Population Structure and Reproductive Biology of the Endangered Crab Deiratonotus japonicus (Brachyura, Camptandriidae) Surveyed for Nine Years in the Kita River, Japan
Abstract
:1. Introduction
2. Materials and Methods
3. Results
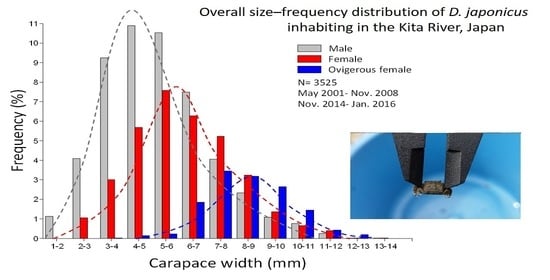
3.1. Population Structure
3.1.1. Sex Ratio
3.1.2. Distribution and Abundance
3.2. Reproductive Biology
3.2.1. Breeding Period and Recruitment
3.2.2. Fecundity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Naruse, T. Species of Moguai Tan and Ng, 1999 (Decapoda: Brachyura: Camptandriidae) from brackish waters in the Ryukyu Islands, Japan, with the description of a new species. Zootaxa 2005, 1044, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Ahyong, S. Paramoguai kavieng, a new genus and species of camptandriid crab from Papua New Guinea (Crustacea: Brachyura). Zootaxa 2014, 3856, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Naruse, T.; Chung, A.Y.C.; Tangah, J. Description of a new genus and a new species of the family Camptandriidae Stimpson, 1858 (Crustacea: Decapoda: Brachyura) from Lower Kinabatangan-Segama Wetlands, Sabah, Malaysia. Raffles Bull. Zool. 2015, 63, 327–333. [Google Scholar]
- Terada, M. Early zoeal stage of Deiratonotus japonicas (Sakai, 1934) (Ocypodidae, Camptandriinae) obtained under laboratory conditions. Crust. Res. 1995, 24, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Kawanae, M.; Wada, K.; Kitaura, J.; Watanabe, K. Taxonomic re-examination of the two camptandriid crab species Deiratonotus japonicus (Sakai, 1934) and D. tondensis Sakai, 1983, and genetic differentiation among their local population. J. Nat. Hist. 2005, 39, 3903–3918. [Google Scholar] [CrossRef]
- Ng, P.K.L.; Guinot, D.; Davie, P.J.F. Systema Brachyurorum: Part I. An annotated checklist of extant brachyuran crabs of the world. Raffles Bull. Zool. 2008, 17, 1–286. [Google Scholar]
- Miura, T.; Kawane, M.; Wada, K. A new Species of Deiratonotus (Crustacea: Brachyura: Camptandriidae) found in the Kumanoe river estuary, Kyushu, Japan. Zool. Sci. 2007, 24, 1045–1050. [Google Scholar] [CrossRef]
- Yamanishi, H.; Kusuda, T.; Hirata, M.; Oh, I.K.; Lee, S.Y. Characteristics of crossing distribution and the preference conditions on habitation of Deiratonotus japonicus. Environ. Eng. Res. 2001, 38, 1–11. [Google Scholar]
- Kawamoto, M.; Wada, K.; Kawane, M.; Kamada, M. Population Subdivision of the Brackish-water Crab Deiratonotus cristatus on the Japanese Coast. Zool. Sci. 2012, 29, 21–29. [Google Scholar] [CrossRef]
- Oh, I.K.; Lee, S.W. Effects of temperature on survival and larval development of Deiratonotus Japonicus (Brachyura, Camptandriidae) as a Biological Indicator. J. Mar. Sci. Eng. 2020, 8, 213. [Google Scholar] [CrossRef] [Green Version]
- Yamanishi, H.; Kusuda, T.; Lee, S.Y.; Hara, A.; Murakami, K. 2000. Study on the influence of water quality and the environmental condition of habitat of Deiratonotus japonicus in the Kita River. Environ. Eng. Res. 2000, 37, 173–181. [Google Scholar]
- Hiu, Y.; Oh, I.K.; Kusuda, T.; Hirata, M. Characterisitics of distribution, changes of the number of individuals and the conditions on habitation of Deiratonotus japonicus. Environ. Eng. Res. 2002, 39, 467–475. [Google Scholar]
- Hiu, Y.; Oh, I.K.; Iyooka, H.; Hirata, M.; Kusuda, T. Natural change in number and habitat characteristics of Deiratonotus japonicus in the Kita River, Japan. In Proceedings of the IWA Asia-Pacific Regional Conference 2003, Bangkok, Thailand, 19–23 October 2003. [Google Scholar]
- Hiu, Y.; Oh, I.K.; Iyooka, H.; Tagomori, K.; Hirata, M.; Lee, S.Y.; Yamanishi, H.; Kusuda, T. Changes of habitat characteristics and evaluation of habitat environment of Deiratonotus japonicus in the Kita river, Japan. In Proceedings of the IWA WWC2004 Conference, Marrakech, Morocco, 19–24 September 2004. [Google Scholar]
- Litulo, C. Population biology of the fiddler crab Uca annulipes (Brachyura: Ocypodidae) in a tropical East African mangrove (Mozambique). Estuar. Coast. Shelf Sci. 2005, 62, 283–290. [Google Scholar] [CrossRef]
- Litulo, C. Population structure and reproductive biology of the fiddler crab Uca inverse (Hoffman, 1874) (Brachyura: Ocypodidae). Acta Oecol. 2005, 27, 135–141. [Google Scholar] [CrossRef]
- Litulo, C.; Mahanjane, Y.; Mantelatto, F.L.M. Population biology of the sand-bubbler crab Dotilla fenestrate (Brachyura: Ocypodidae) from Southern Mozambique. Aquat. Ecol. 2005, 39, 305–313. [Google Scholar] [CrossRef]
- Nakayama, M.; Wada, K. Life history and behavior of a rare brackish- water crab, Ilyograpsus nodulosus (Sakai, 1983) (Macrophthalmidae). Crust. Res. 2015, 44, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Dugan, J.E.; Wenner, A.M.; Hubbard, D.M. Geographic variation in the reproductive biology of the sand crab Emerita analoga (Stimpson) on the California coast. J. Exp. Mar. Biol. Ecol. 1991, 150, 63–81. [Google Scholar] [CrossRef]
- Anger, K. The conquest of freshwater and land by marine crabs: Adaptions in life-history patterns and larval bioenergetics. J. Exp. Mar. Biol. Ecol. 1995, 193, 119–145. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, A.; Drake, P.; Arias, A.M. Reproductive periods and larval abundance patterns of the crabs Panopeus africanus and Uca tangeri in a shallow inlet (SW Spain). Mar. Ecol. Prog. Ser. 1997, 149, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Luis, E.A.B.; Helena, M.C. Population and reproductive biology of the fiddler crab Uca thayeri Rathbun, 1900 (Crustacea: Ocypodidae) in a tropical mangrove from Northeast Brazil. Acta Oecol. 2007, 31, 251–258. [Google Scholar]
- Litulo, C. Population structure and reproductive biology of the fiddler crab Uca urvillei (Hoffman, 1874) (Brachyura: Ocypodidae) in Maputo Bay (South Mozambique). J. Nat. Hist. 2005, 39, 2307–2318. [Google Scholar] [CrossRef]
- Serène, R. Note on the genera and species of the Camptandriinae Stimpson 1858 (Decapoda, Brachyura: Ocypodidae). Treubia 1974, 28, 59–68. [Google Scholar]
- Tan, C.G.S.; Ng, P.K.L. A revision of the genus Camptandrium Stimpson, 1858 (Crustacea: Decapoda: Brachyura: Camptandriidae). Raffles Bull. Zool. 1999, 47, 193–219. [Google Scholar]
- Fukui, Y.; Wada, K. Distribution and reproduction of intertidal crabs (Crustacea, Brachyura) in the Tonda River Estuary, Japan. Mar. Ecol. Prog. Ser. 1986, 30, 229–241. [Google Scholar] [CrossRef]
- Johnson, P.T.J. Biased sex ratios in fiddler crabs (Brachyura, Ocypodidae): A review and evaluation of the influence of sampling method, size class and sex-specific mortality. Crustaceana 2003, 76, 559–580. [Google Scholar] [CrossRef]
- Wenner, A.M. Sex ratio as a function of size in marine crustacea. Am. Nat. 1972, 106, 321–350. [Google Scholar] [CrossRef]
- Montague, C.L. A natural history of temperate western Atlantic fiddler crabs (genus Uca) with reference to their impact on the salt marsh. Contrib. Mar. Sci. 1980, 23, 25–55. [Google Scholar]
- Emmerson, W.D. Seasonal breeding cycles and sex ratios of eight species of crabs from Mgazana, a mangrove estuary in Transkei, South Africa. J. Crust. Biol. 1994, 14, 158–168. [Google Scholar] [CrossRef]
- Lardies, M.A.; Rojas, J.M.; Wehrtman, I.S. Breeding biology and population structure of the intertidal crab Petrolisthes laevigatus (Anomura: Porcellanidae) in central-southern Chile. J. Nat. Hist. 2004, 38, 375–388. [Google Scholar] [CrossRef]
- Hartnoll, R.G. Reproductive investment in Brachyura. Hydrobiologia 2006, 557, 31–40. [Google Scholar] [CrossRef]
- Eggleston, D.B.; Armstrong, D.A. Pre- and post-settlement determinant of estuarine Dungeness crab recruitment. Ecol. Monogr. 1995, 65, 193–216. [Google Scholar] [CrossRef]
- Waiho, K.; Fazhan, H.; Quinitio, E.T.; Baylon, J.C.; Fujaya, Y.; Azmie, G.; Wu, Q.; Shi, X.; Ikhwanuddin, M.; Ma, H. Larval rearing of mud crab (Scylla): What lies ahead. Aquaculture 2018, 493, 37–50. [Google Scholar] [CrossRef]
- Azra, M.N.; Aaqillah-Amr, M.A.; Ikhwanuddin, M.; Ma, H.; Waiho, K.; Ostrensky, A.; Tavares, C.P.D.S.; Abol-Munafi, A.B. Effects of climate- induced water temperature changes on the life history of brachyuran crabs. Rev. Aquacult. 2020, 12, 1211–1216. [Google Scholar] [CrossRef]
- Thurman, C.L. Reproductive biology and population structure of the fiddler crab Uca subcylindrica (Stimpson). Biol. Bull. 1985, 169, 515–529. [Google Scholar] [CrossRef]
- Litulo, C. Reproductive aspects of a tropical population of the fiddler crab Uca annulipes (H. Milne Edwards, 1837) (Brachyura: Ocypodidae) at Costa do Sol mangrove, Maputo Bay, southern Mozambique. Hydrobiologia 2004, 525, 167–173. [Google Scholar] [CrossRef]
- De Vries, M.C.; Tankersley, R.A.; Forward, R.B., Jr.; Kirby-Smith, W.W.; Luettich, R.A. Abundance of estuarine crab larvae is associated with tidal hydrologic variables. Mar. Biol. 1994, 118, 403–413. [Google Scholar] [CrossRef]
- Zeng, C.; Naylor, E. Occurrence in coastal waters and endogenous tidal swimming rhythms of late megalopae of the shore crab Carcinus maenas: Implications for onshore recruitment. Mar. Ecol. Prog. Ser. 1996, 136, 69–79. [Google Scholar] [CrossRef]
- Crane, J. Fiddler Crabs of the World. Ocypodidae: Genus Uca; Princeton University Press: Princeton, NJ, USA, 1975; p. 736. [Google Scholar]
- Kinne, O. Temperature: Animal-Invertebrates. In Marine Ecology, Environmental Factors v. 1; Kinne, O., Ed.; Wiley-Interscience: London, UK, 1970; pp. 407–414. [Google Scholar]
- Harrison, K.E. The role of nutrition in maturation, reproduction and embryonic development of decapod crustaceans: A review. J. Shellfish Res. 1990, 9, 1–28. [Google Scholar]
- Litulo, C. Fecundity of the Pantropical fiddler crab Uca annulipes (H. Milne Edwards, 1837) (Brachyura: Ocypodidae) at Costa do Sol mangrove, Maputo Bay, Southern Mozambique. West. Ind. Ocean J. Mar. Sci. 2004, 3, 87–91. [Google Scholar]









| Month | Male 1 | Female | Total | Sex Ratio | |||||
|---|---|---|---|---|---|---|---|---|---|
| Non-Ovigerous | Ovigerous | ||||||||
| N | % | N | % | N | % | N | % | ||
| May | 114 | 50.0 | 114 | 50.0 | - 2 | - | 228 | 6.5 | 1:1.00 |
| July | 32 | 34.8 | 60 | 65.2 | - | - | 92 | 2.6 | 1:1.88 |
| September | 86 | 61.9 | 53 | 38.1 | - | - | 139 | 3.9 | 1:0.62 |
| November | 58 | 55.2 | 47 | 44.8 | - | - | 105 | 3.0 | 1:0.81 |
| January | 40 | 39.6 | 59 | 58.4 | 2 | 2.0 | 101 | 2.9 | 1:1.53 |
| March | 67 | 43.8 | 67 | 43.8 | 19 | 12.4 | 153 | 4.3 | 1:1.28 |
| May | 52 | 41.6 | 33 | 26.4 | 40 | 32.0 | 125 | 3.6 | 1:1.40 |
| July | 56 | 46.3 | 15 | 12.4 | 50 | 41.3 | 121 | 3.4 | 1:1.16 |
| September | 31 | 52.5 | 5 | 8.5 | 23 | 39.0 | 59 | 1.7 | 1:0.90 |
| November | 14 | 51.9 | 13 | 48.2 | 0 | 0.0 | 27 | 0.8 | 1:0.93 |
| January | 27 | 45.0 | 32 | 53.3 | 1 | 1.7 | 60 | 1.7 | 1:1.22 |
| March | 44 | 43.6 | 52 | 51.5 | 5 | 5.0 | 101 | 2.9 | 1:1.30 |
| May | 61 | 46.9 | 45 | 34.6 | 24 | 18.5 | 130 | 3.7 | 1:1.13 |
| July | 31 | 38.8 | 17 | 21.2 | 32 | 40.0 | 80 | 2.3 | 1:1.58 |
| September | 18 | 50.0 | 10 | 27.8 | 8 | 22.2 | 36 | 1.0 | 1:1.00 |
| November | 52 | 59.1 | 30 | 34.1 | 6 | 6.8 | 88 | 2.5 | 1:0.69 |
| January | 54 | 58.7 | 37 | 40.2 | 1 | 1.1 | 92 | 2.6 | 1:0.70 |
| March | 36 | 46.8 | 36 | 46.8 | 5 | 6.5 | 77 | 2.2 | 1:1.14 |
| May | 30 | 39.0 | 31 | 40.3 | 16 | 20.8 | 77 | 2.2 | 1:1.57 |
| July | 35 | 46.7 | 14 | 18.6 | 26 | 34.7 | 75 | 2.1 | 1:1.43 |
| September | 9 | 60.0 | 2 | 13.3 | 4 | 26.7 | 15 | 0.4 | 1:0.67 |
| November | 10 | 66.7 | 5 | 33.3 | 0 | 0.0 | 15 | 0.4 | 1:0.50 |
| January | 4 | 36.4 | 6 | 54.6 | 1 | 9.1 | 11 | 0.3 | 1:1.75 |
| March | 8 | 53.3 | 4 | 26.7 | 3 | 20.0 | 15 | 0.4 | 1:0.88 |
| May | 12 | 52.2 | 4 | 17.4 | 7 | 30.4 | 23 | 0.6 | 1:0.92 |
| July | 7 | 41.2 | 2 | 11.8 | 8 | 47.1 | 17 | 0.5 | 1:1.43 |
| September | 18 | 54.6 | 5 | 15.2 | 10 | 30.3 | 33 | 0.9 | 1:0.83 |
| November | 23 | 62.2 | 14 | 37.8 | 0 | 0.0 | 37 | 1.0 | 1:0.61 |
| January | 32 | 42.1 | 40 | 52.6 | 4 | 5.3 | 76 | 2.2 | 1:1.38 |
| March | 17 | 50.0 | 13 | 38.2 | 4 | 11.8 | 34 | 0.9 | 1:1.00 |
| May | 15 | 42.9 | 8 | 22.9 | 12 | 34.2 | 35 | 1.0 | 1:1.33 |
| July | 12 | 44.4 | 3 | 11.1 | 12 | 44.4 | 27 | 0.8 | 1:1.25 |
| September | 13 | 61.9 | 2 | 9.5 | 6 | 28.6 | 21 | 0.6 | 1:0.62 |
| November | 39 | 56.5 | 30 | 43.5 | 0 | 0.0 | 69 | 2.0 | 1:0.77 |
| January | 35 | 50.0 | 35 | 50.0 | 0 | 0.0 | 70 | 2.0 | 1:1.00 |
| March | 17 | 56.7 | 11 | 36.7 | 2 | 6.6 | 30 | 0.9 | 1:0.77 |
| May | 6 | 40.0 | 5 | 33.3 | 4 | 26.7 | 15 | 0.4 | 1:1.50 |
| July | 17 | 48.6 | 6 | 17.1 | 12 | 34.3 | 35 | 1.0 | 1:1.06 |
| September | 8 | 88.9 | 0 | 0.0 | 1 | 11.1 | 9 | 0.3 | 1:0.13 |
| November | 35 | 77.8 | 9 | 20.0 | 1 | 2.2 | 45 | 1.3 | 1:0.29 |
| January | 64 | 66.7 | 31 | 32.3 | 1 | 1.0 | 96 | 2.7 | 1:0.50 |
| March | 30 | 51.7 | 27 | 46.6 | 1 | 1.7 | 58 | 1.7 | 1:0.93 |
| May | 16 | 66.7 | 5 | 20.8 | 3 | 12.5 | 24 | 0.7 | 1:0.50 |
| July | 18 | 75.0 | 5 | 20.8 | 1 | 4.2 | 24 | 0.7 | 1:0.33 |
| November | 87 | 82.1 | 15 | 14.2 | 4 | 3.7 | 106 | 3.0 | 1:0.22 |
| November | 50 | 54.9 | 35 | 38.5 | 6 | 6.6 | 91 | 2.6 | 1:0.82 |
| January | 43 | 58.9 | 28 | 38.4 | 2 | 2.7 | 73 | 2.1 | 1:0.70 |
| March | 33 | 53.2 | 22 | 35.5 | 7 | 11.3 | 62 | 1.8 | 1:0.88 |
| May | 51 | 51.5 | 23 | 23.2 | 25 | 25.3 | 99 | 2.8 | 1:0.94 |
| July | 38 | 43.2 | 7 | 7.9 | 43 | 48.9 | 88 | 2.5 | 1:1.32 |
| September | 38 | 53.5 | 6 | 8.5 | 27 | 38.0 | 71 | 2.0 | 1:0.87 |
| November | 38 | 47.5 | 35 | 43.7 | 7 | 8.8 | 80 | 2.3 | 1:1.11 |
| January | 25 | 45.5 | 27 | 49.1 | 3 | 5.4 | 55 | 1.6 | 1:1.20 |
| Total | 1806 | 51.2 | 1240 | 35.2 | 479 | 13.6 | 3525 | 100.0 | 1:0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, I.-K.; Lee, S.-W. Population Structure and Reproductive Biology of the Endangered Crab Deiratonotus japonicus (Brachyura, Camptandriidae) Surveyed for Nine Years in the Kita River, Japan. J. Mar. Sci. Eng. 2020, 8, 921. https://doi.org/10.3390/jmse8110921
Oh I-K, Lee S-W. Population Structure and Reproductive Biology of the Endangered Crab Deiratonotus japonicus (Brachyura, Camptandriidae) Surveyed for Nine Years in the Kita River, Japan. Journal of Marine Science and Engineering. 2020; 8(11):921. https://doi.org/10.3390/jmse8110921
Chicago/Turabian StyleOh, Il-Kweun, and Seung-Woo Lee. 2020. "Population Structure and Reproductive Biology of the Endangered Crab Deiratonotus japonicus (Brachyura, Camptandriidae) Surveyed for Nine Years in the Kita River, Japan" Journal of Marine Science and Engineering 8, no. 11: 921. https://doi.org/10.3390/jmse8110921