A Novel Glass Polyalkenoate Cement for Fixation and Stabilisation of the Ribcage, Post Sternotomy Surgery: An ex-Vivo Study
Abstract
:1. Introduction
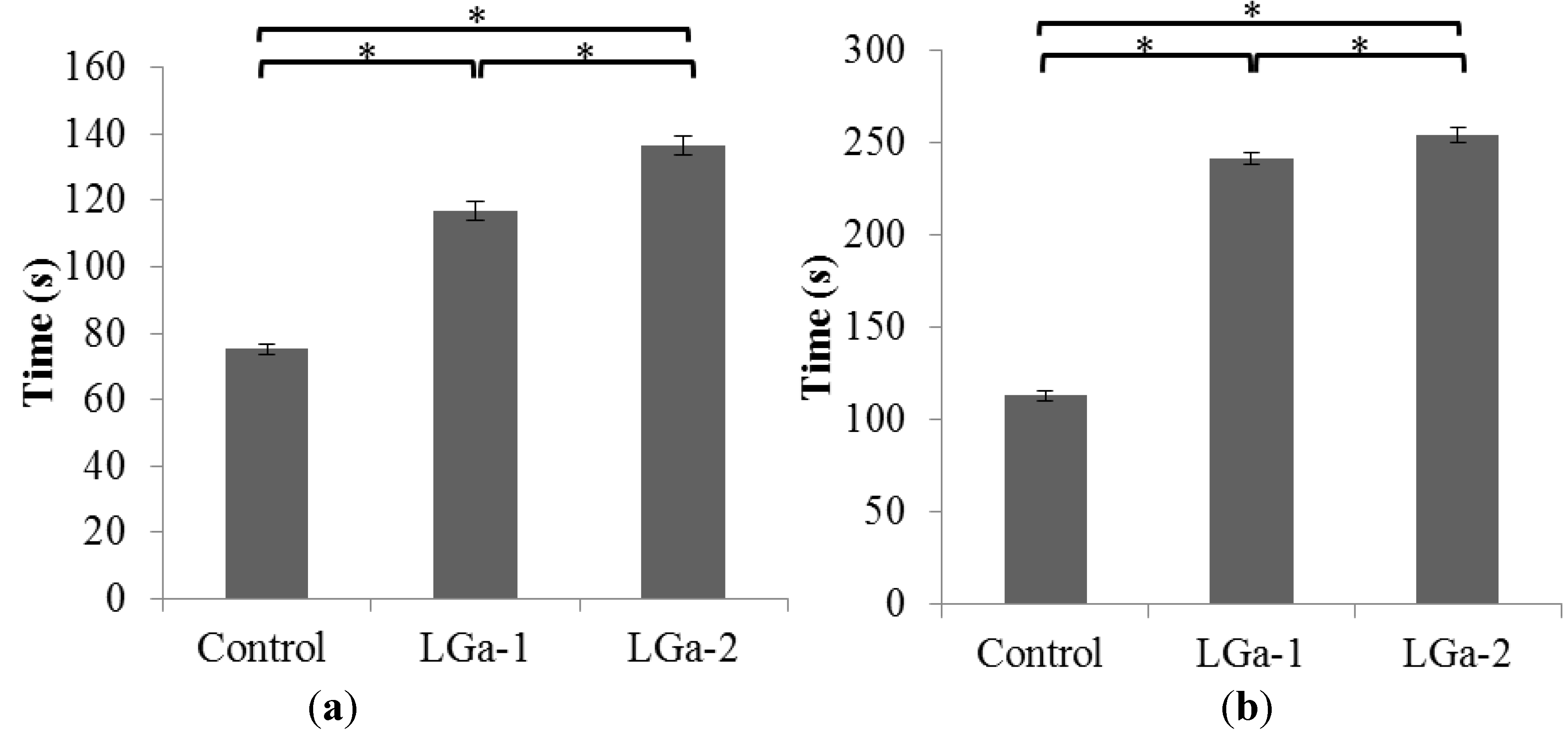
2. Results and Discussion








| Ion Species | Control | LGa-1 | LGa-2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 7 | 30 | 1 | 7 | 30 | 1 | 7 | 30 | |
| Ca2+ (wt %) | 17.1 | 22.4 | 32 | 10.4 | 19.6 | 19.2 | 11.1 | 17.3 | 16.7 |
| Zn2+ (wt %) | 55.5 | 45.6 | 48.2 | 38.9 | 37.2 | 37.7 | 23.0 | 23.8 | 24.5 |
| Si4+ (wt %) | 27.4 | 32.0 | 36.1 | 25.7 | 25.1 | 24.8 | 37.4 | 25.8 | 22.9 |
| Ga3+ (wt %) | 0.0 | 0.0 | 0.0 | 25.1 | 18.2 | 18.3 | 28.4 | 39.3 | 35.9 |
3. Experimental Section
3.1. Glass Synthesis
| Composition | Control | LGa-1 | LGa-2 |
|---|---|---|---|
| SiO2 | 0.48 | 0.48 | 0.48 |
| Ga2O3 | 0.00 | 0.08 | 0.16 |
| ZnO | 0.40 | 0.32 | 0.24 |
| CaO | 0.12 | 0.12 | 0.12 |
3.2. Glass Characterization
3.2.1. X-Ray Diffraction
3.2.2. Particle Size Analysis
3.3. Cement Preparation
3.4. Rheological Properties
3.4.1. Net Setting Time
3.4.2. Working Time
3.5. Cement Characterization
3.5.1. Fourier Transform Infrared Spectroscopy
3.5.2. Ion Release Studies
3.5.3. Contact Angle Measurements
3.5.4. Atomic Force Microscopy
3.6. Mechanical Properties
3.6.1. Compressive Strength
3.6.2. Flexural Strength
3.7. Ex-vivo Study
3.7.1. Sample Collection and Preparation


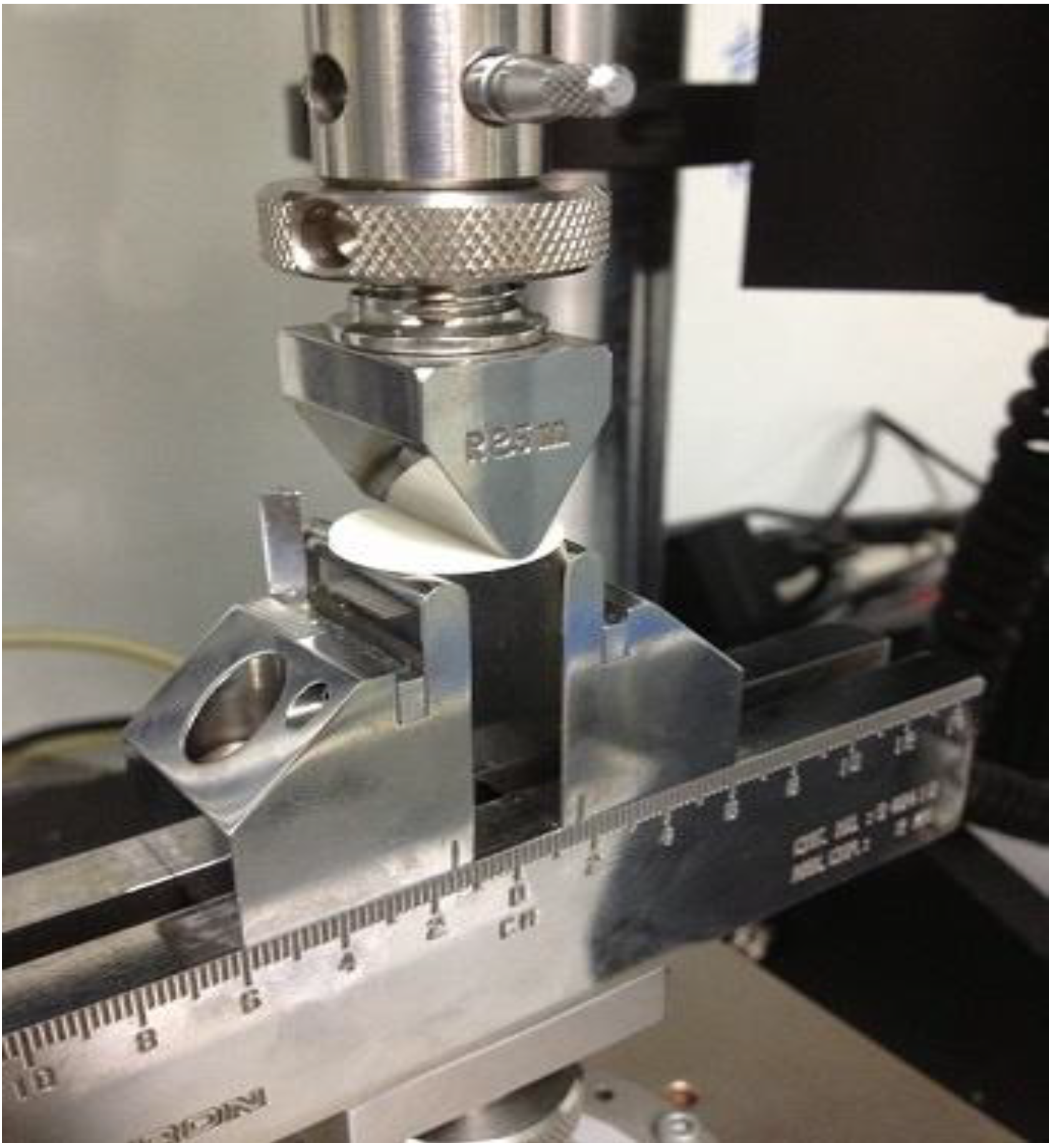
3.7.2. Tensile Failure Test

3.7.3. Scanning Electron Microscopy and Energy Dispersive x-ray Analysis
3.8. Statistical Analysis
4. Limitations of the Study
- During the FTIR experiment, it was not possible to identify a band below 650 cm−1 due to the limited wavelength band of the instrument ranging between 650 and 4000 cm−1. Further research is required since Ga3+ particles might absorb the IR at lower wavelengths around 450 cm−1 and there is no research into the IR absorption for the stretching vibration between Ga3+ and the PAA.
- Results from ion release studies formed the hypothesis that LGa-2 will contribute to a significant reduction in the post-operative complications, post sternotomy. However, further anti-bacterial and cell culture studies are required to test this hypothesis.
- Our ex-vivo study does not consider osteoporosis, bleeding and bone resorption; complications regularly encountered during sternotomy fixation and repair.
- The tensile tests were performed on small samples with an average cross-sectional-area of 350 mm2. However, the application of Ga-cements on the complete sternum would be expected to provide higher strength due to the bond that will be formed across the whole sternum. In the same context, due to the small sample size, authors performed several pilot studies to find the best way to perform the tensile failure test. The Authors found it difficult to test the samples by attaching the sternal ribs to the Instron jaws and hence this is considered as one of the main reasons for the low strengths achieved. The forces imposed by the sternal ribs distribute across the area while in our case, the force is exerted on a small cross-sectional area of the sternum.
5. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Dalton, M.L.; Connally, S.R.; Sealy, W.C. Julian’s reintroduction of Milton’s operation. Ann. Thorac. Surg. 1992, 53, 532–533. [Google Scholar] [CrossRef]
- Ott, D.A.; Cooley, D.A.; Solis, R.T.; Harrison, C.B., III. Wound complications after median sternotomy: A study of 61 patients from a consecutive series of 9279. Cardiovasc. Dis. 1980, 7, 104–111. [Google Scholar]
- Choukairi, F.; Ring, J.; Thekkudan, J.; Enoch, S. Management of sternal wound dehiscence. Wounds 2011, 7, 99–105. [Google Scholar]
- Fedak, P.W.; Kolb, E.; Borsato, G.; Frohlich, D.E.; Kasatkin, A.; Narine, K.; Akkarapaka, N.; King, K.M. Kryptonite bone cement prevents pathologic sternal displacement. Ann. Thorac. Surg. 2010, 90, 979–985. [Google Scholar] [CrossRef]
- Mossad, S.B.; Serkey, J.M.; Longworth, D.L.; Cosgrove, D.M.; Gordon, S.M. Coagulase-negative staphylococcal sternal wound infections after open heart operations. Ann. Thorac. Surg. 1997, 63, 395–401. [Google Scholar] [CrossRef]
- Jolly, S.; Flom, B.; Dyke, C. Cabled butterfly closure: A novel technique for sternal closure. Ann. Thorac. Surg. 2012, 94, 1359–1361. [Google Scholar] [CrossRef]
- Schimmer, C.; Reents, W.; Berneder, S.; Eigel, P.; Sezer, O.; Scheld, H.; Sahraoui, K.; Gansera, B.; Deppert, O.; Rubio, A.; et al. Prevention of sternal dehiscence and infection in high-risk patients: A prospective randomized multicenter trial. Ann. Thorac. Surg. 2008, 86, 1897–1904. [Google Scholar] [CrossRef]
- López Almodóvar, L.F.; Bustos, G.; Lima, P.; Cañas, A.; Paredes, I.; Buendía, J.A. Transverse plate fixation of sternum: A new sternal-sparing technique. Ann. Thorac. Surg. 2008, 86, 1016–1017. [Google Scholar] [CrossRef]
- Schimmer, C.; Ozkur, M.; Sinha, B.; Hain, J.; Gorski, A.; Hager, B.; Leyh, R. Gentamicin-collagen sponge reduces sternal wound complications after heart surgery: A controlled, prospectively randomized, double-blind study. J. Thorac. Cardiovasc. Surg. 2012, 143, 194–200. [Google Scholar] [CrossRef]
- Mavros, M.N.; Mitsikostas, P.K.; Alexiou, V.G.; Peppas, G.; Falagas, M.E. Gentamicin collagen sponges for the prevention of sternal wound infection: A meta-analysis of randomized controlled trials. J. Thorac. Cardiovasc. Surg. 2012, 144, 1235–1240. [Google Scholar] [CrossRef]
- Lee, T.Y.; Estrera, A.L.; Safi, H.J.; Khalil, K.G. Total sternal reconstruction using a titanium plate-supported methyl methacrylate sandwich. Ann. Thorac. Surg. 2007, 84, 664–666. [Google Scholar] [CrossRef]
- Porter, K.; Roplekar, R.; Mohanna, P. A five year audit study on deep sternal wound infections and associated dehiscence post median Sternotomy: An analysis of patient outcome, risk factors and a proposed management strategy. J. Surg. 2012, 10, S16–S17. [Google Scholar]
- Cahalin, L.P.; LaPier, T.K.; Shaw, D.K. Sternal precautions: Is it time for change? Precautions versus restrictions—A review of literature and recommendations for revision. Cardiopulm. Phys. Ther. J. 2011, 22, 5–15. [Google Scholar]
- Alhalawani, A.M.F.; Towler, M.R. A review of sternal closure techniques. J. Biomater. Appl. 2013, 28, 483–497. [Google Scholar] [CrossRef]
- Wilson, A.D.; Nicholson, J.W. Acid-Base Cements—Their Biomedical and Industrial Applications (Chemistry of Solid State Materials); West, A.R., Baxter, H., Eds.; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Moshaverinia, A.; Roohpour, N.; Chee, W.W.L.; Schricker, S.R. A review of powder modifications in conventional glass-ionomer dental cements. J. Mater. Chem. 2011, 21, 1319–1328. [Google Scholar]
- Moshaverinia, A.; Roohpour, N.; Chee, W.W.L.; Schricker, S.R. A review of polyelectrolyte modifications in conventional glass-ionomer dental cements. J. Mater. Chem. 2012, 22, 2824–2833. [Google Scholar]
- Wren, A.W.; Coughlan, A.; Laffir, F.R.; Towler, M.R. Comparison of a SiO2–CaO–ZnO–SrO glass polyalkenoate cement to commercial dental materials: Glass structure and physical properties. J. Mater. Sci.: Mater. Med. 2013, 24, 271–280. [Google Scholar] [CrossRef]
- Dickey, B.T.; Kehoe, S.; Boyd, D. Novel adaptations to zinc–silicate glass polyalkenoate cements: The unexpected influences of germanium based glasses on handling characteristics and mechanical properties. J. Mech. Behav. Biomed. Mater. 2013, 23, 8–21. [Google Scholar] [CrossRef]
- Wren, A.W.; Keenan, T.; Coughlan, A.; Laffir, F.R.; Boyd, D.; Towler, M.R.; Hall, M.M. Characterisation of Ga2O3–Na2O–CaO–ZnO–SiO2 bioactive glasses. J. Mater. Sci. 2013, 48, 3999–4007. [Google Scholar] [CrossRef]
- Boyd, D.; Clarkin, O.M.; Wren, A.W.; Towler, M.R. Zinc-based glass polyalkenoate cements with improved setting times and mechanical properties. Acta. Biomater. 2008, 4, 425–431. [Google Scholar] [CrossRef]
- Nicholson, J.W. Chemistry of glass-ionomer cements: A review. Biomaterials 1998, 19, 485–494. [Google Scholar] [CrossRef]
- Wren, A.W.; Hansen, J.P.; Hayakawa, S.; Towler, M.R. Aluminium-free glass polyalkenoate cements: Ion release and in vitro antibacterial efficacy. J. Mater. Sci. Mater. Med. 2013, 24, 1167–1178. [Google Scholar] [CrossRef]
- Wren, A.W.; Coughlan, A.; Placek, L.; Towler, M.R. Gallium containing glass polyalkenoate anti-cancerous bone cements: glass characterization and physical properties. J. Mater. Sci. Mater. Med. 2012, 23, 1823–1833. [Google Scholar]
- Brook, I.M.; Hatton, P.V. Glass-ionomers: Bioactive implant materials. Biomaterials 1998, 19, 565–571. [Google Scholar] [CrossRef]
- Ana, I.D.; Matsuya, S.; Ohta, M.; Ishikawa, K. Effects of added bioactive glass on the setting and mechanical properties of resin-modified glass ionomer cement. Biomaterials 2003, 24, 3061–3067. [Google Scholar] [CrossRef]
- Wakayama, I.; Song, K.J.; Nerurkar, V.R.; Yoshida, S.; Garruto, R.M. Slow dendritic transport of dissociated mouse hippocampal neurons exposed to aluminum. Brain Res. 1997, 748, 237–240. [Google Scholar] [CrossRef]
- Polizzi, S.; Pira, E.; Ferrara, M.; Bugiani, M.; Papaleo, A.; Albera, R.; Palmi, S. Neurotoxic effects of aluminium among foundry workers and Alzheimer's disease. Neurotoxicology 2002, 23, 761–774. [Google Scholar] [CrossRef]
- Exley, C. A molecular mechanism of aluminium-induced Alzheimer’s disease? J. Inorg. Biochem. 1999, 76, 133–140. [Google Scholar] [CrossRef]
- Guo, G.W.; Liang, Y.X. Aluminum-induced apoptosis in cultured astrocytes and its effect on calcium homeostasis. Brain Res. 2001, 888, 221–226. [Google Scholar] [CrossRef]
- Carter, D.H.; Sloan, P.; Brook, I.M.; Hatton, P.V. Role of exchanged ions in the integration of ionomeric (glass polyalkenoate) bone substitutes. Biomaterials 1997, 18, 459–466. [Google Scholar] [CrossRef]
- Paul, A. Chemistry of glasses, 2nd ed.; Springer: New York, USA, 1989. [Google Scholar]
- Yamaguchi, M.; Matsui, T. Stimulatory effect of zinc-chelating dipeptide on deoxyribonucleic acid synthesis in osteoblastic MC3T3-E1 cells. Peptides 1996, 17, 1207–1211. [Google Scholar] [CrossRef]
- Ovesen, J.; Moller-Madsen, B.; Thomsen, J.S.; Danscher, G.; Mosekilde, L. The positive effects of zinc on skeletal strength in growing rats. Bone 2001, 29, 565–570. [Google Scholar] [CrossRef]
- Boyd, D.; Li, H.; Tanner, D.A.; Towler, M.R.; Wall, J.G. The antibacterial effects of zinc ion migration from zinc-based glass polyalkenoate cements. J. Mater. Sci. Mater. Med. 2006, 17, 489–494. [Google Scholar]
- Warrell, R.P.; Bockman, R.S.; Coonley, C.J.; Isaacs, M.; Staszewski, H. Gallium nitrate inhibits calcium resorption from bone and is effective treatment for cancer-related hypercalcemia. J. Clin. Invest. 1984, 73, 1487–1490. [Google Scholar] [CrossRef]
- DeVita, V.T.; Hellman, S.; Rosenberg, S.A. Important Advances in Oncology; Lippincott: Philadelphia, USA, 1988; pp. 205–220. [Google Scholar]
- Bernstein, L.R. Mechanisms of Therapeutic Activity for Gallium. Pharmacol. Rev. 1998, 50, 665–682. [Google Scholar]
- Ortega, R.; Suda, A.; Devès, G. Nuclear microprobe imaging of gallium nitrate in cancer cells. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Inter Mater. At. 2003, 210, 364–367. [Google Scholar] [CrossRef]
- Chitambar, C.R. Medical applications and toxicities of gallium compounds. Int. J. Environ. Res. Public Health 2010, 7, 337–361. [Google Scholar] [CrossRef]
- Collery, P.; Keppler, B.; Madoulet, C.; Desoize, B. Gallium in cancer treatment. Crit. Rev. Oncol. Hematol. 2002, 42, 283–296. [Google Scholar] [CrossRef]
- Valappil, S.; Ready, D.; Abou Neel, E.; Pickup, D.; Chrzanowski, W.; O’Dell, L.; Newport, R.; Smith, M.; Wilson, M.; Knowles, J. Antimicrobial Gallium-doped Phosphate-based Glasses. Adv. Funct. Mater. 2008, 18, 732–741. [Google Scholar] [CrossRef] [Green Version]
- Warrell, R.P.; Alcock, N.W.; Bockman, R.S. Gallium nitrate inhibits accelerated bone turnover in patients with bone metastases. J. Clin. Oncol. 1987, 5, 292–298. [Google Scholar]
- Bastosa, T.O.; Soares, B.; Cisalpino, P.; Mendesc, I.; dosSantosa, R.; Beraldoc, H. Coordination to gallium(III) strongly enhances the potency of 2-pyridineformamide thiosemicarbazones against Cryptococcus opportunistic fungi. Microbio. Res. 2010, 165, 573–577. [Google Scholar] [CrossRef]
- Valappil, S.P.; Ready, D.; Abou Neel, E.A.; Pickup, D.M.; O’Dell, L.A.; Chrzanowski, W.; Pratten, J.; Newport, R.J.; Smith, M.E.; Wilson, M.; Knowles, J.C. Controlled delivery of antimicrobial gallium ions from phosphate-based glasses. Acta. Biomater. 2009, 5, 1198–1210. [Google Scholar] [CrossRef]
- Da Silva, J.G.; Azzolini, L.S.; Wardell, S.M.S.V.; Wardell, J.L.; Beraldo, H. Increasing the antibacterial activity of gallium(III) against Pseudomonas aeruginosa upon coordination to pyridine-derived thiosemicarbazones. Polyhedron 2009, 28, 2301–2305. [Google Scholar] [CrossRef]
- Banin, E.; Lozinski, A.; Brady, K.M.; Berenshtein, E.; Butterfield, P.W.; Moshe, M.; Chevion, M.; Greenberg, E.P.; Banin, E. The potential of desferrioxamine-gallium as an anti-Pseudomonas therapeutic agent. Proc. Natl. Acad. Sci. USA 2008, 105, 16761–16766. [Google Scholar] [CrossRef]
- Hart, M.M.; Smith, C.F.; Yancey, S.T.; Adamson, R.H. Toxicity and antitumor activity of gallium nitrate and periodically related metal salts. J. Natl. Cancer Inst. 1971, 47, 1121–1127. [Google Scholar]
- Coughlan, A.; Scanlon, K.; Mahon, B.P.; Towler, M.R. Zinc and silver glass polyalkenoate cements: An evaluation of their antibacterial nature. Biomed. Mater. Eng. 2012, 20, 99–106. [Google Scholar]
- Rasey, J.S.; Nelson, N.J.; Larson, S.M. Tumor cell toxicity of stable gallium nitrate: Enhancement by transferrin and protection by iron. Eur. J. Cancer Clin. Oncol. 1982, 18, 661–668. [Google Scholar] [CrossRef]
- Fedak, P.W.; Kieser, T.M.; Maitland, A.M.; Holland, M.; Kasatkin, A.; LeBlanc, P.; Kim, J.K.; King, K.M. Adhesive-enhanced sternal closure to improve postoperative functional recovery: a pilot, randomized controlled trial. Ann. Thorac. Surg. 2011, 92, 1444–1450. [Google Scholar] [CrossRef]
- di Nuzzo, G.; Luongo, M.; Parlato, C.; Moraci, A. Cranial reconstruction using bioabsorbable calcified triglyceride bone cement. J. Craniofac. Surg. 2010, 21, 1170–1174. [Google Scholar] [CrossRef]
- Lee, K.; Shamie, A.; Halevi, L.; Lee, S.; Wang, J. P129. The efficacy of kryptonite in a rat model of posterolateral spine fusion. Spine J. 2005, 5, S172. [Google Scholar]
- Mastrobuoni, S.; Dennie, C.; Rubens, F.; Lapierre, H.; Mesana, T.; Ruel, M. 405 kryptonite, a novel bone cement for primary sternal closure: Mechanistic study using computerized tomography. Can. J. Cardiol. 2012, 28, S251. [Google Scholar]
- Driessen, M.D.; Miller, T.M.; Grassian, V.H. Photocatalytic oxidation of trichloroethylene on zinc oxide: characterization of surface-bound and gas-phase products and intermediates with FT-IR spectroscopy. J. Mol. Catal. A Chem. 1998, 131, 149–156. [Google Scholar] [CrossRef]
- Crisp, S.; Pringuer, M.A.; Wardleworth, D.; Wilson, A.D. Reaction in glass ionomer cements: II. An infrared spectroscopic study. J. Dent. Res. 1974, 53, 1414–1419. [Google Scholar] [CrossRef]
- Wilson, A.D. Alumino-silicate polyacrylic acid and related cements. Brit. Poly J. 1974, 6, 165–179. [Google Scholar]
- Powis, D.R.; Follerås, T.; Merson, S.A.; Wilson, A.D. Improved adhesion of a glass ionomer cement to dentin and enamel. J. Dent. Res. 1982, 61, 1416–1422. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, F.; Zhang, J.; Xia, L. Converting layered zinc acetate nanobelts to one-dimensional structured ZnO nanoparticle aggregates and their photocatalytic activity. Nanoscale. Res. Lett. 2008, 3, 201–204. [Google Scholar] [CrossRef]
- Rajamathi, J.T.; Britto, S.; Rajamathi, M. Synthesis and anion exchange reactions of a layered copper–zinc hydroxy double salt, Cu1-6Zn0-4(OH)3(OAc)∙H2O. J. Chem. Sci. 2005, 117, 629–633. [Google Scholar] [CrossRef]
- Matsuya, S.; Matsuya, Y.; Ohta, M. Structure of bioactive glass and its application to glass ionomer cement. Dent. Mater. J. 1999, 18, 155–166. [Google Scholar] [CrossRef]
- Maltsev, A.A.; Shevelkov, V.F. Infrared absorption spectra of aluminum, gallium and indium suboxidevapors. Some regularities in frequencies of oscillations of suboxides of elements of the III group and evaluation of molecular constants of B2O. National Technical Information Service: Springerfield, MO, USA, 1972; pp. 89–92. [Google Scholar]
- Cook, W.D. Dental polyelectrolyte cements. I. Chemistry of the early stages of the setting reaction. Biomaterials 1982, 3, 232–236. [Google Scholar] [CrossRef]
- Young, A.M.; Sherpa, A.; Pearson, G.; Schottlander, B.; Waters, D.N. Use of Raman spectroscopy in the characterisation of the acid-base reaction in glass-ionomer cements. Biomaterials 2000, 21, 1971–1979. [Google Scholar] [CrossRef]
- Tsybeskov, L.; Vandyshev, J.V.; Fauchet, P.M. Blue emission in porous silicon: Oxygen-related photoluminescence. Phys. Rev. B Condens. Matter 1994, 49, 7821–7824. [Google Scholar] [CrossRef]
- Tajmir-Riahi, H.A.; Naoui, M.; Ahmad, R. A Comparative Study of Calf-Thymus DNA Binding Trivalent Al, Ga, Cr and Fe Ions in Aqueous Solution. In Metal Ions in Biology and Medicine; John Libbey Eurotext: Paris, France, 1992; pp. 98–101. [Google Scholar]
- Moschèn; Schweizer, K.; Wagner, C.A.; Geis-Gerstorfer, J.; Lang, F. Effects of gallium and mercury ions on transport systems. J. Dent. Res. 2001, 80, 1753–1757. [Google Scholar] [CrossRef]
- Mittal, K.L. Contact Angle, Wettability and Adhesion, 4th ed.; CRC: New York, NY, USA, 2006. [Google Scholar]
- Casha, A.R.; Yang, L.; Cooper, G.J. Measurement of chest wall forces on coughing with the use of human cadavers. J. Thorac. Cardiovasc. Surg. 1999, 118, 1157–1158. [Google Scholar] [CrossRef]
- ISO 9917–1:2007. Dentistry–Water–Based Cements–Part 1: Powder/liquid acid-base cements. ISO: Geneva, Switzerland. Available online: http://www.iso.org/iso/catalogue_detail.htm?csnumber=45818 (Accessed on 1 May 2013).
- Kao, E.C.; Culbertson, B.M.; Xie, D. Preparation of glass ionomer cement using N-acryloyl-substituted amino acid monomers-Evaluation of physical properties. Dent. Mater. 1996, 12, 44–51. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Chee, W.W.; Brantley, W.A.; Schricker, S.R. Surface properties and bond strength measurements of N-vinylcaprolactam (NVC)-containing glass-ionomer cements. J. Prosthet. Dent. 2011, 105, 185–193. [Google Scholar] [CrossRef]
- Williams, J.A.; Billington, R.W.; Pearson, G.J. The effect of the disc support system on biaxial tensile strength of a glass ionomer cement. Dent. Mater. 2002, 18, 376–379. [Google Scholar] [CrossRef]
- Steiner, M.; Ramp, W.K. Short-term storage of freshly harvested bone. J. Oral Maxillofac. Surg. 1988, 46, 868–871. [Google Scholar] [CrossRef]
- Van Haaren, E.H.; van der Zwaard, B.C.; van der Veen, A.J.; Heyligers, I.C.; Wuisman, P.I.; Smit, T.H. Effect of long-term preservation on the mechanical properties of cortical bone in goats. Acta. Orthopaedica. 2008, 79, 708–716. [Google Scholar] [CrossRef]
- Öhman, C.; Dall’Ara, E.; Baleani, M.; Van Sint Jan, S.; Viceconti, M. The effects of embalming using a 4% formalin solution on the compressive mechanical properties of human cortical bone. Clin. Biomech. 2008, 23, 1294–1298. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Alhalawani, A.M.F.; Curran, D.J.; Pingguan-Murphy, B.; Boyd, D.; Towler, M.R. A Novel Glass Polyalkenoate Cement for Fixation and Stabilisation of the Ribcage, Post Sternotomy Surgery: An ex-Vivo Study. J. Funct. Biomater. 2013, 4, 329-357. https://doi.org/10.3390/jfb4040329
Alhalawani AMF, Curran DJ, Pingguan-Murphy B, Boyd D, Towler MR. A Novel Glass Polyalkenoate Cement for Fixation and Stabilisation of the Ribcage, Post Sternotomy Surgery: An ex-Vivo Study. Journal of Functional Biomaterials. 2013; 4(4):329-357. https://doi.org/10.3390/jfb4040329
Chicago/Turabian StyleAlhalawani, Adel M.F., Declan J. Curran, Belinda Pingguan-Murphy, Daniel Boyd, and Mark R. Towler. 2013. "A Novel Glass Polyalkenoate Cement for Fixation and Stabilisation of the Ribcage, Post Sternotomy Surgery: An ex-Vivo Study" Journal of Functional Biomaterials 4, no. 4: 329-357. https://doi.org/10.3390/jfb4040329





