Influence of Calcined Clay Reactivity on the Mechanical Properties and Chloride Diffusion Resistance of Limestone Calcined Clay Cement (LC3) Concrete
Abstract
:1. Introduction
2. Materials and Methodology
2.1. Materials, Concrete Mix Design and Curing Condition
2.2. Characteristics of Calcined Clay
2.3. Mechanical Properties of LC3 Concrete
2.4. Bulk Diffusion Test
3. Results and Discussion
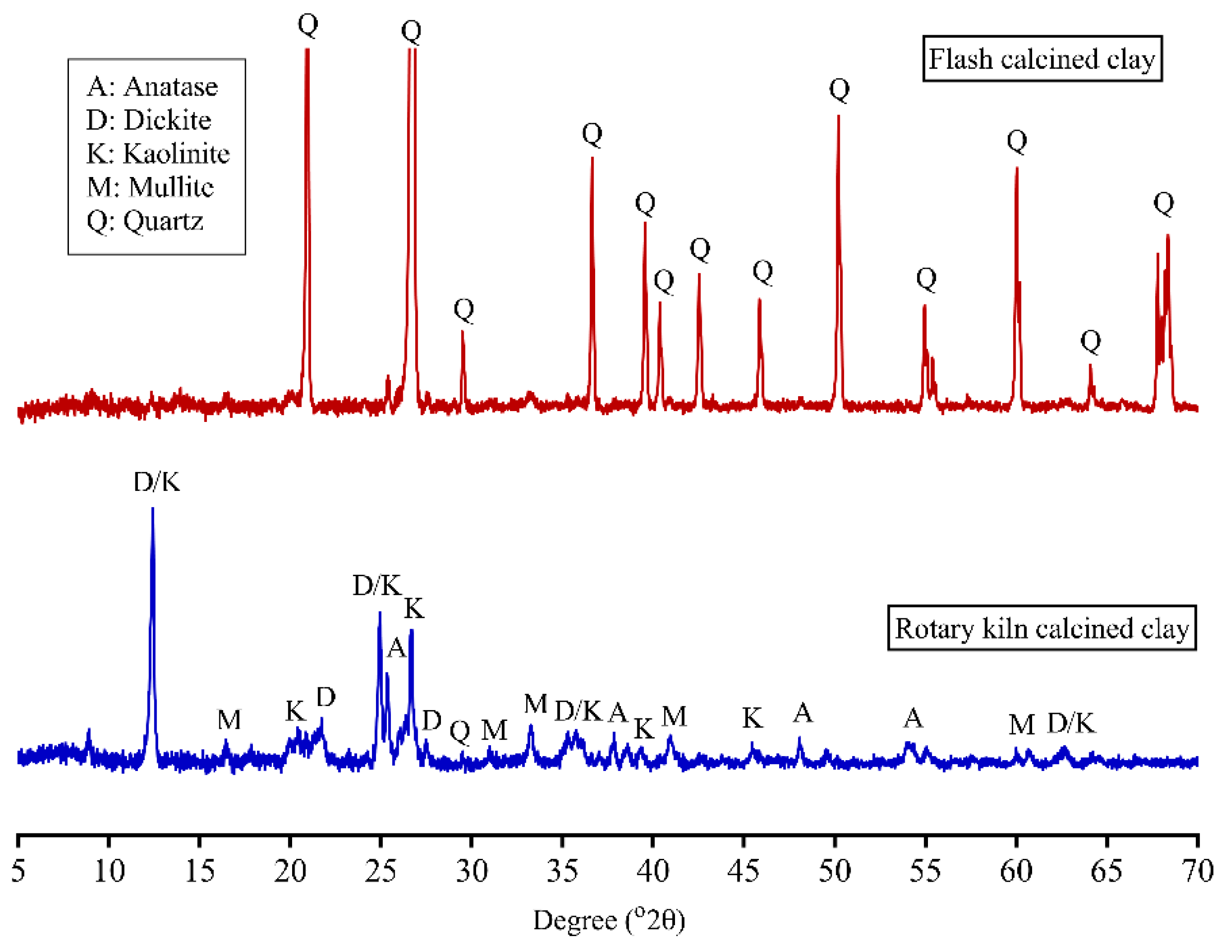
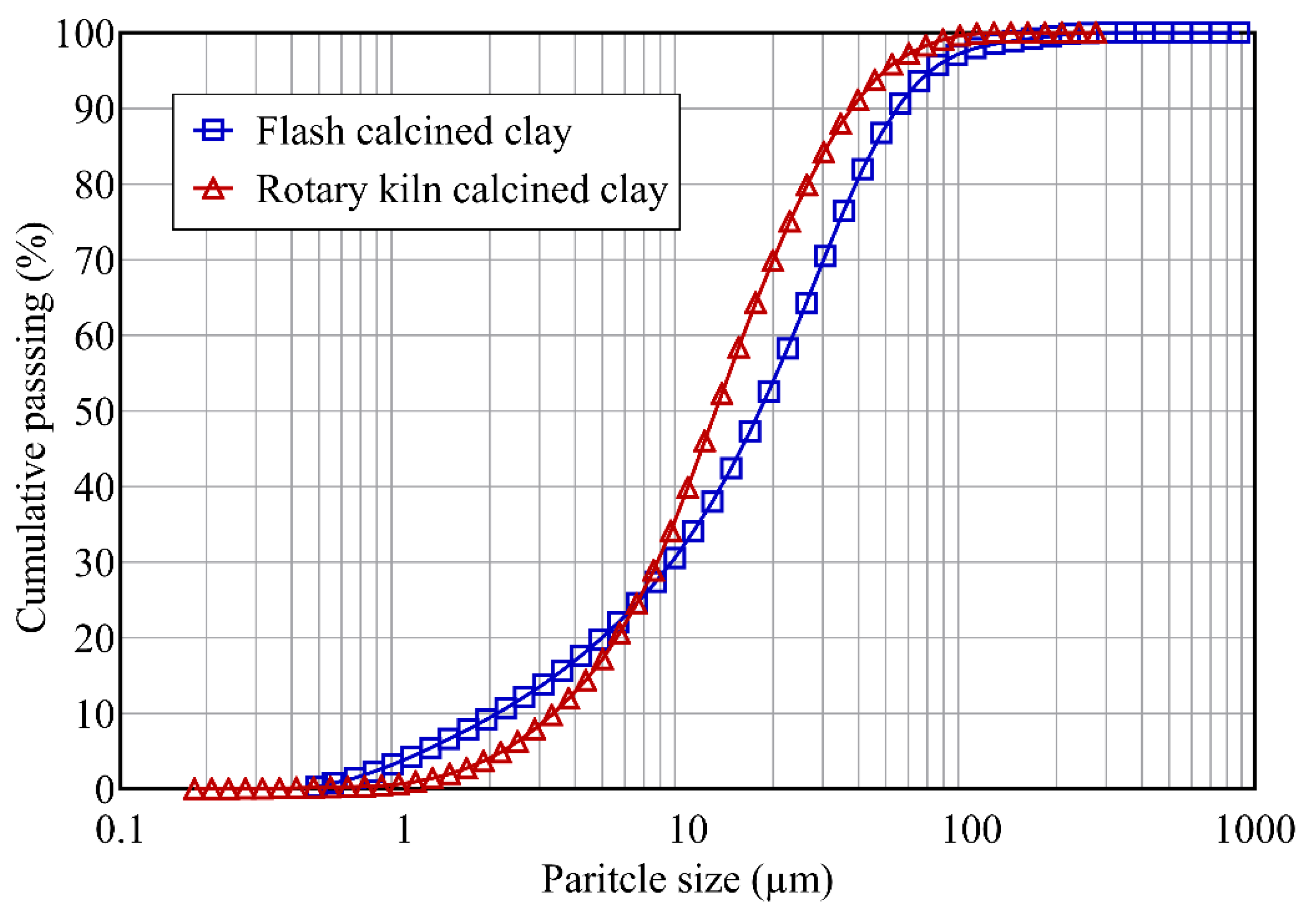
3.1. Characteristics of Calcined Clays
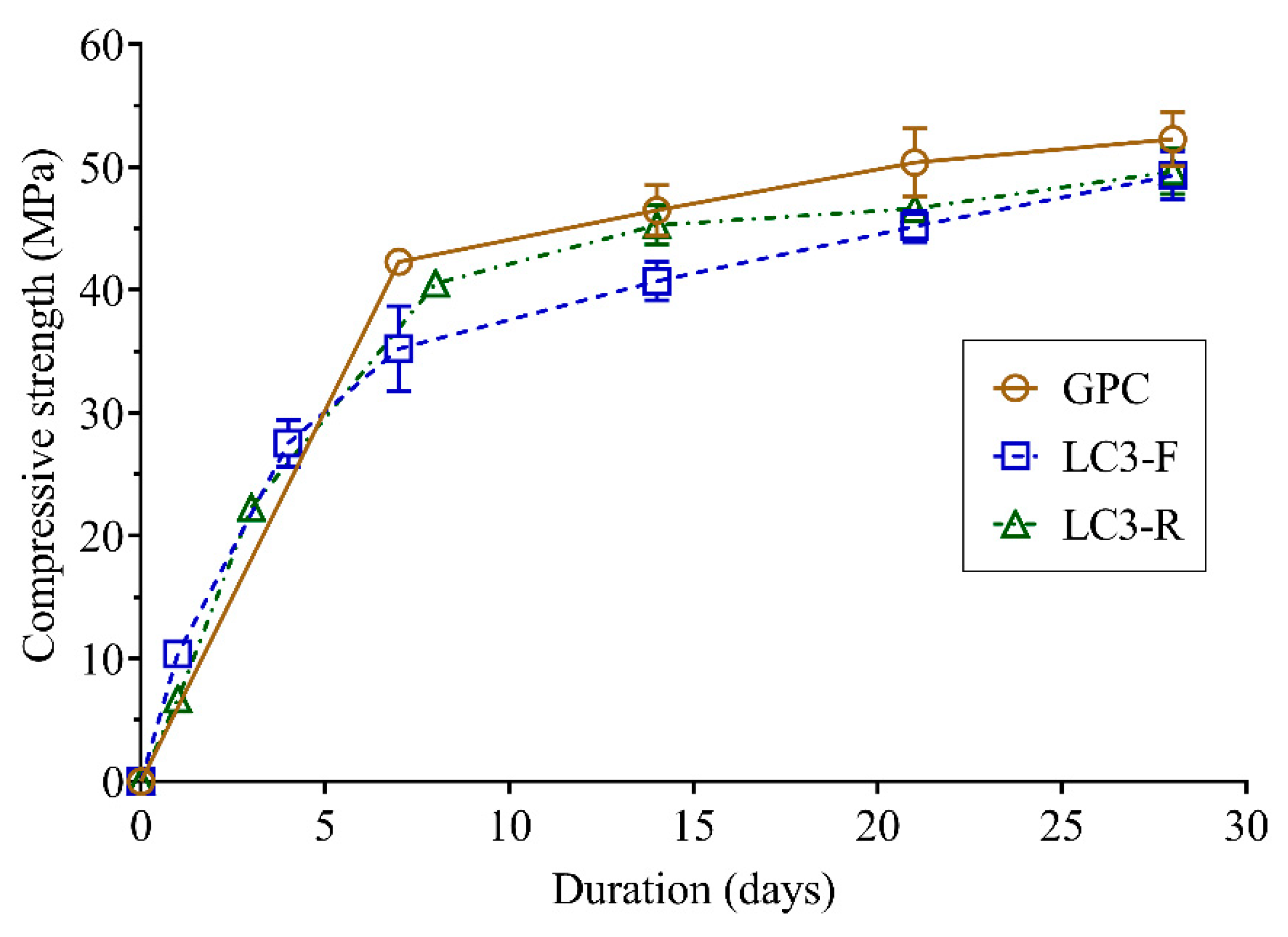
3.2. Mechanical Properties
3.3. Bulk Diffusion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Madlool, N.A.; Saidur, R.; Hossain, M.S.; Rahim, N.A. A critical review on energy use and savings in the cement industries. Renew. Sustain. Energy Rev. 2011, 15, 2042–2060. [Google Scholar] [CrossRef]
- Schneider, M. The cement industry on the way to a low-carbon future. Cem. Concr. Res. 2019, 124, 105792. [Google Scholar] [CrossRef]
- United Nations. World Urbanization Prospects: The 2011 Revision; Department of Economic and Social Affairs: New York, NY, USA, 2011.
- Angst, U.M. Challenges and opportunities in corrosion of steel in concrete. Mater. Struct. 2018, 51, 4. [Google Scholar] [CrossRef] [Green Version]
- Tinnea, J. Corrosion Control Plan for Bridges; A NACE International White Paper; NACE International: Houston, TX, USA, 2012. [Google Scholar]
- Bertolini, L.; Elsener, B.; Pedeferri, P.; Redaelli, E.; Polder, R.B. Corrosion of Steel in Concrete: Prevention, Diagnosis, Repair, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013. [Google Scholar]
- Andrade, C. Propagation of reinforcement corrosion: Principles, testing and modelling. Mater. Struct. 2018, 52, 2. [Google Scholar] [CrossRef] [Green Version]
- Favier, A.; De Wolf, C.; Scrivener, K.; Habert, G. A Sustainable Future for the European Cement and Concrete Industry: Technology Assessment for Full Decarbonisation of the Industry by 2050; ETH Zurich: Zurich, Switzerland, 2018. [Google Scholar]
- Yoshioka, K.; Obata, D.; Nanjo, H.; Yokozeki, K.; Torichigai, T.; Morioka, M.; Higuchi, T. New Ecological Concrete that Reduces CO2 Emissions Below Zero Level ∼ New Method for CO2 Capture and Storage. Energy Procedia 2013, 37, 6018–6025. [Google Scholar] [CrossRef] [Green Version]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- San Nicolas, R.; Cyr, M.; Escadeillas, G. Performance-based approach to durability of concrete containing flash-calcined metakaolin as cement replacement. Constr. Build. Mater. 2014, 55, 313–322. [Google Scholar] [CrossRef]
- San Nicolas, R.; Cyr, M.; Escadeillas, G. Characteristics and applications of flash metakaolins. Appl. Clay Sci. 2013, 83, 253–262. [Google Scholar] [CrossRef]
- Lothenbach, B.; Le Saout, G.; Gallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cem. Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]
- Mishra, G.; Emmanuel, A.C.; Bishnoi, S. Influence of temperature on hydration and microstructure properties of limestone-calcined clay blended cement. Mater. Struct. 2019, 52, 91. [Google Scholar] [CrossRef]
- Cancio Díaz, Y.; Sánchez Berriel, S.; Heierli, U.; Favier, A.R.; Sánchez Machado, I.R.; Scrivener, K.L.; Martirena Hernández, J.F.; Habert, G. Limestone calcined clay cement as a low-carbon solution to meet expanding cement demand in emerging economies. Dev. Eng. 2017, 2, 82–91. [Google Scholar] [CrossRef]
- Antoni, M.; Rossen, J.; Martirena, F.; Scrivener, K. Cement substitution by a combination of metakaolin and limestone. Cem. Concr. Res. 2012, 42, 1579–1589. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Khan, M.S.H.; Castel, A. Engineering Properties of Limestone Calcined Clay Concrete. J. Adv. Concr. Technol. 2018, 16, 343–357. [Google Scholar] [CrossRef] [Green Version]
- Tironi, A.; Scian, A.N.; Irassar, E.F. Blended Cements with Limestone Filler and Kaolinitic Calcined Clay: Filler and Pozzolanic Effects. J. Mater. Civ. Eng. 2017, 29, 04017116. [Google Scholar] [CrossRef]
- Dhandapani, Y.; Sakthivel, T.; Santhanam, M.; Gettu, R.; Pillai, R.G. Mechanical properties and durability performance of concretes with Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2018, 107, 136–151. [Google Scholar] [CrossRef]
- Medjigbodo, G.; Roziere, E.; Charrier, K.; Izoret, L.; Loukili, A. Hydration, shrinkage, and durability of ternary binders containing Portland cement, limestone filler and metakaolin. Constr. Build. Mater. 2018, 183, 114–126. [Google Scholar] [CrossRef]
- Khan, M.S.H.; Nguyen, Q.D.; Castel, A. Performance of limestone calcined clay blended cement-based concrete against carbonation. Adv. Cem. Res. 2019, 0, 1–11. [Google Scholar] [CrossRef]
- Shi, Z.G.; Lothenbach, B.; Geiker, M.R.; Kaufmann, J.; Leemann, A.; Ferreiro, S.; Skibsted, J. Experimental studies and thermodynamic modeling of the carbonation of Portland cement, metakaolin and limestone mortars. Cem. Concr. Res. 2016, 88, 60–72. [Google Scholar] [CrossRef]
- Muzenda, T.R.; Hou, P.; Kawashima, S.; Sui, T.; Cheng, X. The role of limestone and calcined clay on the rheological properties of LC3. Cem. Concr. Compos. 2020, 107, 103516. [Google Scholar] [CrossRef]
- Vizcaíno Andrés, L.M.; Antoni, M.G.; Alujas Diaz, A.; Martirena Hernández, J.F.; Scrivener, K.L. Effect of fineness in clinker-calcined clays-limestone cements. Adv. Res. 2015, 27, 546–556. [Google Scholar] [CrossRef]
- Khan, M.S.H.; Nguyen, Q.D. Castel, Carbonation of Limestone Calcined Clay Cement Concrete; Springer: Dordrecht, The Netherlands, 2018; pp. 238–243. [Google Scholar]
- Nguyen, Q.D.; Castel, A. Reinforcement corrosion in limestone flash calcined clay cement-based concrete. Cem.Concr. Res. 2020, 132, 106051. [Google Scholar] [CrossRef]
- Shi, Z.G.; Geiker, M.R.; De Weerdt, K.; Ostnor, T.A.; Lothenbach, B.; Winnefeld, F.; Skibsted, J. Role of calcium on chloride binding in hydrated Portland cement-metakaolin-limestone blends. Cem. Concr. Res. 2017, 95, 205–216. [Google Scholar] [CrossRef]
- Maraghec hi, H.; Avet, F.; Wong, H.; Kamyab, H.; Scrivener, K. Performance of Limestone Calcined Clay Cement (LC3) with various kaolinite contents with respect to chloride transport. Mater. Struct. 2018, 51, 125. [Google Scholar] [CrossRef]
- Sui, S.; Georget, F.; Maraghechi, H.; Sun, W.; Scrivener, K. Towards a generic approach to durability: Factors affecting chloride transport in binary and ternary cementitious materials. Cem. Concr. Res. 2019, 124, 105783. [Google Scholar] [CrossRef]
- Avet, F.; Scrivener, K. Influence of pH on the chloride binding capacity of Limestone Calcined Clay Cements (LC3). Cem. Concr. Res. 2020, 131, 106031. [Google Scholar] [CrossRef]
- Yang, P.; Dhandapani, Y.; Santhanam, M.; Neithalath, N. Simulation of chloride diffusion in fly ash and limestone-calcined clay cement (LC3) concretes and the influence of damage on service-life. Cem. Concr. Res. 2020, 130, 106010. [Google Scholar] [CrossRef]
- Tironi, A.; Castellano, C.C.; Bonavetti, V.L.; Trezza, M.A.; Scian, A.N.; Irassar, E.F. Kaolinitic calcined clays–Portland cement system: Hydration and properties. Constr. Build. Mater. 2014, 64, 215–221. [Google Scholar] [CrossRef]
- Tironi, A.; Trezza, M.A.; Scian, A.N.; Irassar, E.F. Thermal analysis to assess pozzolanic activity of calcined kaolinitic clays. J. Therm. Anal. Calorim. 2014, 117, 547–556. [Google Scholar] [CrossRef]
- Avet, F.; Scrivener, K. Investigation of the calcined kaolinite content on the hydration of Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2018, 107, 124–135. [Google Scholar] [CrossRef]
- Gbozee, M.; Zheng, K.; He, F.; Zeng, X. The influence of aluminum from metakaolin on chemical binding of chloride ions in hydrated cement pastes. Appl. Clay Sci. 2018, 158, 186–194. [Google Scholar] [CrossRef]
- AS 3600: Concrete Structures; Standards Australia: Sydney, Australia, 2018.
- AS 3972: General Purpose and Blended Cements; Standards Australia: Sydney, Australia, 2010.
- ASTM C39/C39M-18: Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens; ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM C469/C469M-14: Standard Test Method for Static Modulus of Elasticity and Poisson’s Ratio of Concrete in Compression; ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM C1556-11a(2016): Standard Test Method for Determining the Apparent Chloride Diffusion Coefficient of Cementitious Mixtures by Bulk Diffusion; ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM C1152/C1152M-04(2012)e1: Standard Test Method for Acid-Soluble Chloride in Mortar and Concrete; ASTM International: West Conshohocken, PA, USA, 2012.
- Noushini, A.; Castel, A.; Aldred, J.; Rawal, A. Chloride diffusion resistance and chloride binding capacity of fly ash-based geopolymer concrete. Cem. Concr. Compos. 2020, 105, 103290. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Chaves Figueiredo, S.; Çopuroğlu, O.; Veer, F.; Schlangen, E. Limestone and Calcined Clay-Based Sustainable Cementitious Materials for 3D Concrete Printing: A Fundamental Study of Extrudability and Early-Age Strength Development. Appl. Sci. 2019, 9, 1809. [Google Scholar] [CrossRef] [Green Version]
- Badogiannis, E.; Papadakis, V.; Chaniotakis, E.; Tsivilis, S. Exploitation of poor Greek kaolins: strength development of metakaolin concrete and evaluation by means of k-value. Cem. Concr. Res. 2004, 34, 1035–1041. [Google Scholar] [CrossRef]
- ASTM C618-19: Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete; ASTM International: West Conshohocken, PA, USA, 2019.
- Claverie, M.; Martin, F.; Tardy, J.; Cyr, M.; De Parseval, P.; Grauby, O.; Le Roux, C. Structural and chemical changes in kaolinite caused by flash calcination: Formation of spherical particles. Appl. Clay Sci. 2015, 114, 247–255. [Google Scholar] [CrossRef]
- Blissett, R.; Rowson, N. A review of the multi-component utilisation of coal fly ash. Fuel 2012, 97, 1–23. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Aboustait, M.; Kim, T.; Ley, M.T.; Davis, J.M. Physical and chemical characteristics of fly ash using automated scanning electron microscopy. Constr. Build. Mater. 2016, 106, 1–10. [Google Scholar] [CrossRef]
- Cepuritis, R.; Garboczi, E.J.; Ferraris, C.F.; Jacobsen, S.; Sørensen, B.E. Measurement of particle size distribution and specific surface area for crushed concrete aggregate fines. Adv. Powder Technol. 2017, 28, 706–720. [Google Scholar] [CrossRef] [Green Version]
- Odler, I. The BET-specific surface area of hydrated Portland cement and related materials. Cem. Concr. Res. 2003, 33, 2049–2056. [Google Scholar] [CrossRef]
- Li, C.; Jiang, L.; Xu, N.; Jiang, S. Pore structure and permeability of concrete with high volume of limestone powder addition. Powder Technol. 2018, 338, 416–424. [Google Scholar] [CrossRef]
- Ishida, T.; Iqbal, P.O.; Anh, H.T.L. Modeling of chloride diffusivity coupled with non-linear binding capacity in sound and cracked concrete. Cem. Concr. Res. 2009, 39, 913–923. [Google Scholar] [CrossRef]
- Stanish, K.; Thomas, M. The use of bulk diffusion tests to establish time-dependent concrete chloride diffusion coefficients. Cem. Concr. Res. 2003, 33, 55–62. [Google Scholar] [CrossRef]
- Luping, T.; Gulikers, J. On the mathematics of time-dependent apparent chloride diffusion coefficient in concrete. Cem. Concr. Res. 2007, 37, 589–595. [Google Scholar] [CrossRef]
- Shafikhani, M.; Chidiac, S. Quantification of concrete chloride diffusion coefficient—A critical review. Cem. Concr. Compos. 2019, 99, 225–250. [Google Scholar] [CrossRef]
- Pillai, R.G.; Gettu, R.; Santhanam, M.; Rengaraju, S.; Dhandapani, Y.; Rathnarajan, S.; Basavaraj, A.S. Service life and life cycle assessment of reinforced concrete systems with limestone calcined clay cement (LC3). Cem. Concr. Res. 2019, 118, 111–119. [Google Scholar] [CrossRef]



| Chemical Composition (wt.%) | GP Cement | Limestone | Flash Calcined Clay | Rotary Kiln Calcined Clay |
|---|---|---|---|---|
| SiO2 | 19.74 | 0.36 | 70.42 | 48.15 |
| Al2O3 | 4.70 | 0.11 | 22.34 | 41.63 |
| Fe2O3 | 2.98 | 0.1 | 2.34 | 2.27 |
| CaO | 64.62 | 57.51 | 0.49 | 0.12 |
| MgO | 1.48 | 0.29 | 0.16 | 0.09 |
| Na2O | 0.21 | - | 0.1 | 0.32 |
| K2O | 0.64 | - | 0.19 | 0.05 |
| TiO2 | 0.31 | - | 1.1 | 3.39 |
| SO3 | 2.24 | - | 0.02 | 0.09 |
| Loss on ignition (LOI) | 3.18 | 42.61 | 1.76 | 3.21 |
| Materials (kg) | GPC | LC3-F | LC3-R |
|---|---|---|---|
| Coarse aggregate | 1221 | 1221 | 1201.5 |
| Fine aggregate | 620.8 | 620.8 | 610.9 |
| Total binder | 388 | 388 | 388 |
| GPC | 388 | 310.4 | 217.3 |
| Flash calcined clay | 0 | 50.44 | 0 |
| Rotary kiln calcined clay | 0 | 0 | 116.4 |
| Limestone | 0 | 27.16 | 54.3 |
| Water/binder ratio | 0.45 | 0.45 | 0.45 |
| Water | 174.5 | 174.5 | 174.5 |
| Minerals (wt.%) | Flash Calcined Clay | Rotary Kiln Calcined Clay |
|---|---|---|
| Quartz | 49.1 ± 0.1 | 2.0 ± 0.1 |
| Mullite | - | 6.4 ± 0.2 |
| Dickite | - | 7.3 ± 0.2 |
| Kaolinite | - | 4.1 ± 0.1 |
| Anatase | - | 1.5 ± 0.1 |
| Amorphous | 50.9 ± 0.1 | 78.8 ± 0.1 |
| Particle size distribution (µm) | ||
| Dv10 | 2.50 | 3.85 |
| Dv50 | 21.19 | 14.40 |
| Dv90 | 64.75 | 43.37 |
| BET surface area (m2/g) | 12.05 ± 0.01 | 8.80 ± 0.49 |
| Concrete Types | Elastic Modulus at 28 Days (GPa) |
|---|---|
| GPC | 31.1 ± 0.9 |
| LC3-F | 34.5 ± 0.5 |
| LC3-R | 41.0 ± 0.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, Q.D.; Afroz, S.; Castel, A. Influence of Calcined Clay Reactivity on the Mechanical Properties and Chloride Diffusion Resistance of Limestone Calcined Clay Cement (LC3) Concrete. J. Mar. Sci. Eng. 2020, 8, 301. https://doi.org/10.3390/jmse8050301
Nguyen QD, Afroz S, Castel A. Influence of Calcined Clay Reactivity on the Mechanical Properties and Chloride Diffusion Resistance of Limestone Calcined Clay Cement (LC3) Concrete. Journal of Marine Science and Engineering. 2020; 8(5):301. https://doi.org/10.3390/jmse8050301
Chicago/Turabian StyleNguyen, Quang Dieu, Sumaiya Afroz, and Arnaud Castel. 2020. "Influence of Calcined Clay Reactivity on the Mechanical Properties and Chloride Diffusion Resistance of Limestone Calcined Clay Cement (LC3) Concrete" Journal of Marine Science and Engineering 8, no. 5: 301. https://doi.org/10.3390/jmse8050301





