Study of the Formation of Hydrates with NaCl, Methanol Additive, and Quartz Sand Particles
Abstract
:1. Introduction
2. Experimental and Data Processing
2.1. Apparatus and Materials
2.2. Procedures and Conditions
2.3. Calculation Methods
3. Results and Discussion
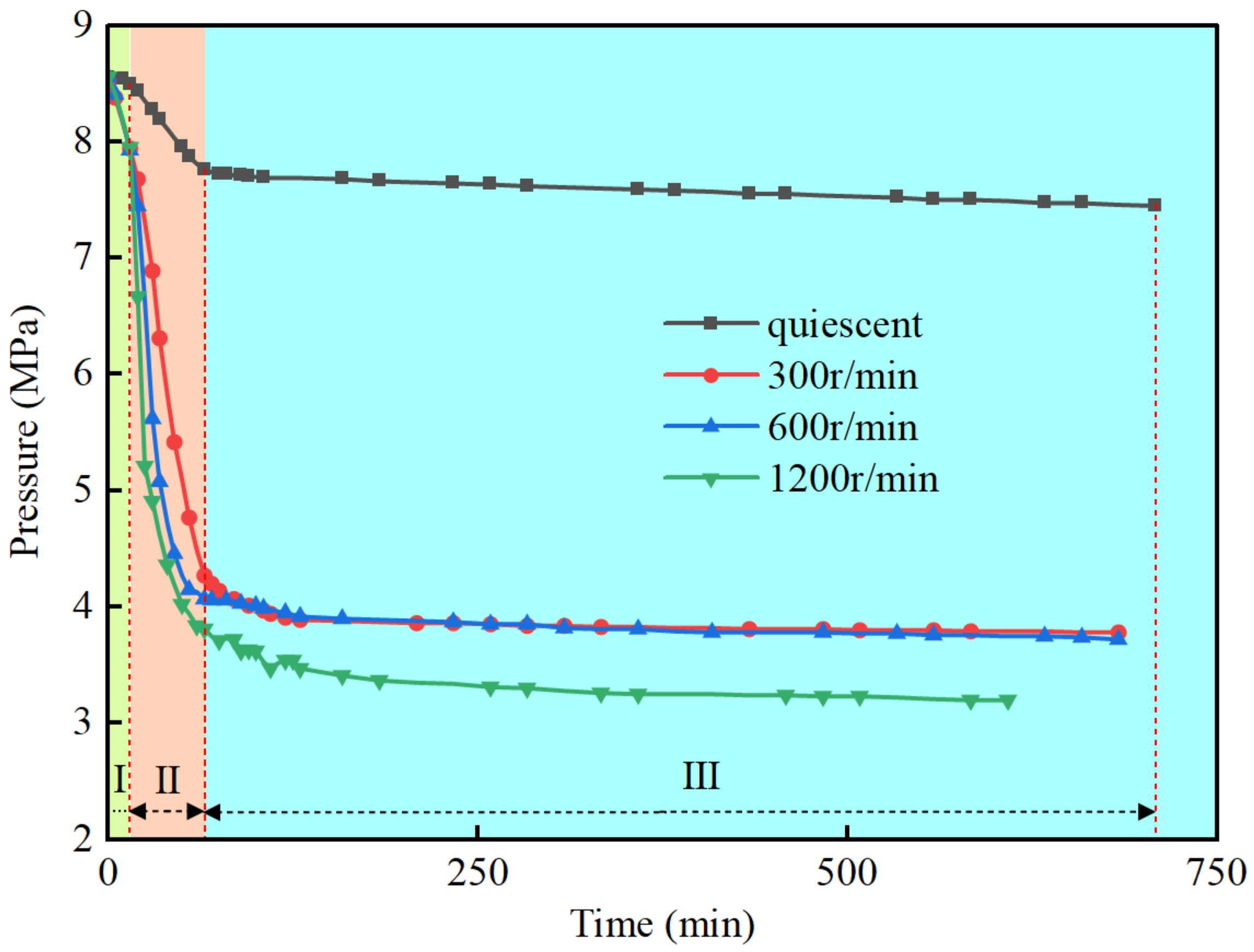
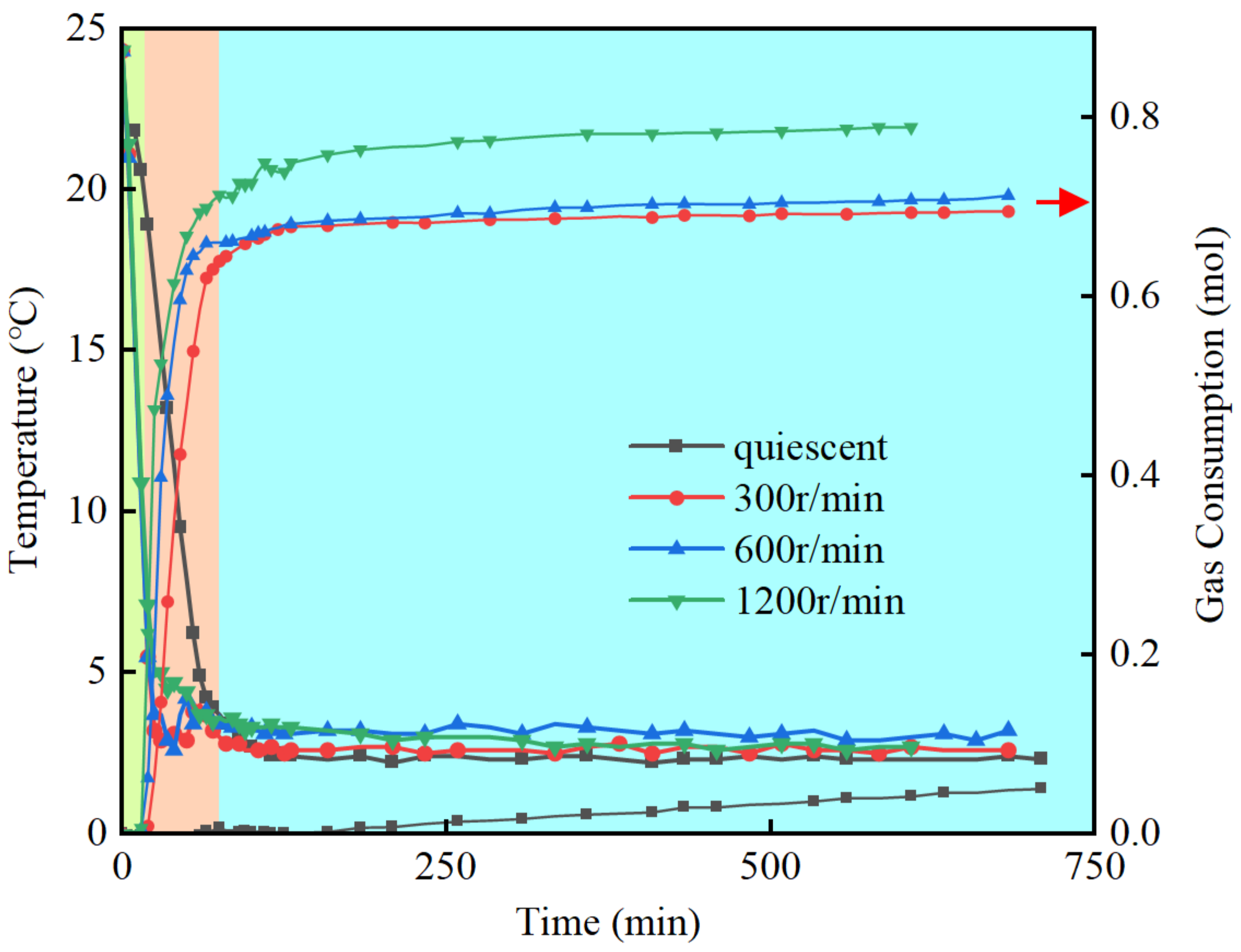
3.1. The Effect of the Stirring Rate
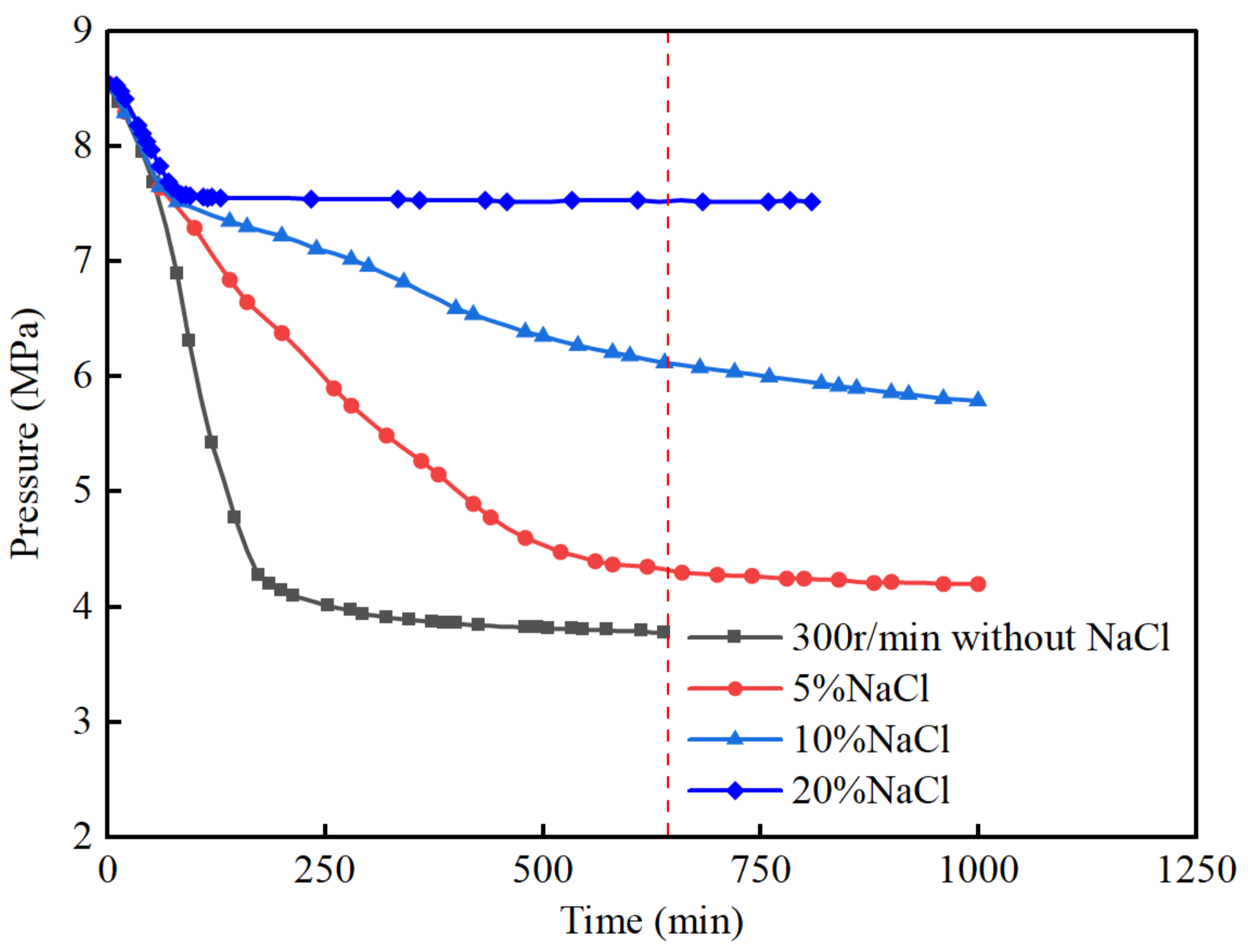
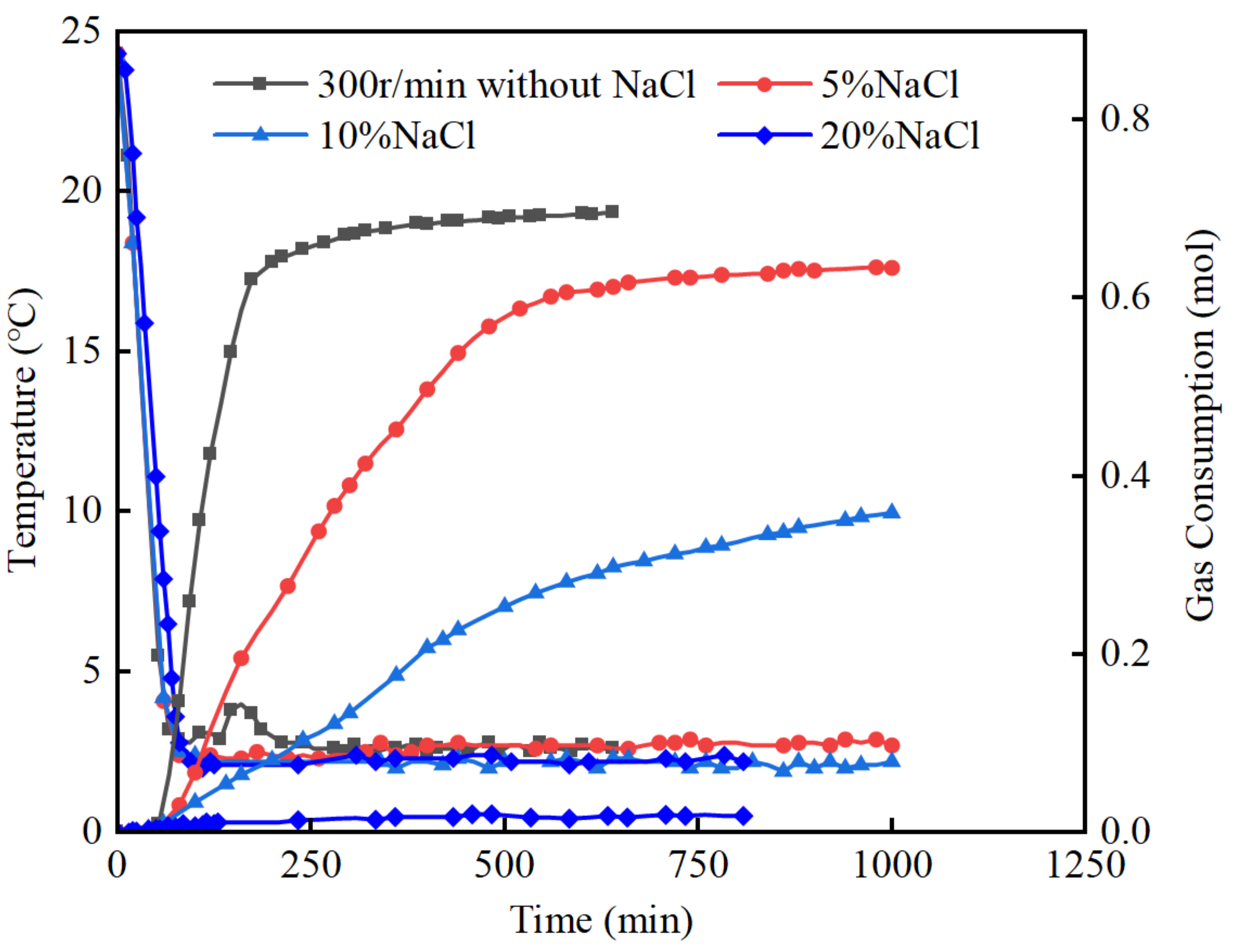
3.2. The Effect of NaCl Additive
3.3. The Effect of Methanol L Additive
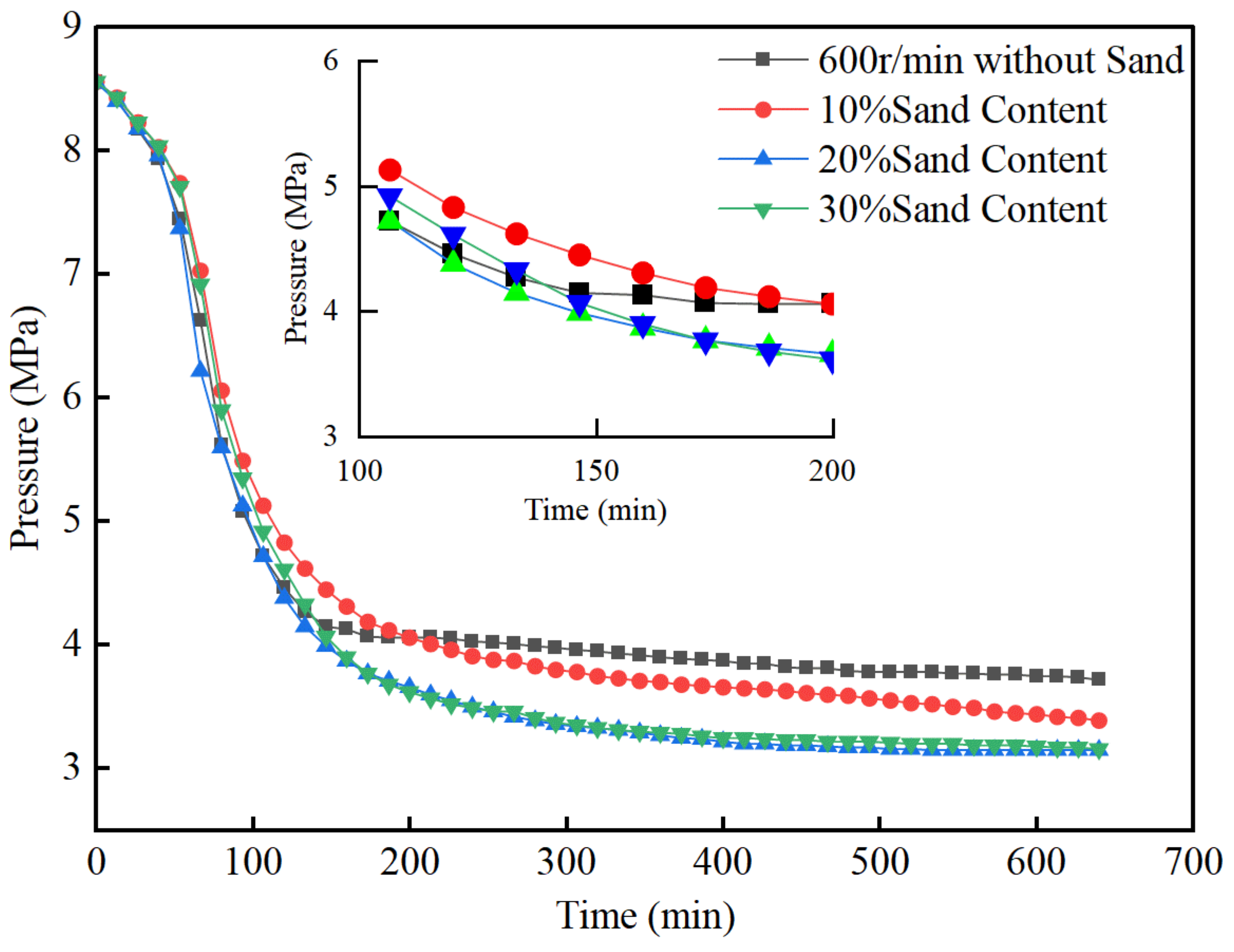
3.4. The Effect of Sand Contents
4. Conclusions
- (1)
- The hydrate formation process can be divided into three stages: the induction stage, the rapid formation stage, and the slow formation stage. During the induction stage, the pressure decreases with a decrease in temperature until a turning point is reached, and hydrate nucleation gradually occurs. In the rapid formation stage, the pressure rapidly decreases, and gas consumption significantly increases. The slow formation stage is characterized by a steady decrease in pressure, and the rate of gas consumption gradually diminishes.
- (2)
- The stirring effect has a significant impact on both the induction stage and the rapid formation stage times. Comparing the static system to systems with stirring at 300 r/min, 600 r/min, and 1200 r/min, the induction stage times are around 40 min for all cases, and the rapid formation stage times are approximately 130 min, 100 min, and 90 min, respectively.
- (3)
- NaCl and methanol have a significant impact on the induction stage and the final gas consumption. In NaCl and methanol solutions, the formation of hydrates is inhibited, and the induction stage time increases with the concentration of NaCl and methanol. The hydrate formation rate remains stable, showing no clear rapid formation stage. The final gas consumption decreases significantly with the increasing concentration of NaCl and methanol, and in the case of a 20% mass concentration, hydrate formation is almost completely inhibited in both NaCl and methanol solutions.
- (4)
- In the quartz sand system, the induction stage time and the rapid generation stage time are relatively close to those in the pure water system under the same conditions. However, in the slow generation stage, the gas consumption is significantly higher than in the pure water system. Within a certain range (in this study, with a sand content of 20%), the final gas consumption increases with the increase in sand content.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seyyedattar, M.; Zendehboudi, S.; Butt, S. Technical and non-technical challenges of development of offshore petroleum reservoirs: Characterization and production. Nat. Resour. Res. 2020, 29, 2147–2189. [Google Scholar] [CrossRef]
- Fraser, G.S. Impacts of offshore oil and gas development on marine wildlife resources. In Peak Oil, Economic Growth, and Wildlife Conservation; Springer: New York, NY, USA, 2014; pp. 191–217. [Google Scholar]
- Osborne, J.J.; Yetginer, A.G.; Halliday, T.; Tjelta, T.I. The future of deepwater site investigation: Seabed drilling technology? In Frontiers in Offshore Geotechnics II; CRC Press: Boca Raton, FL, USA, 2010; pp. 317–322. [Google Scholar]
- Shicun, W.; Nong, H. The energy security of China and oil and gas exploitation in the disputed South China Sea Area. In Recent Developments in the Law of the Sea and China; Brill Nijhoff: Leiden, The Netherlands, 2005; pp. 145–154. [Google Scholar]
- Metelitsa, A.; Kupfer, J. Oil and Gas Resources and Transit Issues in the South China Sea; Asia Society: New York, NY, USA, 2022. [Google Scholar]
- Aird, P. Deepwater Drilling; Gulf Professional Publishing: Houston, TX, USA; Elsevier: Amsterdam, The Netherlands, 2019; Volume 441, 475p. [Google Scholar]
- Abimbola, M.; Khan, F.; Khakzad, N. Dynamic safety risk analysis of offshore drilling. J. Loss Prev. Process Ind. 2014, 30, 74–85. [Google Scholar] [CrossRef]
- Millett, J.; Wilkins, A.; Campbell, E.; Hole, M.; Taylor, R.; Healy, D.; Jerram, D.; Jolley, D.; Planke, S.; Archer, S.; et al. The geology of offshore drilling through basalt sequences: Understanding operational complications to improve efficiency. Mar. Pet. Geol. 2016, 77, 1177–1192. [Google Scholar] [CrossRef]
- Hannegan, D. Offshore Drilling Hazard Mitigation: Controlled Pressure Drilling Redefines What Is Drillable. Drilling Contractor. 2009, pp. 84–89. Available online: https://drillingcontractor.org/offshore-drilling-hazard-mitigation-controlled-pressure-drilling-redefines-what-is-drillable-3178 (accessed on 1 January 2024).
- Zhiyuan, W.; Jianbo, Z.; Wenbo, M.; Baojiang, S.; Jinsheng, S.; Jintang, W.; Dahui, L.; Jinbo, W. Formation, deposition characteristics and prevention methods of gas hydrates in deepwater gas wells. Acta Pet. Sin. 2021, 42, 776. [Google Scholar]
- Cha, S.B.; Ouar, H.; Wildeman, T.R.; Sloan, E.D. A third-surface effect on hydrate formation. J. Phys. Chem. 1988, 92, 6492–6494. [Google Scholar] [CrossRef]
- Lai, D.T.; Dzialowski, A.K. Investigation of natural gas hydrates in various drilling fluids. In Proceedings of the SPE/IADC Drilling Conference, Miami, FL, USA, 21–23 February 1989. [Google Scholar]
- Waite, W.F.; Winters, W.J.; Mason, D.H. Methane hydrate formation in partially water-saturated Ottawa sand. Am. Mineral. 2004, 89, 1202–1207. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, Q.; Mu, C. Influence of temperature on methane hydrate formation. Sci. Rep. 2017, 7, 7904. [Google Scholar] [CrossRef]
- Zhou, Y.; Castaldi, M.J.; Yegulalp, T.M. Experimental investigation of methane gas production from methane hydrate. Ind. Eng. Chem. Res. 2009, 48, 3142–3149. [Google Scholar] [CrossRef]
- Filarsky, F.; Hagelstein, M.; Schultz, H.J. Influence of different stirring setups on mass transport, gas hydrate formation, and scale transfer concepts for technical gas hydrate applications. Appl. Res. 2023, 2, e202200050. [Google Scholar] [CrossRef]
- Wang, P.; Wang, S.; Song, Y.; Yang, M. Dynamic measurements of methane hydrate formation/dissociation in different gas flow direction. Appl. Energy 2018, 227, 703–709. [Google Scholar] [CrossRef]
- Huang, T.; Li, X.; Zhang, Y.; Wang, Y.; Chen, Z. Experimental study of the drilling process in hydrate-bearing sediments under different circulation rates of drilling fluid. J. Pet. Sci. Eng. 2020, 189, 107001. [Google Scholar] [CrossRef]
- Dong, S.; Song, Y.; Pang, W.; Zhao, J.; Zheng, J.-N.; Yang, M. Experimental Study on the Decomposition Characteristics of Methane Hydrate for the Pressure Regulation of Production Processes. Energy Fuels 2023, 37, 2019–2029. [Google Scholar] [CrossRef]
- Yu, Y.S.; Sun, W.Z.; Chen, C.; Li, X.S.; Chen, Z.Y. Kinetics studies of methane hydrate formation and dissociation in the presence of cyclopentane. Fuel 2024, 356, 129651. [Google Scholar] [CrossRef]
- Dubey, S.; Gurjar, P.; Kumar, U.; Sahai, M.; Kumar, S.; Kumar, A. Elucidating the Impact of Thermodynamic Hydrate Inhibitors and Kinetic Hydrate Inhibitors on a Complex System of Natural Gas Hydrates: Application in Flow Assurance. Energy Fuels 2023, 37, 6533–6544. [Google Scholar] [CrossRef]
- Jiang, G.; Liu, T.; Ning, F.; Tu, Y.; Zhang, L.; Yu, Y.; Kuang, L. Polyethylene glycol drilling fluid for drilling in marine gas hydrates-bearing sediments: An experimental study. Energies 2011, 4, 140–150. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Huang, H.; Qi, D.; Zhang, Y.; Ren, S.; Wu, Z.; Fang, M. Gas hydrate risks and prevention for deep water drilling and completion: A case study of well QDN-X in Qiongdongnan Basin, South China Sea. Pet. Explor. Dev. 2014, 41, 824–832. [Google Scholar] [CrossRef]
- Farahani, M.V.; Hassanpouryouzband, A.; Yang, J.; Tohidi, B. Insights into the climate-driven evolution of gas hydrate-bearing permafrost sediments: Implications for prediction of environmental impacts and security of energy in cold regions. RSC Adv. 2021, 11, 14334–14346. [Google Scholar] [CrossRef] [PubMed]
- Farahani, M.V.; Guo, X.; Zhang, L.; Yang, M.; Hassanpouryouzband, A.; Zhao, J.; Yang, J.; Song, Y.; Tohidi, B. Effect of thermal formation/dissociation cycles on the kinetics of formation and pore-scale distribution of methane hydrates in porous media: A magnetic resonance imaging study. Sustain. Energy Fuels 2021, 5, 1567–1583. [Google Scholar] [CrossRef]
- Okwananke, A.; Hassanpouryouzband, A.; Farahani, M.V.; Yang, J.; Tohidi, B.; Chuvilin, E.; Istomin, V.; Bukhanov, B. Methane recovery from gas hydrate-bearing sediments: An experimental study on the gas permeation characteristics under varying pressure. J. Pet. Sci. Eng. 2019, 180, 435–444. [Google Scholar] [CrossRef]
- Handa, Y.P. A calorimetric study of naturally occurring gas hydrates. Ind. Eng. Chem. Res. 1988, 27, 872–874. [Google Scholar] [CrossRef]
- Uchida, T.; Hirano, T.; Ebinuma, T.; Narita, H.; Gohara, K.; Mae, S.; Matsumoto, R. Raman spectroscopic determination of hydration number of methane hydrates. AIChE J. 1999, 45, 2641–2645. [Google Scholar] [CrossRef]
- Qi, Y.; Wu, W.; Liu, Y.; Xie, Y.; Chen, X. The influence of NaCl ions on hydrate structure and thermodynamic equilibrium conditions of gas hydrates. Fluid Phase Equilibria 2012, 325, 6–10. [Google Scholar] [CrossRef]
- Mimachi, H.; Takeya, S.; Gotoh, Y.; Yoneyama, A.; Hyodo, K.; Takeda, T.; Murayama, T. Dissociation behaviors of methane hydrate formed from NaCl solutions. Fluid Phase Equilibria 2016, 413, 22–27. [Google Scholar] [CrossRef]
- Madygulov, M.S.; Vlasov, V.A. Kinetics of methane hydrate formation from stirred aqueous NaCl solutions. Chem. Eng. Res. Des. 2024, 202, 267–271. [Google Scholar] [CrossRef]
- Anderson, F.E.; Prausnitz, J.M. Inhibition of gas hydrates by methanol. AIChE J. 1986, 32, 1321–1333. [Google Scholar] [CrossRef]
- Vysniauskas, A.; Bishnoi, P.R. A kinetic study of methane hydrate formation. Chem. Eng. Sci. 1983, 38, 1061–1072. [Google Scholar] [CrossRef]
- You, K.; Flemings, P.; Malinverno, A.; Collett, T.; Darnell, K. Mechanisms of methane hydrate formation in geological systems. Rev. Geophys. 2019, 57, 1146–1196. [Google Scholar] [CrossRef]
- Lan, W.-P.; Lin, D.-C.; Huang, F.-F.; Shi, B.-H.; Chen, Y.-C.; Wu, H.-H.; Gong, J. Experimental Study on Formation and Decomposition of Methane Hydrate in the Presence of Sand. Contemp. Chem. Ind. 2020, 49, 1839–1846. [Google Scholar]
- Hu, G.W.; Bu, Q.T. Research progress on the effects of particle size and pore size distribution on natural gas hydrate formation. Geol. Sci. Technol. Inf. 2019, 38, 41–52. [Google Scholar]
- Taylor, C.J.; Miller, K.T.; Koh, C.A.; Sloan, E.D. Macroscopic investigation of hydrate film growth at the hydrocarbon/water interface. Chem. Eng. Sci. 2007, 62, 6524–6533. [Google Scholar] [CrossRef]
- Turner, D.J.; Miller, K.T.; Sloan, E.D. Direct conversion of water droplets to methane hydrate in crude oil. Chem. Eng. Sci. 2009, 64, 5066–5072. [Google Scholar] [CrossRef]






| Name | Standard | Source |
|---|---|---|
| deionized water | 18.2 MΩ.cm (25 °C) | Laboratory |
| methane | Purity ≥ 99.999% | Qingdao Xinkeyuan Technology Co., Ltd. (Qingdao, China) |
| NaCl | AR | Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) |
| methanol | AR | Sinopharm Chemical Reagent Co., Ltd. |
| quartz sand | - | Laboratory |
| Project | Unit | Data |
|---|---|---|
| specific gravity | g·cm−3 | 2.66 |
| Weight | g·cm−3 | 1.75 |
| Porosity | % | 43 |
| Mohs hardness | / | 7.5 |
| Serial Number | Constituencies | Content |
|---|---|---|
| 1 | Control group (quiescent) | Deionized water |
| 2 | Stirring Rate | 300 r/min |
| 3 | 600 r/min | |
| 4 | 1200 r/min | |
| 5 | Inorganic salts | 5% NaCl |
| 6 | 10% NaCl | |
| 7 | 20% NaCl | |
| 8 | Alcohol | 5% methanol |
| 9 | 10% methanol | |
| 10 | 20% methanol | |
| 11 | Quartz sand | 10% Sand content |
| 12 | 20% Sand content | |
| 13 | 30% Sand content |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Gao, Y.; Zhang, L.; Su, X.; Guo, Y. Study of the Formation of Hydrates with NaCl, Methanol Additive, and Quartz Sand Particles. J. Mar. Sci. Eng. 2024, 12, 364. https://doi.org/10.3390/jmse12030364
Qi Y, Gao Y, Zhang L, Su X, Guo Y. Study of the Formation of Hydrates with NaCl, Methanol Additive, and Quartz Sand Particles. Journal of Marine Science and Engineering. 2024; 12(3):364. https://doi.org/10.3390/jmse12030364
Chicago/Turabian StyleQi, Yaqiang, Yonghai Gao, Lei Zhang, Xinyao Su, and Yanli Guo. 2024. "Study of the Formation of Hydrates with NaCl, Methanol Additive, and Quartz Sand Particles" Journal of Marine Science and Engineering 12, no. 3: 364. https://doi.org/10.3390/jmse12030364







