New Studies of the Physical Properties of Metallic Amorphous Membranes for Hydrogen Purification
Abstract
:1. Introduction
2. Results and Discussion
2.1. Changes of the Physical Properties as a Function of the Zr Content
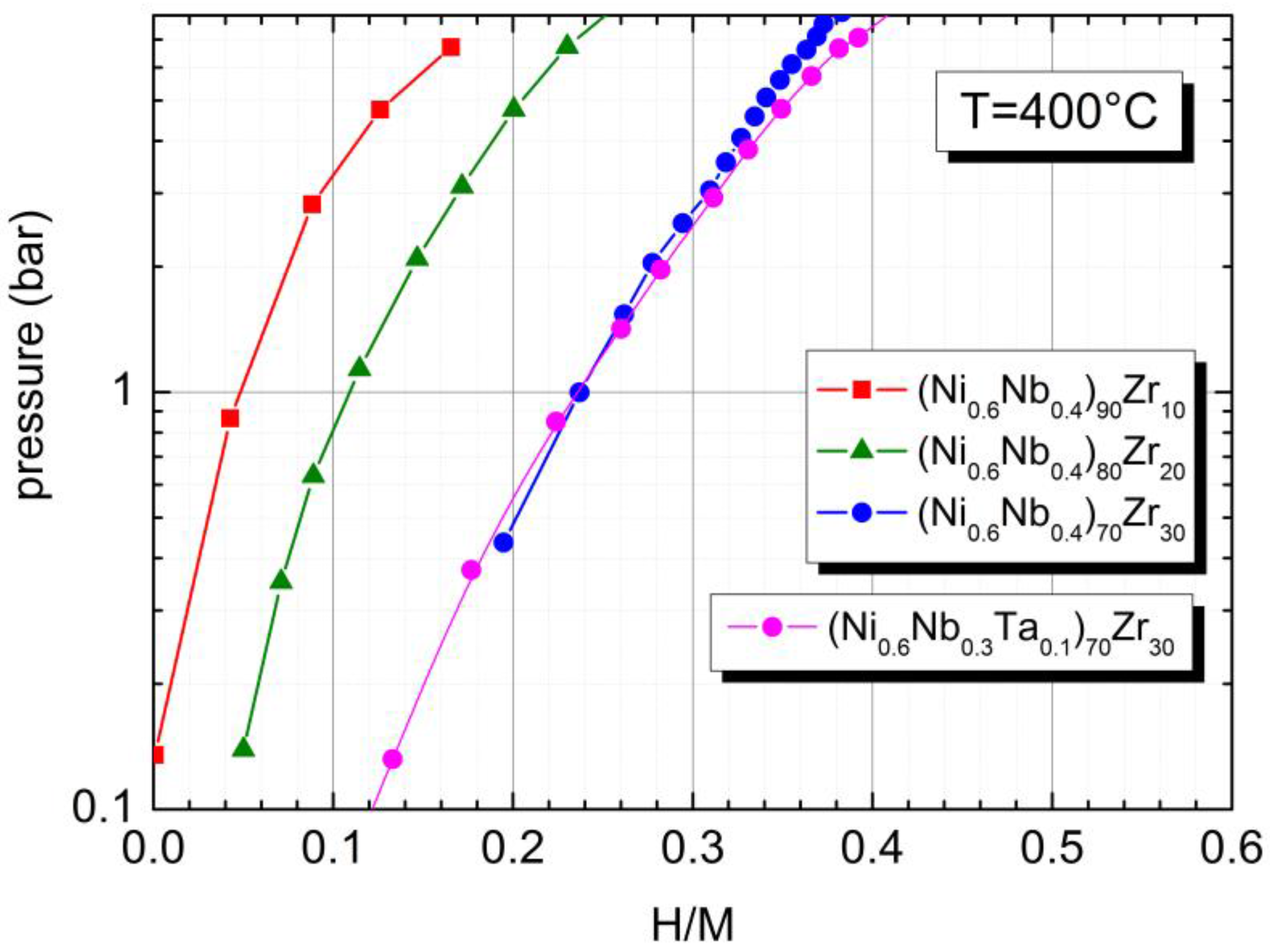
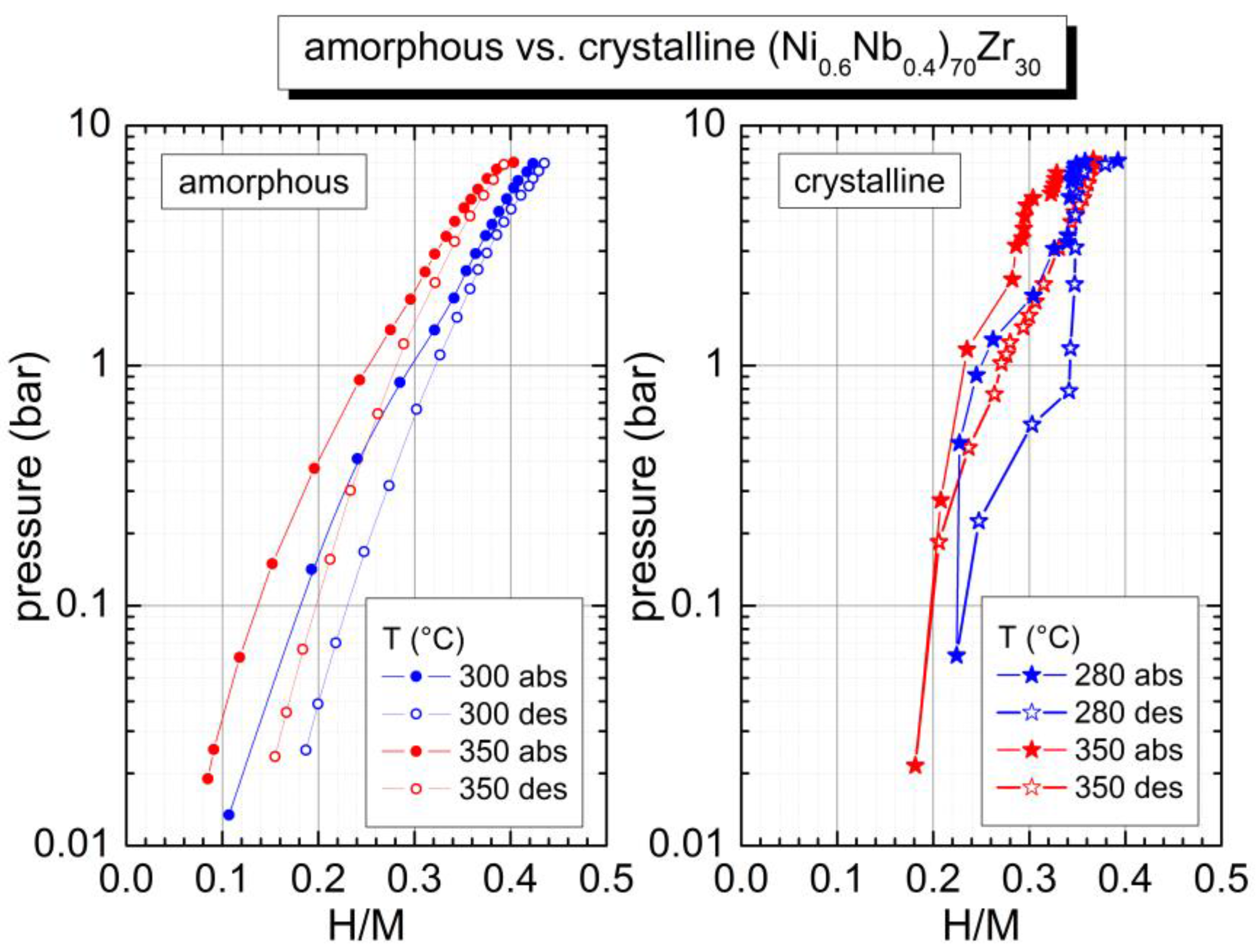
2.2. Hydrogen Sorption Properties in the Amorphous and in the Crystalline State
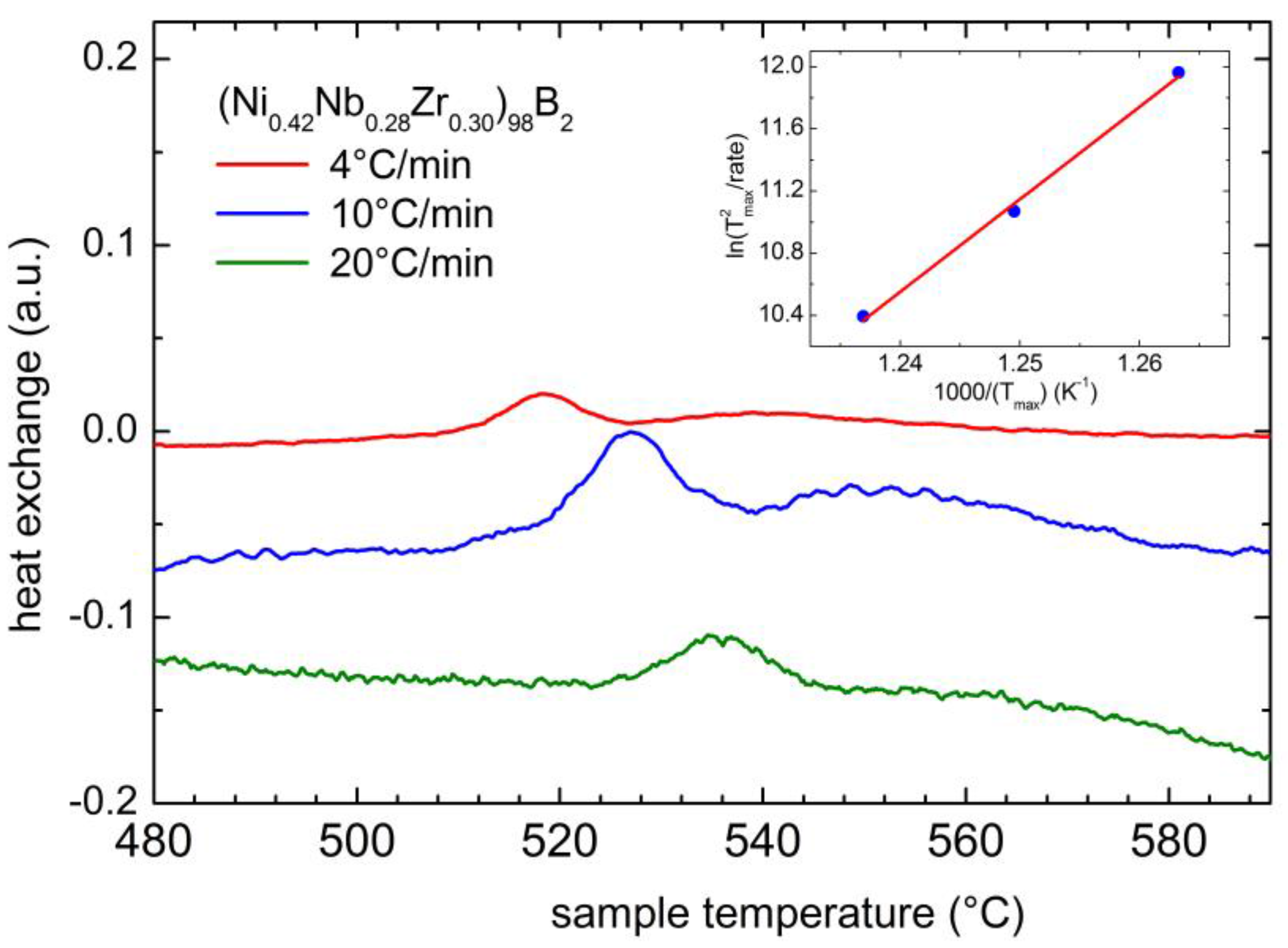
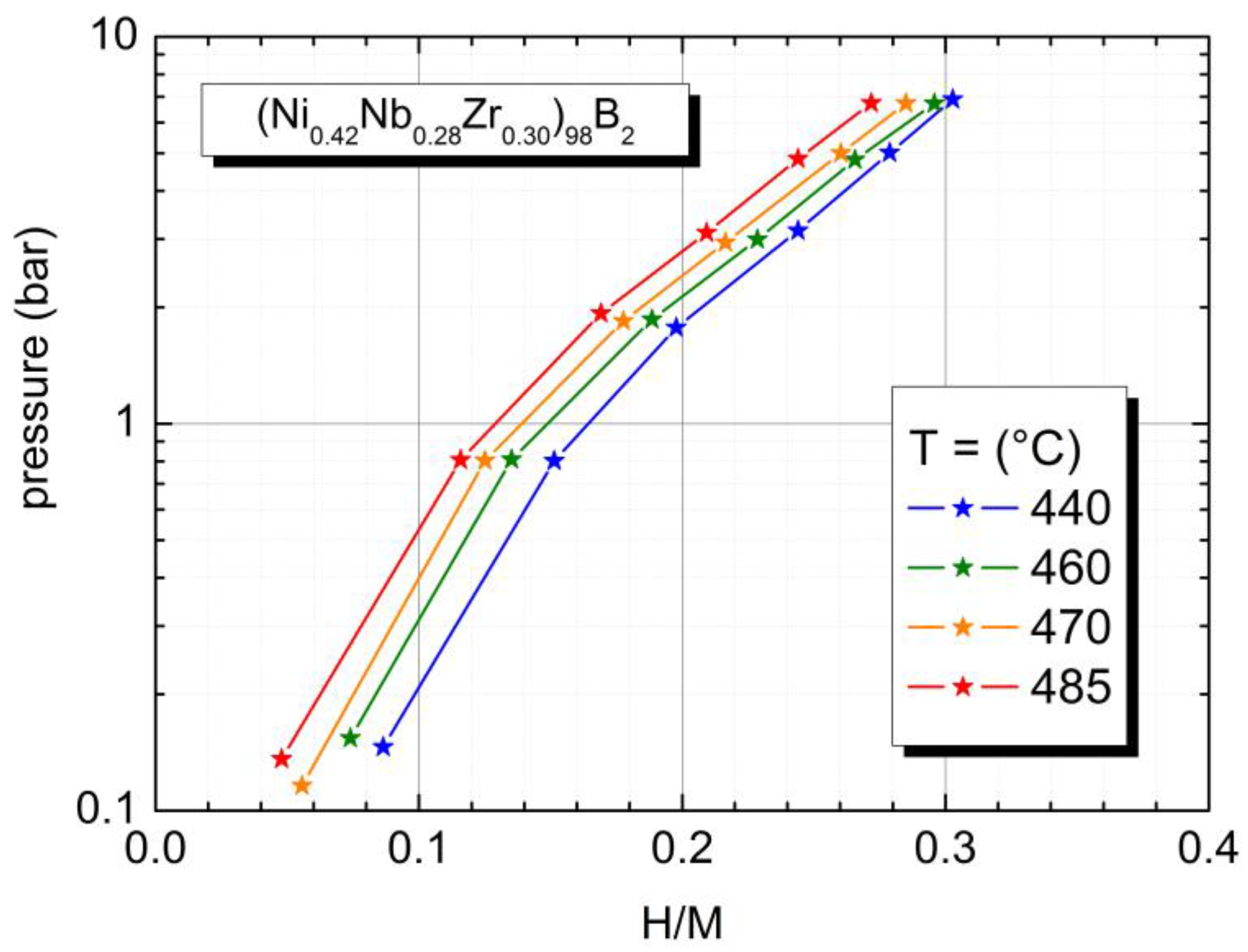
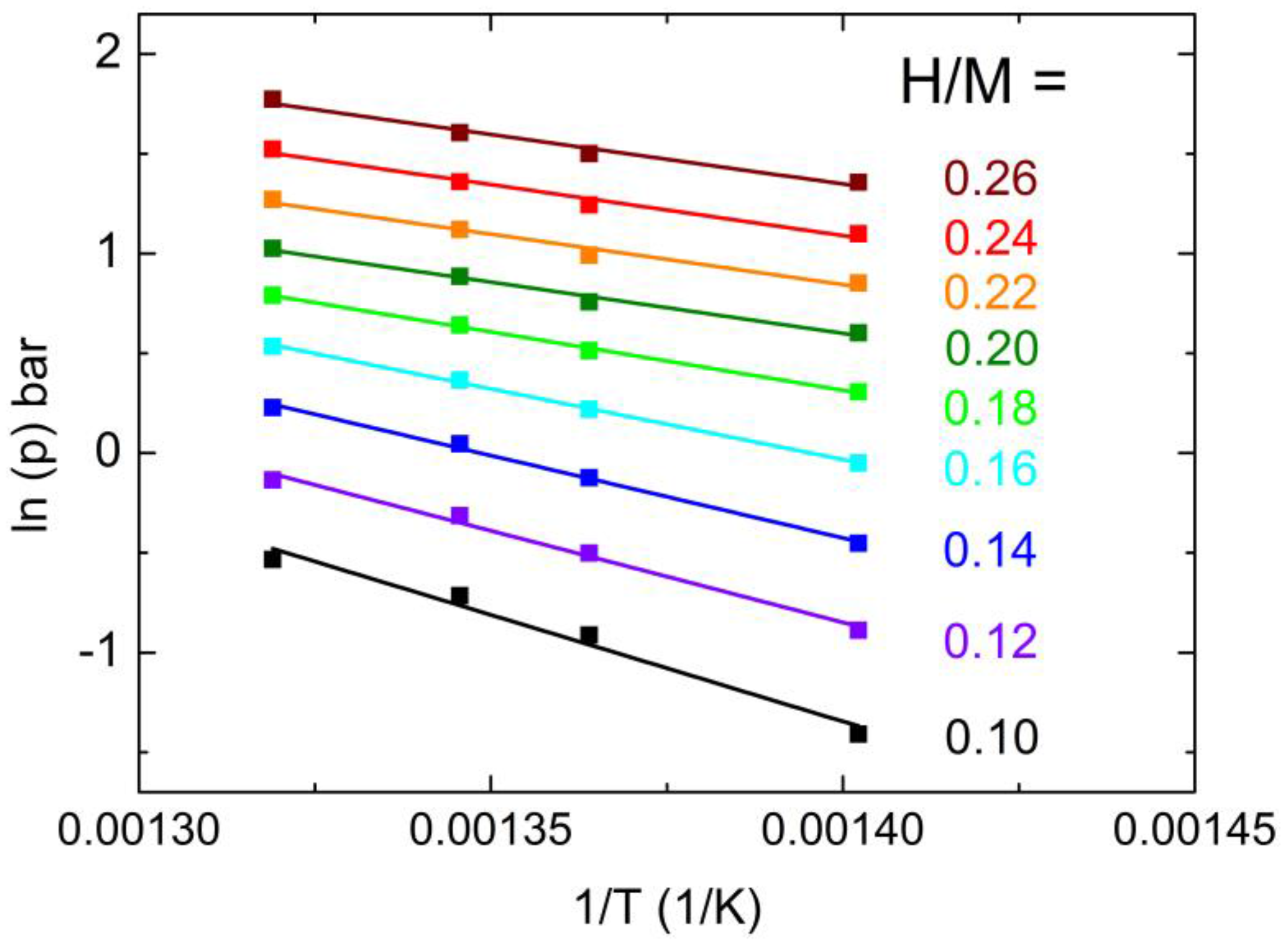
2.3. Physical Properties of a Boron Containing Sample
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ockwig, N.W.; Nenoff, T.M. Membranes for Hydrogen Separation. Chem. Rev. 2007, 107, 4078–4110. [Google Scholar] [CrossRef] [PubMed]
- Budd, P.M.; McKeown, N.B. Highly permeable polymers for gas separation membranes. Polym. Chem. 2010, 1, 63–68. [Google Scholar] [CrossRef]
- Yampolskii, Y. Polymer Gas Separation Membranes. Macromolecule 2012, 45, 3298–3311. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymer gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef]
- Razali, M.; Kim, J.F.; Attfield, M.; Budd, P.M.; Drioli, E.; Lee, Y.M.; Szekely, G. Sustainable wastewater treatment and recycling in membrane manufacturing. Green Chem. 2015, 17, 5196–5205. [Google Scholar] [CrossRef]
- Steward, S.A. Review of Hydrogen Isotope Permeability through Metals; U.S. National Laboratory Report: UCRL-53441; Lawrence Livermore National Lab.: Livermore, CA, USA, 1983. [Google Scholar]
- Dolan, M.D.; Dave, N.C.; Ilyushechkin, A.Y.; Morpeth, L.D.; McLennan, K.G. Composition and operation of hydrogen-selective amorphous alloy membrane. J. Membr. Sci. 2006, 285, 30–55. [Google Scholar] [CrossRef]
- Sarker, S.; Chandra, D.; Hirscher, M.; Dolan, M.; Isheim, D.; Wermer, J.; Viano, D.; Baricco, M.; Udovic, T.J.; Grant, D.; et al. Developments in the Ni-Nb-Zr Amorphous Alloy Membranes. Appl. Phys. A 2016, 122, 168:1–168:9. [Google Scholar] [CrossRef]
- Hara, S.; Hatakeyma, N.; Itoh, N.; Kimura, H.-M.; Inoue, A. Hydrogen permeation through amorphous Zr36-xHfxNi64 alloy membranes. J. Membr. Sci. 2003, 211, 149–156. [Google Scholar] [CrossRef]
- Hara, S.; Sakaki, K.; Itoh, N.; Kimura, H.-M.; Asami, K.; Inoue, A. An amorphous alloy membrane without noble metals for gaseous hydrogen separation. J. Membr. Sci. 2000, 164, 289–294. [Google Scholar] [CrossRef]
- Hara, S.; Hatakeyma, N.; Itoh, N.; Kimura, H.-M.; Inoue, A. Hydrogen permeation through palladium-coated amorphous Zr-M-Ni (M = Ti, Hf) alloy membranes. Desalination 2002, 144, 115–120. [Google Scholar] [CrossRef]
- Yamaura, S.-I.; Simpo, Y.; Okouchi, H.; Nishida, M.; Kajita, O.; Inoue, A. The Effect of Additional Elements on Hydrogen permeation Properties of Melt-spun Ni-Nb-Zr amorphous alloys. Mater. Trans. 2004, 45, 330–333. [Google Scholar] [CrossRef]
- Kimura, H.; Inoue, A.; Yamaura, S.-I.; Sasamori, K.; Nishida, M.; Shinpo, Y.; Okouchi, H. Thermal stability and mechanical properties of glassy and amorphous Ni-Nb-Zr alloys produced by rapid solidification. Mater. Trans. JIM 2003, 44, 1167–1171. [Google Scholar] [CrossRef]
- Suzuki, A.; Yukawa, H.; Nambu, T.; Matsumoto, Y.; Murata, Y. Consistent description of hydrogen permeability through metal membrane based on hydrogen chemical potential. Int. J. Hydrogen Energ. 2014, 39, 7919–7924. [Google Scholar] [CrossRef]
- Palumbo, O.; Trequattrini, F.; Hulyalkar, M.; Sarker, S.; Pal, N.; Chandra, D.; Flanagan, T.; Dolan, M.; Paolone, A. Hydrogen induced abrupt structural expansion at high temperatures of a Ni32Nb28Zr30Cu10 membrane for H2 purification. Membranes 2016, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Niu, J.; Zhang, Z.; Ge, W.; Shang, C.; Wang, Y. Effects of B addition on glass formation, mechanical properties and corrosion resistance of the Zr66.7-xNi33.3Bx (x = 0, 1, 3, and 5 at%) metallic glasses. JOM 2016, 68, 682–691. [Google Scholar] [CrossRef]
- Li, H.-W.; Yan, Y.; Orimo, S.-I.; Züttel, A.; Jensen, C.M. Recent Progress in Metal Borohydrides for Hydrogen Storage. Energies 2011, 4, 185–214. [Google Scholar] [CrossRef]
- Kim, S.-M.; Chandra, D.; Pal, N.K.; Dolan, M.D.; Chien, W.-M.; Talekar, A.; Lamb, J.; Paglieri, S.N.; Flanagan, T.B. Hydrogen permeability and crystallization kinetics in amorphous Ni-Nb-Zr alloys. Int. J. Hydrogen Energ. 2012, 37, 3904–3913. [Google Scholar] [CrossRef]
- Shimpo, Y.; Yamaura, S.I.; Nishida, M.; Kimura, H.; Inoue, A. Development of melt-spun Ni–Nb–Zr–Co amorphous alloy for high-performance hydrogen separating membrane. J. Membr. Sci. 2006, 286, 170–173. [Google Scholar] [CrossRef]
- Palumbo, O.; Trequattrini, F.; Pal, N.; Hulyalkar, M.; Sarker, S.; Chandra, D.; Flanagan, T.; Dolan, M.; Paolone, A. Hydrogen absorption properties of amorphous (Ni0.6Nb0.4-yTay)100-xZrx membranes. Prog. Nat. Sci. 2017. [Google Scholar] [CrossRef]
- Qiang, J.B.; Zhang, W.; Yamaura, S.; Inoue, A. Thermal Stability and Hydrogen Permeation of Ni42Zr30Nb28-xTax Amorphous Alloys. Mater. Trans. 2009, 50, 1236–1239. [Google Scholar] [CrossRef]
- Rabkin, E.; Skripnyuk, V.N. On pressure hysteresis during hydrogenation of metallic powders. Scripta Mater. 2003, 49, 477–483. [Google Scholar] [CrossRef]
- Kirchheim, R.; Sommer, F.; Schluckebier, G. Hydrogen in amorphous metal I. Acta Metall. 1982, 30, 1069–1078. [Google Scholar] [CrossRef]
- Karger, B.L.; Snyder, L.R.; Horvath, C. An Introduction to Separation Science; Wiley: New York, NY, USA, 1973. [Google Scholar]
- Jayalakshmi, S.; Choi, Y.G.; Kim, Y.C.; Kim, Y.B.; Fleury, E. Hydrogenation properties of Ni-Nb-Zr–Ta amorphous ribbons. Intermetallics 2010, 18, 1988–1993. [Google Scholar] [CrossRef]
- Paglieri, S.N.; Pal, N.K.; Dolan, M.D.; Kim, S.-M.; Chien, W.-M.; Lamb, J.; Chandra, D.; Hubbard, K.M.; Moore, D.P. Hydrogen permeability, thermal stability and hydrogen embrittlement of Ni-Nb-Zr and Ni-Nb-Ta-Zr amorphous alloy membranes. J. Membr. Sci. 2011, 378, 42–50. [Google Scholar] [CrossRef]
- Palumbo, O.; Brutti, S.; Trequattrini, F.; Sarker, S.; Dolan, M.; Chandra, D.; Paolone, A. Temperature dependence of the Elastic Modulus of (Ni0.6Nb0.4)1−xZrx Membranes: Effects of Thermal Treatments and Hydrogenation. Energies 2015, 8, 3944–3954. [Google Scholar] [CrossRef]
- Palumbo, O.; Paolone, A.; Rispoli, P.; Cantelli, R.; Autrey, T. Decomposition of NH3BH3 at sub-ambient pressures: A combined thermogravimetry–differential thermal analysis–mass spectrometry study. J. Power Sources 2010, 195, 1615–1618. [Google Scholar] [CrossRef]
- Palumbo, O.; Trequattrini, F.; Vitucci, F.M.; Bianchin, A.; Paolone, A. Study of the hydrogenation/dehydrogenation process in the Mg-Ni-C-Al system. J. Alloys Compd. 2015, 645, S239–S241. [Google Scholar] [CrossRef]
- Recommended Best Practices for the Characterization of Storage Properties of Hydrogen Storage Materials: V3.34 21 February 2012. Available online: https://energy.gov/eere/fuelcells/downloads/recommended-best-practices-characterization-storage-properties-hydrogen-0 (accessed on 9 February 2017).

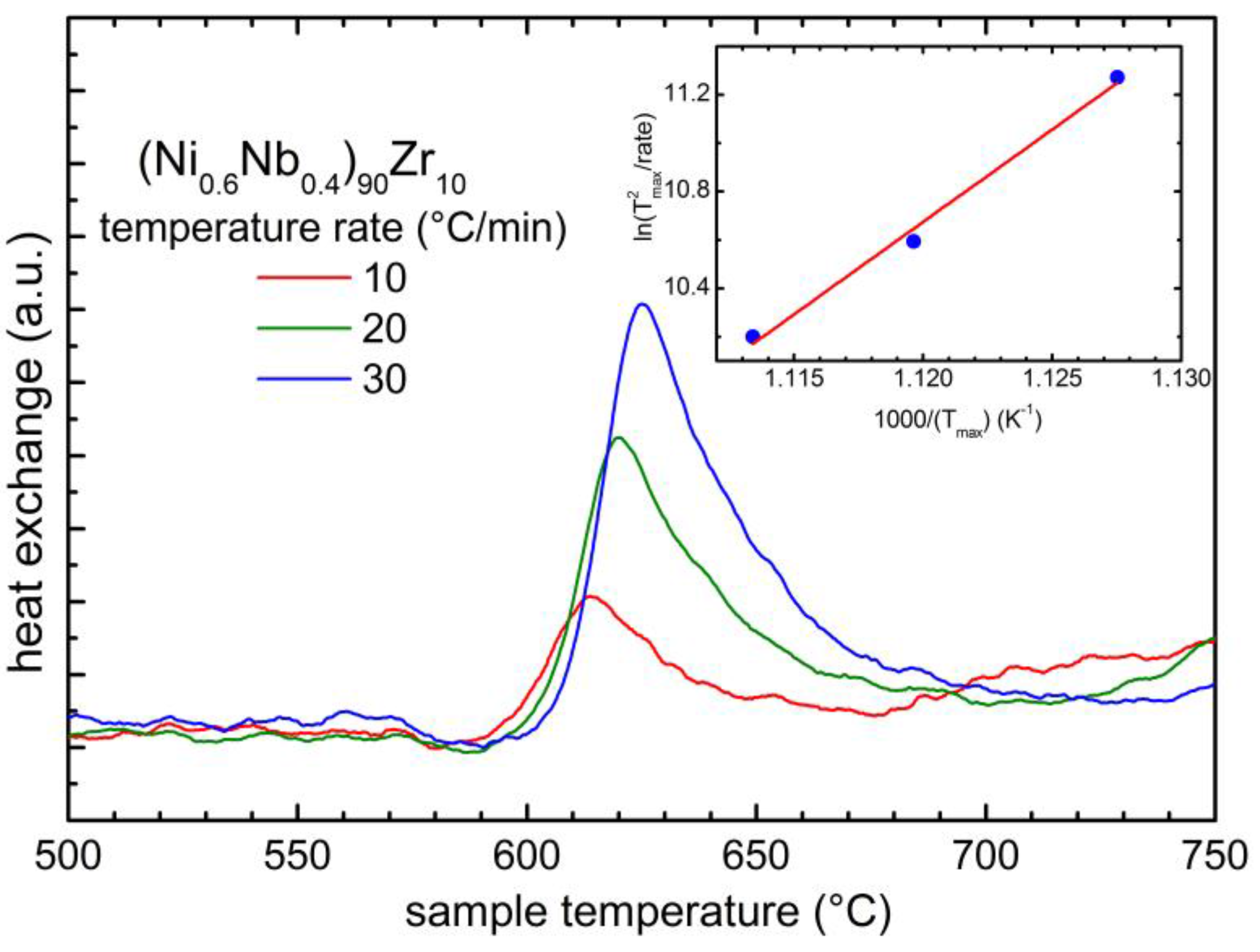
| X | Tc,onset (°C) |
|---|---|
| 0 | 648 ± 2 |
| 10 | 591 ± 3 |
| 20 | 547 ± 3 |
| 30 | 506 ± 2 |
| H/M | ΔHhyd (kJ/mol) |
|---|---|
| 0.10 | 89 ± 9 |
| 0.12 | 76 ± 5 |
| 0.14 | 69 ± 3 |
| 0.16 | 59 ± 3 |
| 0.18 | 49 ± 2 |
| 0.20 | 43 ± 3 |
| 0.22 | 43 ± 3 |
| 0.24 | 43 ± 3 |
| 0.26 | 43 ± 3 |
| Elemental Composition | Tc (°C) | Solubility (H/M) | ΔH (kJ/mol) |
|---|---|---|---|
| (Ni0.6Nb0.4)90Zr10 | 615 | 0.16 | -- |
| (Ni0.6Nb0.4)80Zr20 | 567 | 0.24 | 14 (H/M = 0.10) [20] 8 (H/M = 0.16) [20] |
| (Ni0.6Nb0.4)70Zr30 | 540 | 0.36 | 41 (H/M = 0.34) [20] 33 (H/M = 0.42) [20] |
| (Ni0.42Nb0.28Zr0.30)98B2 | 526 | 0.31 (T = 440 °C) | 89 (H/M = 0.10) 43 (H/M = 0.20) |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palumbo, O.; Trequattrini, F.; Sarker, S.; Hulyakar, M.; Pal, N.; Chandra, D.; Dolan, M.; Paolone, A. New Studies of the Physical Properties of Metallic Amorphous Membranes for Hydrogen Purification. Challenges 2017, 8, 4. https://doi.org/10.3390/challe8010004
Palumbo O, Trequattrini F, Sarker S, Hulyakar M, Pal N, Chandra D, Dolan M, Paolone A. New Studies of the Physical Properties of Metallic Amorphous Membranes for Hydrogen Purification. Challenges. 2017; 8(1):4. https://doi.org/10.3390/challe8010004
Chicago/Turabian StylePalumbo, Oriele, Francesco Trequattrini, Suchismita Sarker, Madhura Hulyakar, Narendra Pal, Dhanesh Chandra, Michael Dolan, and Annalisa Paolone. 2017. "New Studies of the Physical Properties of Metallic Amorphous Membranes for Hydrogen Purification" Challenges 8, no. 1: 4. https://doi.org/10.3390/challe8010004






