2.1. Formulation and Characterization of “Smart” Particles
The ability of P(EAA-
co-BMA)-
b-PNASI-
g-P(HMA-
co-TMAEMA)comb-like polymer to condense anti-Bcl-2 siRNA molecules into pH-sensitive particles was analyzed using the standard gel retardation assay. Comb-like polymer was mixed with a fixed amount (0.7 µg) of anti-Bcl-2 siRNA molecules at different N/P (+/−) ratios. The loaded RNA molecules were encapsulated into stable particles as a result of the electrostatic interaction between the cationic quaternary amine (N/+) groups of the TMAEMA monomers and the anionic phosphate (P/−) groups of the RNA molecules. Results showed that P(EAA-
co-BMA)-
b-PNASI-
g-P(HMA-
co-TMAEMA) comb-like polymer successfully complexed the loaded siRNA molecules at all N/P ratios, which is indicated by their retention in the loading wells, while free siRNA molecules migrate towards the positive electrode (
Scheme IB).
Results show that the polymer can condense siRNA molecules at lower N/P ratios compared to those of other acrylic acid-based polymers [
12,
13], thus reducing the amount of comb-like polymer needed to complex a given dose of therapeutic nucleic acids and consequently minimizing the toxicity commonly associated with excess cationic carriers [
14,
15]. Size and surface charge of the particles prepared at N/P ratios of 2.5/1, 4/1, and 5/1 were measured using dynamic light scattering and zeta potential measurements, respectively. Results show that particles prepared at N/P ratios of 2.5/1, 4/1, and 5/1 have an average size of 245, 373, and 313 nm, respectively (
Figure 1). These particles carry positive surface charges of 22.4, 24.9, and 32.3 mV at N/P ratios of 2.5/1, 4/1, and 5/1, respectively. siPORT amine, which is a commercial polymer-based transfection reagent was used a positive control in our studies. Results show that the size of “smart” particles is slightly larger than siPORT amine-based complexes, while the surface charges are relatively similar. The size of “smart” particles proved to fall below the size cut off size of 400–600 nm for tumor vasculature [
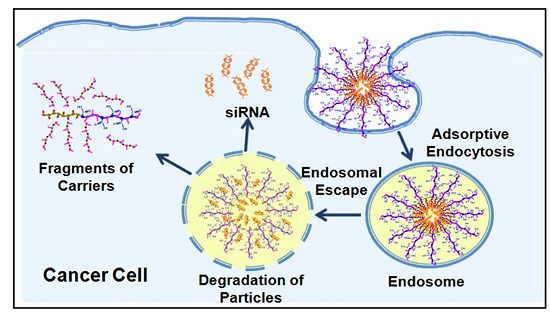
16], which when coupled with their cationic nature will facilitate particle’s interaction and internalization into target cells via adsorptive endocytosis.
Figure 1.
The size and zeta potential of siPORT amine-based complexes and “smart” particles prepared by complexation of P(EAA-co-BMA)-b-PNASI-g-P(HMA-co-TMAEMA) comb-like polymer with 1.14 µg of anti-GAPDH siRNA at N/P (+/−) ratios of 2.5/1, 4/1, and 5/1. The plotted results are the average ± the standard error of the mean of two independent experiments each carried out in triplicates.
Figure 1.
The size and zeta potential of siPORT amine-based complexes and “smart” particles prepared by complexation of P(EAA-co-BMA)-b-PNASI-g-P(HMA-co-TMAEMA) comb-like polymer with 1.14 µg of anti-GAPDH siRNA at N/P (+/−) ratios of 2.5/1, 4/1, and 5/1. The plotted results are the average ± the standard error of the mean of two independent experiments each carried out in triplicates.
2.2. Uptake of “Smart” Particles by HeLa and UM-SCC-17B Cells
We evaluated the internalization of fluorescently-labeled “smart” particles prepared at different N/P ratios into HeLa cervical carcinoma and UM-SCC-17B head and neck squamous cell carcinoma using flow cytometry. Complexes prepared using commercial siPORT amine transfection agents were used as positive controls.
Figure 2 shows that “smart” particles are efficiently (>97%) taken up into HeLa and UM-SCC-17B cells at N/P ratios higher than 2.5/1, and siPORT amine-based complexes also showed high internalization (~100%) into both cell types. The relatively lower uptake into HeLa cells using particles prepared at N/P of 1.5/1 could be due to the different cell membrane compositions between HeLa and UM-SCC-17B cancer cells [
17]. These results indicate that our “smart” particles can be successfully internalized by HeLa and UM-SCC-17B cancer cells through adsorptive endocytosis due to the positive surface charge of these particles. Earlier research showed that the increase of particle’s positive surface charge is typically associated with toxicity or low transfection efficiency due to poor decomplexation of the loaded DNA/RNA molecules [
18,
19,
20]. Consequently, we decided to evaluate the transfection efficiency of the particles prepared at N/P ratios of 2.5/1, 4/1 and 6/1, which will have a sufficient number of cationic TMAEMA residues to complex the loaded siRNA molecules, while eliminating cellular toxicity without preventing cytoplasmic decomplexation of the loaded siRNA molecules.
Figure 2.
Percentage of HeLa and UM-SCC-17B cancer cells that internalize siPORT amine-based complexes and “smart” nanoparticles prepared by complexation of P(EAA-co-BMA)-b-PNASI-g-P(HMA-co-TMAEMA) comb-like polymer with 1.14 µg of fluorescently-labeled anti-GAPDH siRNA at different N/P (+/−) ratios upon incubation for 6 h in a serum-free culture medium. Results are the average + the standard error of the mean of three replicates.
Figure 2.
Percentage of HeLa and UM-SCC-17B cancer cells that internalize siPORT amine-based complexes and “smart” nanoparticles prepared by complexation of P(EAA-co-BMA)-b-PNASI-g-P(HMA-co-TMAEMA) comb-like polymer with 1.14 µg of fluorescently-labeled anti-GAPDH siRNA at different N/P (+/−) ratios upon incubation for 6 h in a serum-free culture medium. Results are the average + the standard error of the mean of three replicates.
2.3. Effect of “Smart” Particles on GAPDH Expression
The ability of “smart” particles to achieve functional delivery of complexed siRNA molecules into the cytoplasm of HeLa and UM-SCC-17B cancer cells was evaluated based on their ability to selectively knockdown GAPDH gene expression at the mRNA and protein levels. We utilized the KDalert GAPDH assay kit to measure the changes in GAPDH protein level upon incubation with particles that encapsulate the anti-GAPDH siRNA molecules. These particles were compared to those encapsulating a scrambled siRNA sequence. We utilized siPORT amine-based complexes encapsulating an equal dose of anti-GAPDH siRNA molecules as a positive control to determine the maximum level of knockdown that can be achieved using robust commercial transfection agents. As shown in
Figure 3A, particles prepared at N/P ratios of 2.5/1 and 4/1 induced 30% and 39% knockdown in GAPDH protein expression in HeLa cells, respectively. This knockdown is better than siPORT amine-based complexes, which inhibited GAPDH protein expression by only 21% with toxicity, since scrambled siRNA molecules also induced 53% GAPDH reduction compared to untreated cells. “Smart” particles prepared at an N/P ratio of 6/1 also induced 39% knockdown in GAPDH protein expression, which was associated with non-specific toxicity possibly due to the use of excess cationic carrier. To eliminate their toxicity, we decided to use particles prepared at 2.5/1 and 4/1 ratios for the rest of the experiments in HeLa cells. We further utilized qRT-PCR to evaluate the changes in GAPDH mRNA level upon incubation with particles that encapsulated the anti-GAPDH siRNA molecules. As can be seen in
Figure 3B, particles prepared at N/P ratios of 2.5/1 and 4/1 induced 40% and 60% knockdown in GAPDH mRNA expression in HeLa cells, respectively, while siPORT amine-based complexes induced 50% knockdown.
Figure 3.
Effect of siPORT amine-based complexes and “smart” nanoparticles prepared by complexation of P(EAA-co-BMA)-b-PNASI-g-P(HMA-co-TMAEMA) comb-like polymer with 1.14 µg of the anti-GAPDH siRNA (+) or scrambled siRNA (−) at N/P (+/−) ratios of 2.5/1, 4/1, and 6/1 (A,B) or 5/1 (C,D) on GAPDH protein (A,C) and mRNA levels (B,D) in HeLa cervical cancer cells (A,B) and in UM-SCC-17B head and neck cancer cells (C,D). Levels of GAPDH mRNA are normalized to the levels of β-actin. Results are the average + the standard error of the mean of five replicates. Statistical difference between particles encapsulating anti-GAPDH siRNA (+) and scrambled siRNA (−) was evaluated using paired t test where * denotes p ≤ 0.05, ** denotes p ≤ 0.01, and *** denotes p ≤ 0.005.
Figure 3.
Effect of siPORT amine-based complexes and “smart” nanoparticles prepared by complexation of P(EAA-co-BMA)-b-PNASI-g-P(HMA-co-TMAEMA) comb-like polymer with 1.14 µg of the anti-GAPDH siRNA (+) or scrambled siRNA (−) at N/P (+/−) ratios of 2.5/1, 4/1, and 6/1 (A,B) or 5/1 (C,D) on GAPDH protein (A,C) and mRNA levels (B,D) in HeLa cervical cancer cells (A,B) and in UM-SCC-17B head and neck cancer cells (C,D). Levels of GAPDH mRNA are normalized to the levels of β-actin. Results are the average + the standard error of the mean of five replicates. Statistical difference between particles encapsulating anti-GAPDH siRNA (+) and scrambled siRNA (−) was evaluated using paired t test where * denotes p ≤ 0.05, ** denotes p ≤ 0.01, and *** denotes p ≤ 0.005.
In comparison, particles prepared at N/P ratios of 2.5/1 and 4/1 did not induce significant knockdown in GAPDH protein expression in UM-SCC-17B (data not shown), and this may be caused by the delayed acidification of endosomal pH thus causing inefficient cytoplasmic release of siRNA molecules in head and neck cells [
21]. Therefore, we evaluated the transfection efficiency of particles prepared at an N/P ratio of 5/1, which induced 14% knockdown in GAPDH protein expression in UM-SCC-17B cells without toxicity while siPORT amine-based complexes induce 36% GAPDH protein knockdown with toxicity (
Figure 3C). The low transfection efficiency of “smart” particles in UM-SCC-17B cells is perhaps a result of poor decomplexation of siRNA from the carrier after endosomal escape since a higher amount of polymer was used at an N/P ratio of 5/1 than 2.5/1 and 4/1.
Figure 3D shows that particles prepared at 5/1 ratio induced 38% knockdown in GAPDH mRNA expression in UM-SCC-17B cells, compared to siPORT amine-based complexes that induced 54% knockdown. These particles proved to transfect HeLa and UM-SCC-17B cells more effectively than the commercial transfection agent without toxicity.
The observed difference in GAPDH knockdown induced by “smart” particles loaded with anti-GAPDH siRNA in HeLa and UM-SCC-17B cells can be explained by the difference in intracellular pH between these cells lines. The literature shows that normal cells generally have neutral cytosolic (pH 7.2) and acidic endosomal (pH 6.0) and lysosomal (pH 5.0) environment [
21,
22,
23]. Whereas, many tumor cells have an acidified cytosol and more alkaline endosomes/lysosomes with both pH values is around 6.7 [
21,
22,
23]. Although the reason for alkalinization of the endosomal and lysosomal compartments remains elusive, the elevated organelle pH in tumor cells has been confirmed in many reports [
21,
23,
24,
25] and proved to dramatically reduce the transfection efficiency of non-viral vectors in tumor cells [
26]. Similarly, low GAPDH knockdown in UM-SCC-17B cancer cells can be attributed to endosomal alkalinization, which will reduce the hydrolysis of the hydrazone linkages connecting the membrane-active P(HMA-
co-TMAEMA) grafts to the polymer backbone. Incomplete release of P(HMA-
co-TMAEMA) grafts will reduce the net disruption of the endosomal membrane, which will limit the delivery of the loaded anti-GAPDH siRNA into the cytoplasm and diminish the associated GAPDH knockdown. This explains lower GAPDH knockdown observed in UM-SCC-17B cancer cells compared to that observed with HeLa cells. Nevertheless, our results collectively show that “smart” comb-like polymers can function as an effective carrier for enhancing the cytoplasmic delivery of siRNA molecules.
2.4. Effect of “Smart” Particles on Bcl-2 Expression
The therapeutic activity of “smart” particles was evaluated based on their ability to selectively knockdown Bcl-2 gene expression at both the mRNA and protein levels. We utilized qRT-PCR to measure the changes in Bcl-2 mRNA level upon incubation with particles that encapsulate the anti-Bcl-2 siRNA and compare to those encapsulating a scrambled siRNA sequence. We utilized siPORT amine-based complexes encapsulating an equal dose of anti-Bcl-2 siRNA as a positive control to determine the maximum level of knockdown that can be achieved using robust commercial transfection agents. As shown in
Figure 4A, particles prepared at N/P ratios of 2.5/1 and 4/1 selectively induced 50% and 60% knockdown in Bcl-2 mRNA expression in HeLa cells, respectively. This knockdown is better than siPORT amine-based complexes which only inhibited Bcl-2 mRNA expression by 40% accompanied with toxicity. In
Figure 4B, particles prepared at N/P ratios of 2.5/1 and 4/1 induced 79% and 81% knockdown in Bcl-2 protein expression in HeLa cells, respectively, while siPORT amine-based complexes induced only 64% knockdown. For UM-SCC-17B cells, in order to exclude the issue of poor decomplexation of particles prepared at an N/P ratio of 5/1, we decreased the N/P ratio to 2.5/1 but increased the incubation time to solve the possible problem of delayed endosomal pH drop. As shown in
Figure 5A, Bcl-2 mRNA expression was inhibited by 30%, 40%, and 20% after treatment with particles for 48, 72, and 96 h, respectively. Inhibition of Bcl-2 protein expression was only shown after treatment for 72 h by 30% knockdown (
Figure 5B). The results suggested that the therapeutic effects of anti-Bcl-2 siRNA delivered by using comb-like polymer is most effective after treatment for 72 h in UM-SCC-17B cancer cells, which matches earlier studies [
27] and suggests the transfection condition should be optimized in different cell types. In summary, these results prove our “smart” comb-like polymer can be utilized as effective carriers for the delivery of therapeutic siRNA molecules into multiple mammalian epithelial cells.
Figure 4.
Effect of siPORT amine-based complexes and “smart” nanoparticles prepared by complexation of P(EAA-co-BMA)-b-PNASI-g-P(HMA-co-TMAEMA) comb-like polymer with 1.14 µg of the anti-Bcl-2 siRNA (+) or scrambled siRNA (−) at N/P (+/−) ratios of 2.5/1 and 4/1 on Bcl-2 mRNA (A) and protein (B) levels after treatment for 48 h in HeLa cervical cancer cells. Levels for Bcl-2 mRNA are normalized to the levels of 18S rRNA. Results are the average + the standard error of the mean of five replicates. Statistical difference between particles encapsulating anti-Bcl-2 siRNA (+) and scrambled siRNA (−) was evaluated using paired t test where ** denotes p ≤ 0.01 and *** denotes p ≤ 0.005. Levels for Bcl-2 protein are quantified by Image J software (NIH, Bethesda, MD, USA) and normalized to the levels of β-actin.
Figure 4.
Effect of siPORT amine-based complexes and “smart” nanoparticles prepared by complexation of P(EAA-co-BMA)-b-PNASI-g-P(HMA-co-TMAEMA) comb-like polymer with 1.14 µg of the anti-Bcl-2 siRNA (+) or scrambled siRNA (−) at N/P (+/−) ratios of 2.5/1 and 4/1 on Bcl-2 mRNA (A) and protein (B) levels after treatment for 48 h in HeLa cervical cancer cells. Levels for Bcl-2 mRNA are normalized to the levels of 18S rRNA. Results are the average + the standard error of the mean of five replicates. Statistical difference between particles encapsulating anti-Bcl-2 siRNA (+) and scrambled siRNA (−) was evaluated using paired t test where ** denotes p ≤ 0.01 and *** denotes p ≤ 0.005. Levels for Bcl-2 protein are quantified by Image J software (NIH, Bethesda, MD, USA) and normalized to the levels of β-actin.
Figure 5.
Effect of “smart” nanoparticles prepared by complexation of P(EAA-co-BMA)-b-PNASI-g-P(HMA-co-TMAEMA) comb-like polymer with 1.14 µg of the anti-Bcl-2 siRNA (+) or scrambled siRNA (−) at an N/P (+/−) ratio of 2.5/1 on Bcl-2 mRNA (A) and protein (B) levels at 48, 72, and 96 h in UM-SCC-17B head and neck cancer cells. Levels for Bcl-2 mRNA are normalized to the levels of 18S rRNA. Results are the average + the standard error of the mean of five replicates. Statistical difference between particles encapsulating anti-Bcl-2 siRNA (+) and scrambled siRNA (−) was evaluated using paired t test where * denotes p ≤ 0.05. Levels for Bcl-2 protein are quantified by Image J software (NIH, Bethesda, MD, USA) and normalized to the levels of β-actin.
Figure 5.
Effect of “smart” nanoparticles prepared by complexation of P(EAA-co-BMA)-b-PNASI-g-P(HMA-co-TMAEMA) comb-like polymer with 1.14 µg of the anti-Bcl-2 siRNA (+) or scrambled siRNA (−) at an N/P (+/−) ratio of 2.5/1 on Bcl-2 mRNA (A) and protein (B) levels at 48, 72, and 96 h in UM-SCC-17B head and neck cancer cells. Levels for Bcl-2 mRNA are normalized to the levels of 18S rRNA. Results are the average + the standard error of the mean of five replicates. Statistical difference between particles encapsulating anti-Bcl-2 siRNA (+) and scrambled siRNA (−) was evaluated using paired t test where * denotes p ≤ 0.05. Levels for Bcl-2 protein are quantified by Image J software (NIH, Bethesda, MD, USA) and normalized to the levels of β-actin.