Natural gas hydrate exploitation by CO2/H2 continuous Injection-Production mode. Appl. Energ. 2018, 226, 10–21. [Google Scholar] [CrossRef]
- Wang, X.H.; Sun, Y.F.; Wang, Y.F.; Li, N.; Sun, C.Y.; Chen, G.J.; Liu, B.; Yang, L.Y. Gas production from hydrates by CH4-CO2/H2 replacement. Appl. Energ. 2017, 188, 305–314. [Google Scholar] [CrossRef]
- Xie, Y.; Zheng, T.; Zhu, Y.J.; Zhong, J.R.; Feng, J.C.; Sun, C.Y.; Chen, G.J. Effects of H2-N2 on CO2 hydrate flm growth: Morphology and microstructure. Chem. Eng. J. 2022, 431, 134004. [Google Scholar]
- Xie, Y.; Zhu, Y.-J.; Zheng, T.; Yuan, Q.; Sun, C.-Y.; Yang, L.-Y.; Chen, G.-J. Replacement in CH4-CO2 hydrate below freezing point based on abnormal self-preservation differences of CH4 hydrate. Chem. Eng. J. 2021, 403, 126283. [Google Scholar] [CrossRef]
- Li, X.; Xu, C.-G.; Zhang, Y.; Ruan, X.-K.; Li, G.; Wang, Y. Investigation into gas production from natural gas hydrate: A review. Appl. Energy 2016, 172, 286–322. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Li, R.; Wang, X.-H.; Zheng, T.; Cui, J.-L.; Yuan, Q.; Qin, H.-B.; Sun, C.-Y.; Chen, G.-J. Review on the accumulation behavior of natural gas hydrates in porous sediments. J. Nat. Gas Sci. Eng. 2020, 83, 103520. [Google Scholar] [CrossRef]
- Rutqvist, J.; Moridis, G.J. Development of a numerical simulator for analyzing the geomechanical performance of hydrate-bearing sediments. In Proceedings of the 42nd US Rock Mechanics Symposium and 2nd US-Canada Rock Mechanics Symposium, San Francisco, CA, USA, 29 June–2 July 2008; Onepetro: San Francisco, CA, USA, 2008. [Google Scholar]
- Moridis, G.J. Numerical Studies of Gas Production from Methane Hydrates. SPE J. 2003, 8, 359–370. [Google Scholar] [CrossRef]
- Feng, J.; Sun, L.; Wang, Y.; Li, X. Advances of experimental study on gas production from synthetic hydrate reservoir in China. Chin. J. Chem. Eng. 2019, 27, 2213–2225. [Google Scholar] [CrossRef]
- Wang, X.; Hutchinson, D.R.; Wu, S.; Yang, S.; Guo, Y. Elevated gas hydrate saturation within silt and silty clay sediments in the Shenhu area, South China Sea. J. Geophys. Res. Solid Earth 2011, 116, B05102. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, X.-S.; Xu, W.-Y.; Li, Q.-P.; Zhang, Y.; Li, G.; Huang, N.-S.; Feng, J.-C. Experimental Investigation into Factors Influencing Methane Hydrate Formation and a Novel Method for Hydrate Formation in Porous Media. Energy Fuels 2013, 27, 3751–3757. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.-C.; Li, X.-S. Pilot-scale experimental test on gas production from methane hydrate decomposition using depressurization assisted with heat stimulation below quadruple point. Int. J. Heat Mass Transf. 2018, 131, 965–972. [Google Scholar] [CrossRef]
- Chen, G.-J.; Guo, T.-M. A new approach to gas hydrate modelling. Chem. Eng. J. 1998, 71, 145–151. [Google Scholar] [CrossRef]
- Fitzgerald, G.C.; Castaldi, M.J.; Zhou, Y. Large scale reactor details and results for the formation and decomposition of methane hydrates via thermal stimulation dissociation. J. Pet. Sci. Eng. 2012, 94-95, 19–27. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.-C.; Li, X.; Zhang, Y. Influence of well pattern on gas recovery from methane hydrate reservoir by large scale experimental investigation. Energy 2018, 152, 34–45. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.-C.; Li, X.; Zhan, L.; Li, X.Y. Pilot-scale experimental evaluation of gas recovery from methane hydrate using cycling-depressurization scheme. Energy 2018, 160, 835–844. [Google Scholar] [CrossRef]
- Feng, J.-C.; Wang, Y.; Li, X. Entropy generation analysis of hydrate dissociation by depressurization with horizontal well in different scales of hydrate reservoirs. Energy 2017, 125, 62–71. [Google Scholar] [CrossRef]
- Yang, X.; Sun, C.-Y.; Su, K.-H.; Yuan, Q.; Li, Q.-P.; Chen, G.-J. A three-dimensional study on the formation and dissociation of methane hydrate in porous sediment by depressurization. Energy Convers. Manag. 2012, 56, 1–7. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Feng, J.; Sun, L.; Wang, J.; Hu, W.; Peng, B.; Wang, Y.; Wang, Y. Coupled Simulation of Hydrate-Bearing and Overburden Sedimentary Layers to Study Hydrate Dissociation and Methane Leakage. J. Mar. Sci. Eng. 2022, 10, 668. https://doi.org/10.3390/jmse10050668
Xie Y, Feng J, Sun L, Wang J, Hu W, Peng B, Wang Y, Wang Y. Coupled Simulation of Hydrate-Bearing and Overburden Sedimentary Layers to Study Hydrate Dissociation and Methane Leakage. Journal of Marine Science and Engineering. 2022; 10(5):668. https://doi.org/10.3390/jmse10050668
Chicago/Turabian StyleXie, Yan, Jingchun Feng, Liwei Sun, Junwen Wang, Weiqiang Hu, Bo Peng, Yujun Wang, and Yi Wang. 2022. "Coupled Simulation of Hydrate-Bearing and Overburden Sedimentary Layers to Study Hydrate Dissociation and Methane Leakage" Journal of Marine Science and Engineering 10, no. 5: 668. https://doi.org/10.3390/jmse10050668





