Hyperthermia Using Antibody-Conjugated Magnetic Nanoparticles and Its Enhanced Effect with Cryptotanshinone
Abstract
:1. Introduction
2. Results and Discussion
2.1. HeLa Cell Growth in the Presence of CH11 Antibody or MNP/Antibody Complexes
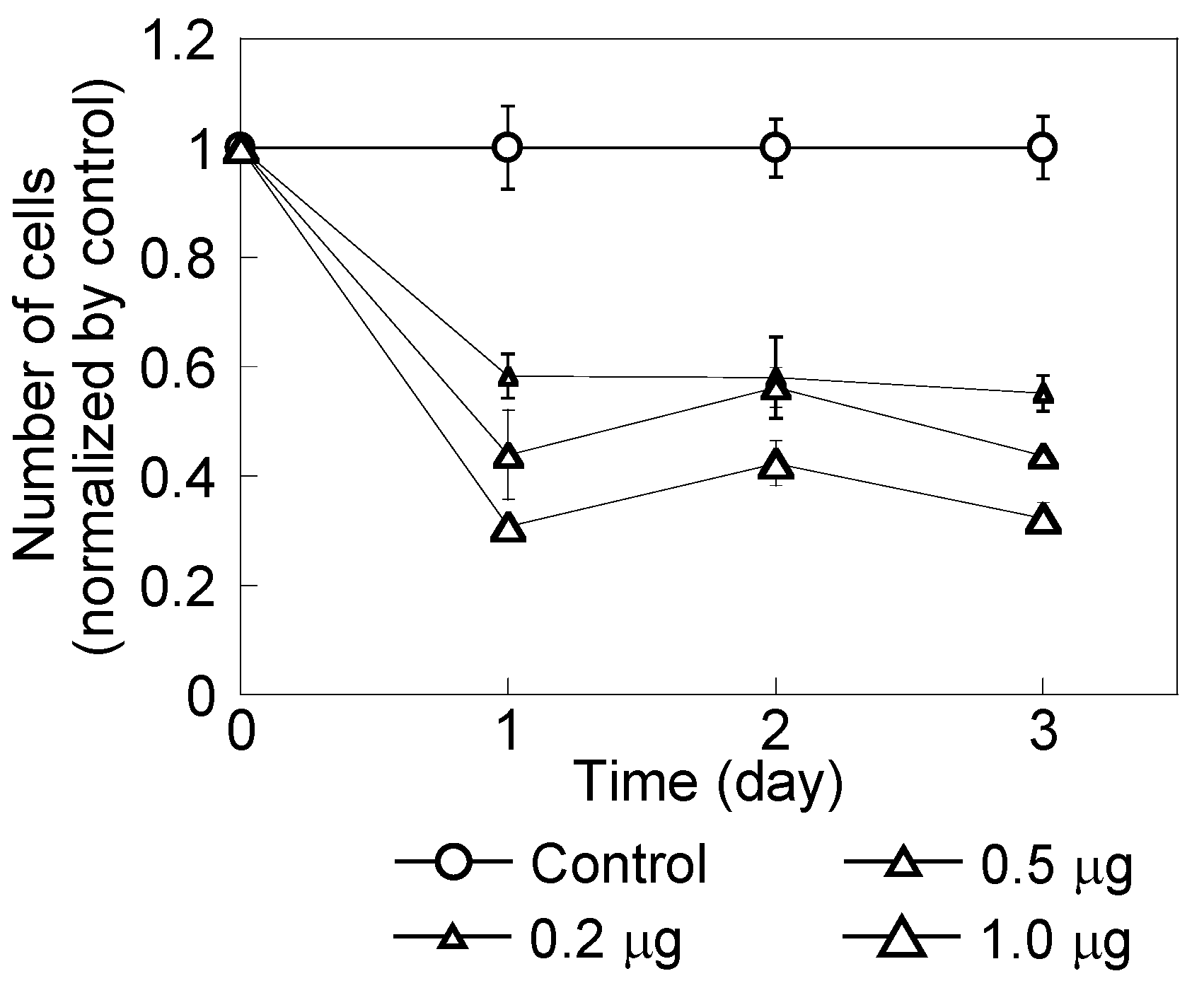

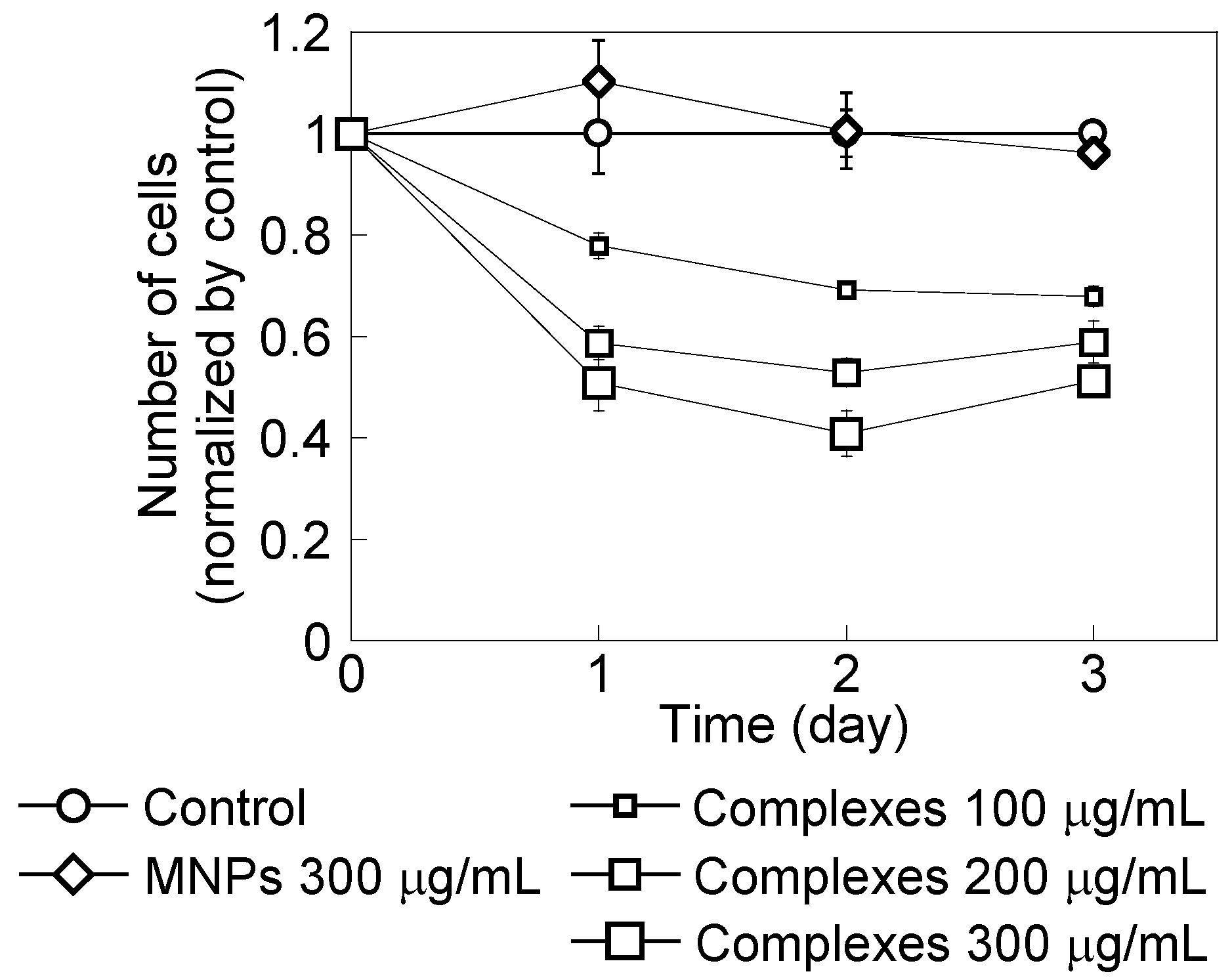
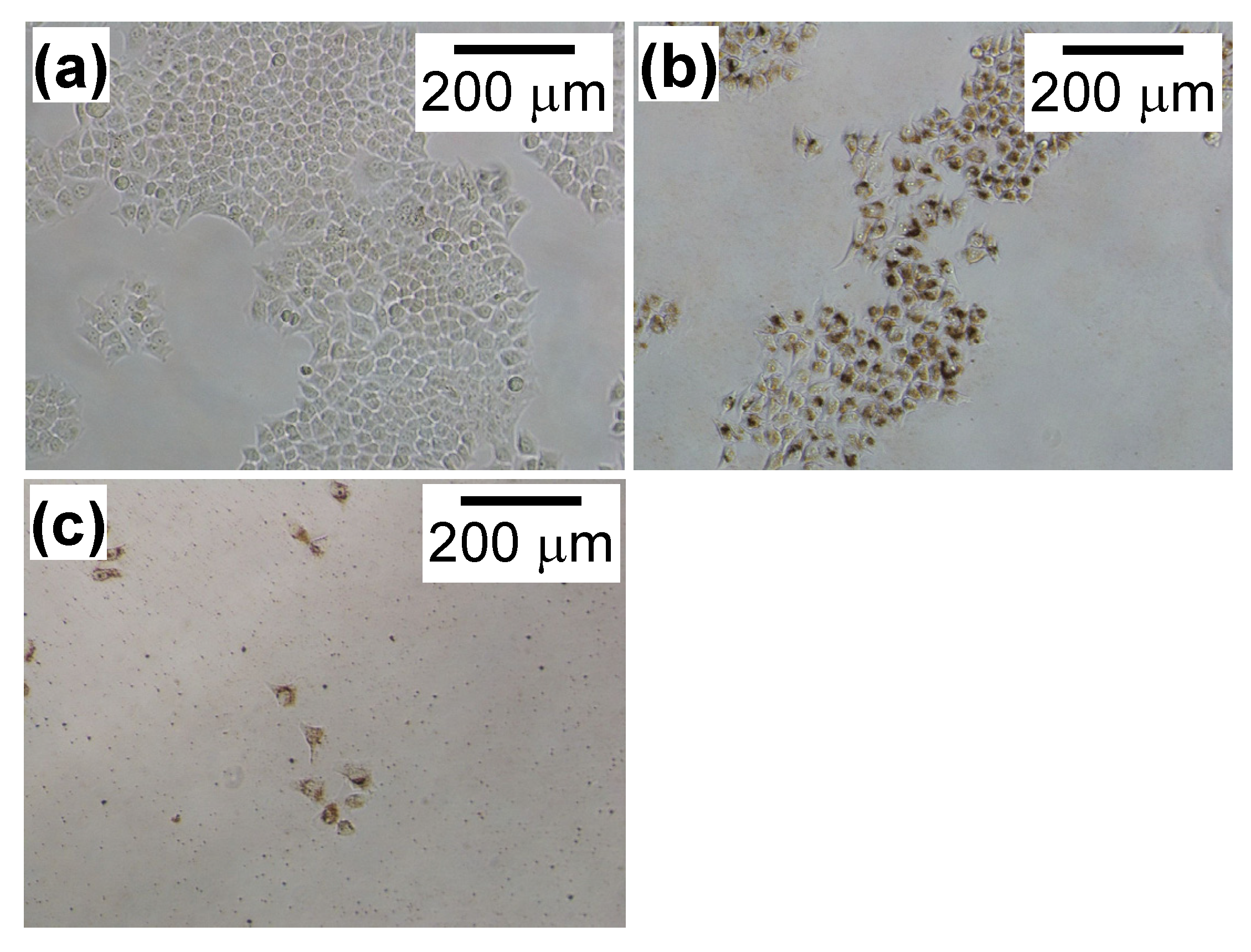
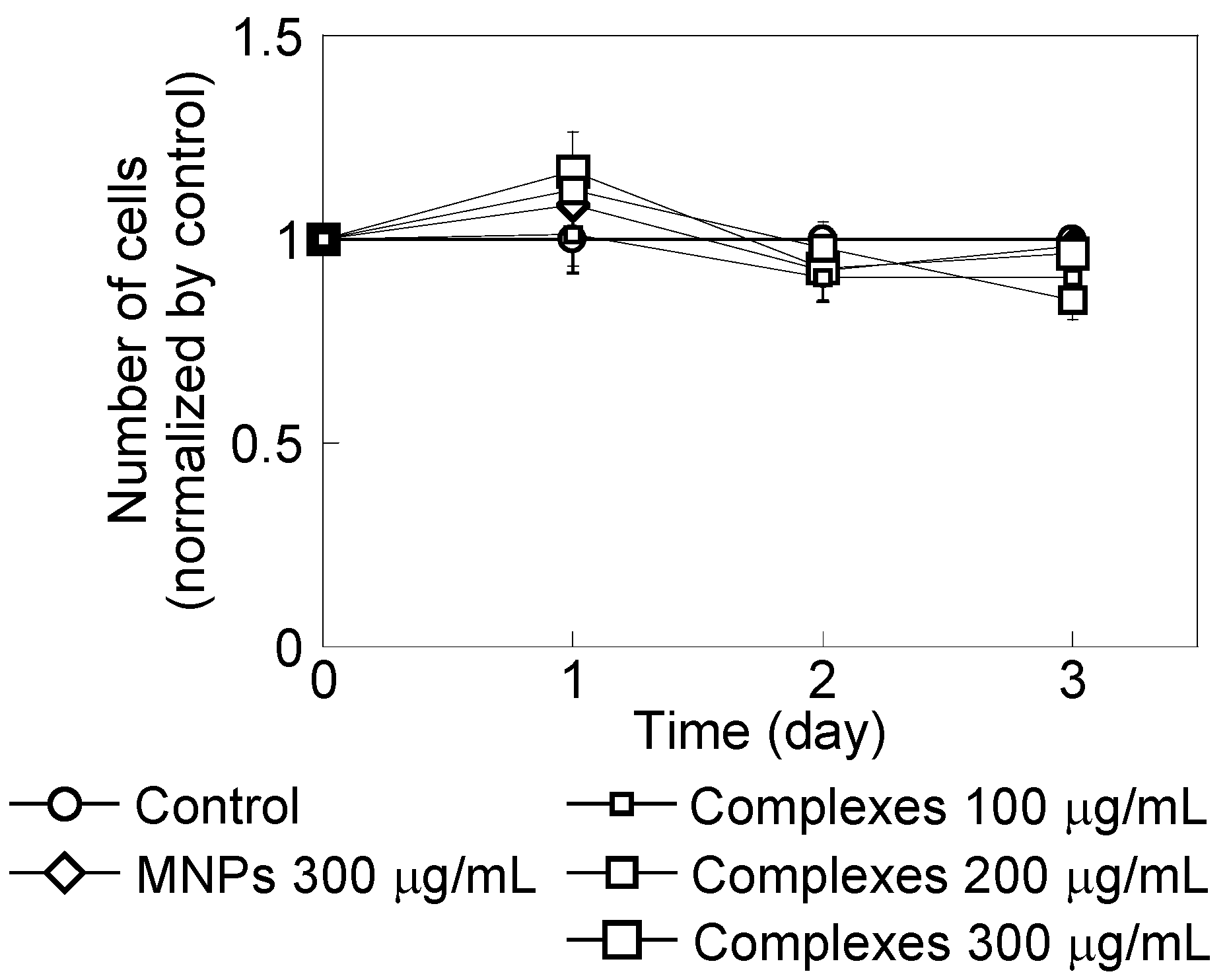
2.2. Effect of Magnetic Hyperthermia Combined with CH11 Antibodies

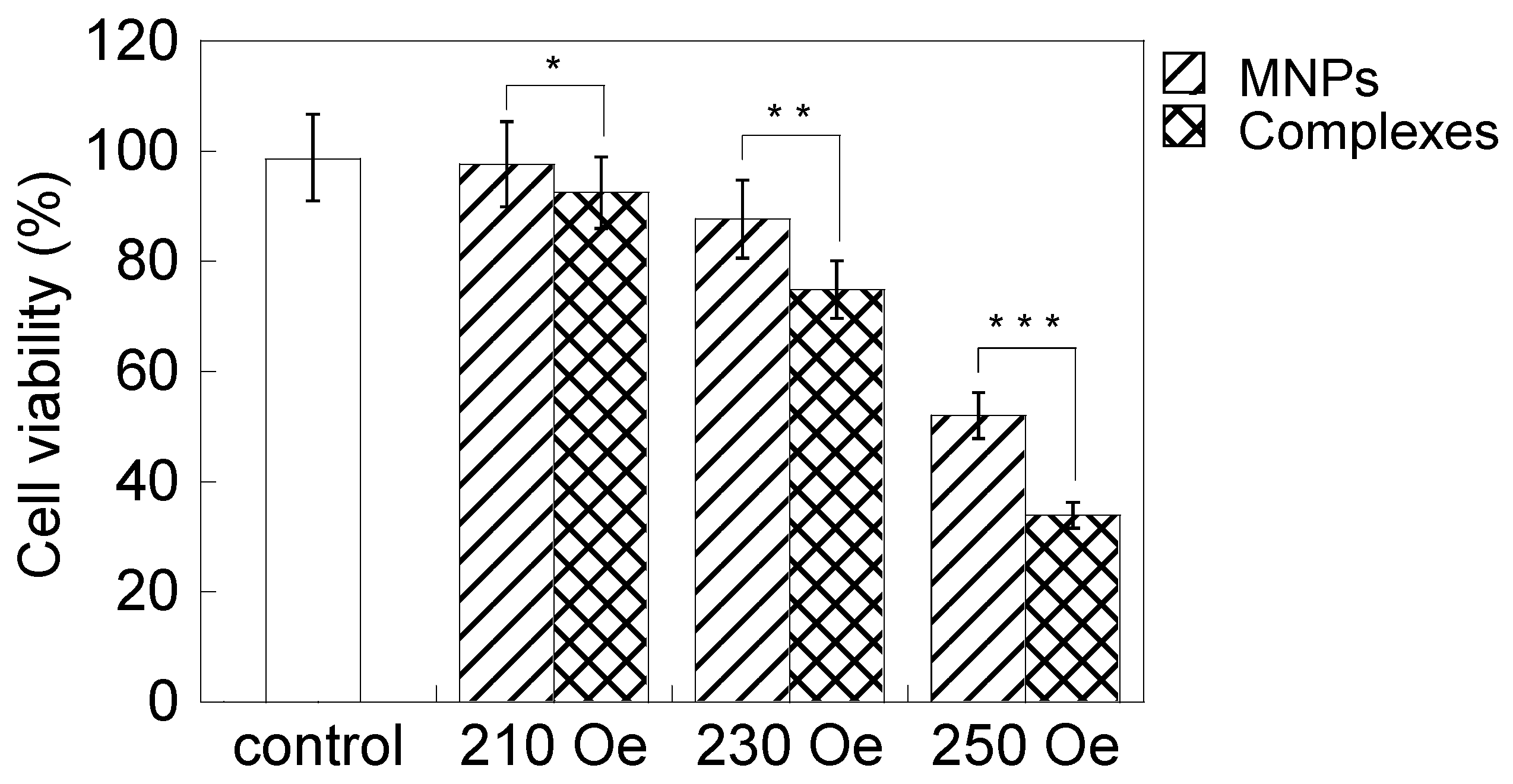
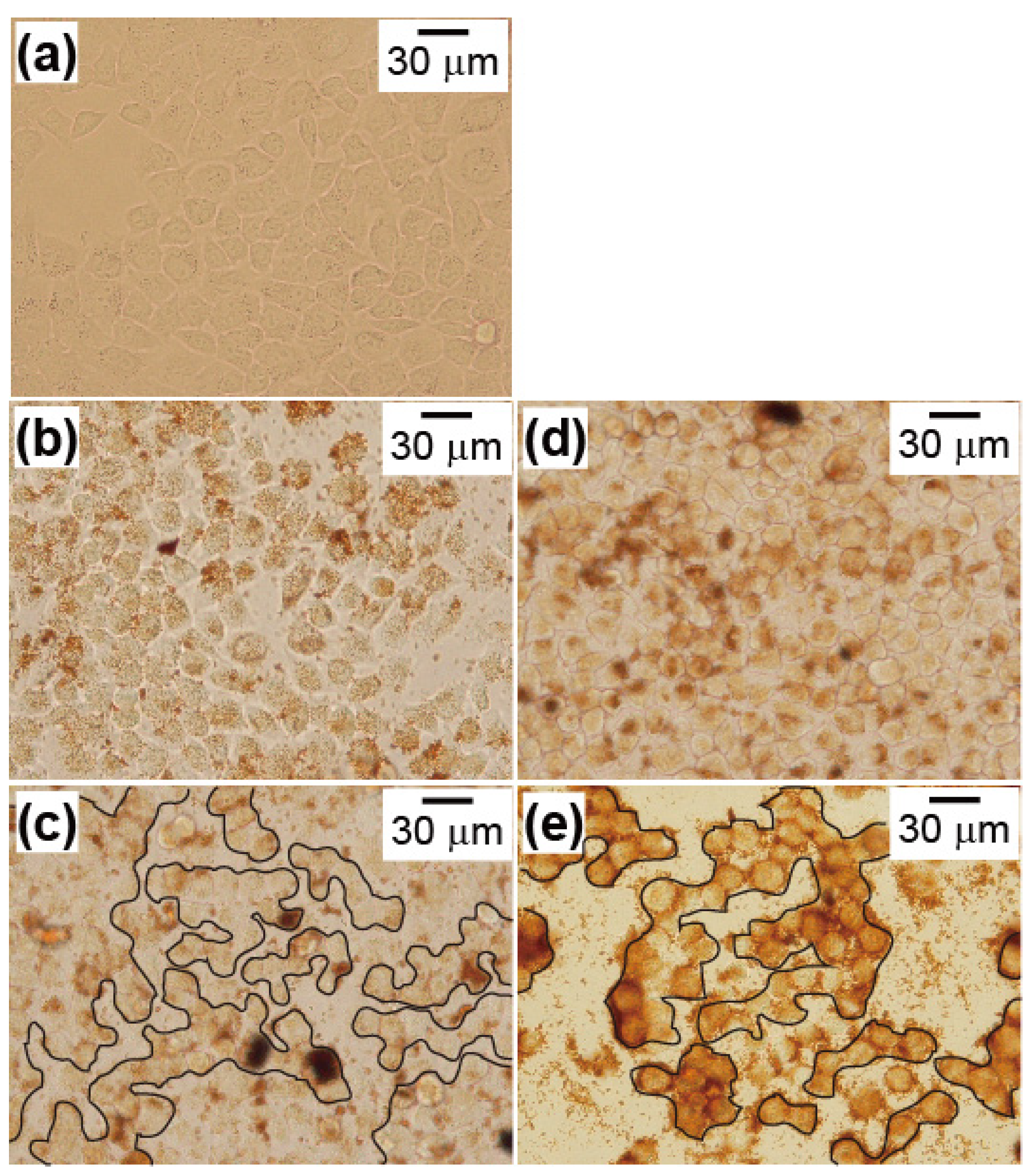
2.3. Cryptotanshinone-Induced Cellular Apoptosis

3. Experimental Section
3.1. Materials and Reagents
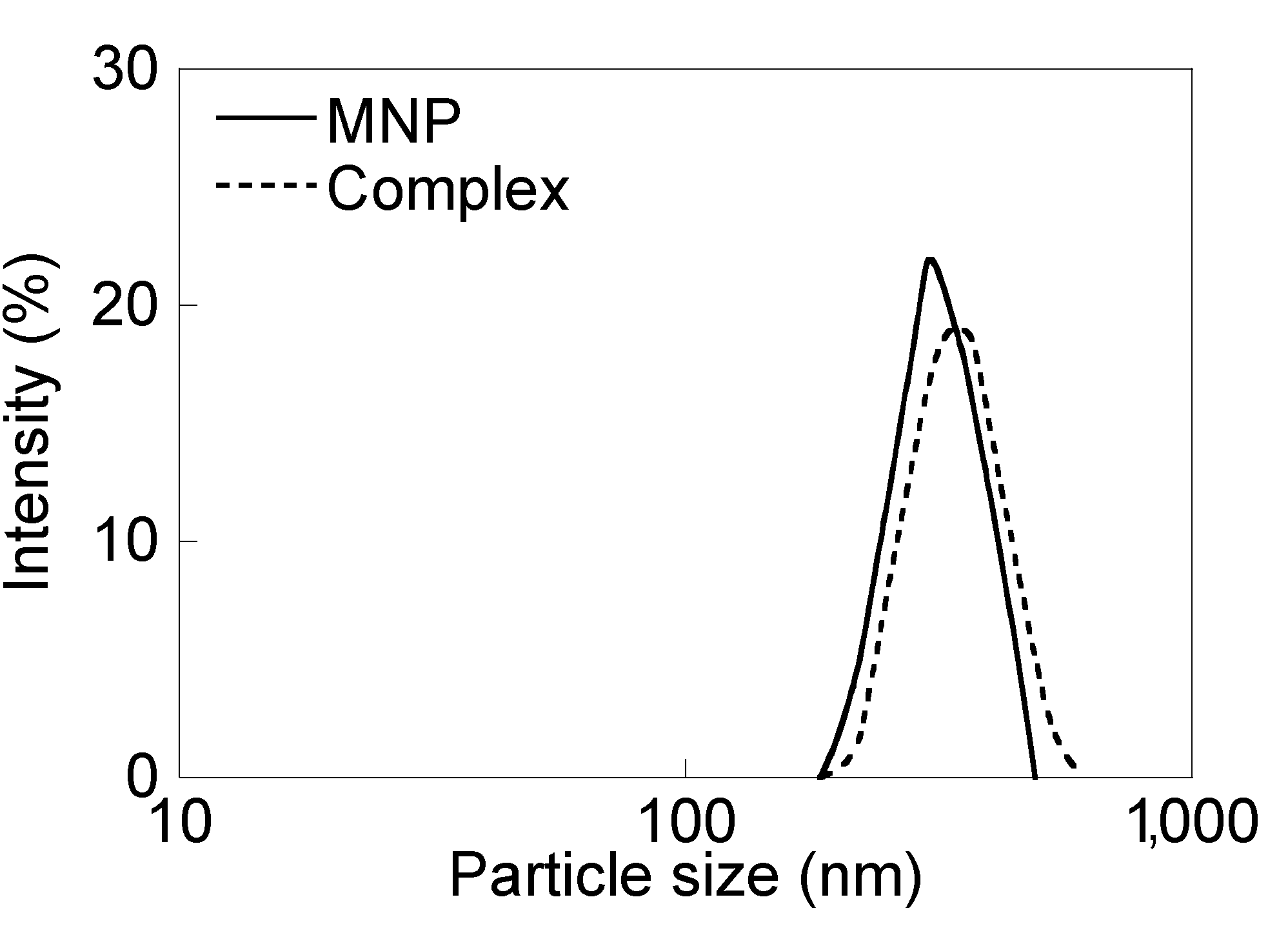
3.2. Surface Coating and Synthesis of MNP/Antibody Complexes
3.3. Cell Culture
3.4. Cell Growth Inhibition
3.5. Hyperthermia and Cryptotanshinone Treatment
4. Conclusions
Conflicts of Interest
References
- Berry, C.C.; Adam, A.S.G. Functionalisation of magnetic nanoparticles for application in biomedicine. J. Phys. D 2003, 36, R198–R206. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D 2003, 36, R167–R181. [Google Scholar] [CrossRef]
- Kozissnik, B.; Bohorquez, A.C.; Dobson, J.; Rinaldi, C. Magnetic fluid hyperthermia: Advances, challenges, and opportunity. Int. J. Hyperthermia 2013, 29, 706–714. [Google Scholar] [CrossRef]
- Fuchigami, T.; Kawamura, R.; Kitamoto, Y.; Nakagawa, M.; Namiki, Y. Ferromagnetic FePt-nanoparticles/polycation hybrid capsules designed for a magnetically guided drug delivery system. Langmuir 2011, 27, 2923–2928. [Google Scholar] [CrossRef]
- Shundo, C.; Zhang, H.; Nakanishi, T.; Osaka, T. Cytotoxicity evaluation of magnetite (Fe3O4) nanoparticles in mouse embryonic stem cells. J. Colloid Surf. B 2012, 97, 221–225. [Google Scholar] [CrossRef]
- Iida, H.; Takayanagi, K.; Nakanishi, T.; Osaka, T. Synthesis of Fe3O4 nanoparticles with various size and magnetic properties by controlled hydrolysis. J. Colloid Interface Sci. 2007, 314, 274–280. [Google Scholar] [CrossRef]
- Tomitaka, A.; Ueda, K.; Yamada, T.; Takemura, Y. Heat dissipation and magnetic properties of surface-coated Fe3O4 nanoparticles for biomedical applicaiton. J. Magn. Magn. Mater. 2012, 324, 3437–3442. [Google Scholar] [CrossRef]
- Johannsen, M.; Gneveckow, U.; Eckelt, L.; Feussner, A.; WaldÖFner, N.; Scholz, R.; Deger, S.; Wust, P.; Loening, S.A.; Jordan, A. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: Presentation of a new interstitial technique. Int. J. Hyperthermia 2005, 21, 637–647. [Google Scholar] [CrossRef]
- Jordan, A.; Scholz, R.; Wust, P.; Fähling, H.; Felix, R. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mater. 1999, 201, 413–419. [Google Scholar] [CrossRef]
- Fairbairn, J.J.; Khan, M.W.; Ward, K.J.; Loveridge, B.W.; Fairbairn, D.W.; O’Neill, K.L. Induction of apoptotic cell DNA fragmentation in human cells after treatment with hyperthermia. Cancer Lett. 1995, 89, 183–188. [Google Scholar] [CrossRef]
- Shubayev, V.I.; Pisanic, T.R., II; Jin, S. Magnetic nanoparticles for theragnostics. Adv. Drug Deliv. Rev. 2009, 61, 467–477. [Google Scholar] [CrossRef]
- Cole, A.J.; Yang, V.C.; David, A.E. Cancer theranostics: The rise of targeted magnetic nanoparticles. Trends Biotechnol. 2011, 29, 323–332. [Google Scholar] [CrossRef]
- Hayashi, K.; Nakamura, M.; Sakamoto, W.; Yogo, T.; Miki, H.; Ozaki, S.; Abe, M.; Matsumoto, T.; Ishimura, K. Superparamagnetic nanoparticles clusters for cancer theranostics combining magnetic resonance imaging and hyperthermia treatment. Theranostics 2013, 3, 366–376. [Google Scholar] [CrossRef]
- Zoil, W.; Ricotti, L.; Tesei, A.; Barzanti, F.; Amadori, D. In vitro preclinical models for a rational design of chemotherapy combinations in human tumors. Crit. Rev. Oncol. Hematol. 2001, 37, 69–82. [Google Scholar] [CrossRef]
- Duan, Y.; Zheng, J.; Han, S.; Wu, Y.; Wang, Y.; Li, D.; Kong, D.; Yu, Y. A tumor targeted gene vector modified with G250 monoclonal antibody for gene therapy. J. Control. Release 2008, 127, 173–179. [Google Scholar] [CrossRef]
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005, 100, 1–11. [Google Scholar] [CrossRef]
- Nagata, S.; Golstein, P. The Fas death factor. Science 1995, 267, 1449–1456. [Google Scholar] [CrossRef]
- Yonehara, S.; Ishii, A.; Yonehara, M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J. Exp. Med. 1989, 169, 1747–1756. [Google Scholar] [CrossRef]
- Nagata, S. Apoptosis by death factor. Cell 1997, 88, 355–365. [Google Scholar] [CrossRef]
- Zhang, L.; Shimizu, S.; Sakamaki, K.; Yonehara, S.; Tsujimoto, Y. A caspase-8-independent signaling pathway activated by Fas ligation leads to exposure of the Bak N terminus. J. Biol. Chem. 2004, 279, 33865–33874. [Google Scholar]
- Itoh, N.; Yonehara, S.; Ishii, A.; Yonehara, M.; Mizushima, S.; Sameshima, M.; Hase, A.; Seto, Y.; Nagata, S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 1991, 66, 233–243. [Google Scholar] [CrossRef]
- Sloand, E.M.; Young, N.S.; Kumar, P.; Weichold, F.F.; Sato, T.; Maciejewski, J.P. Role of Fas ligand and receptor in the mechanism of T-cell depletion in acquired immunodeficiency syndrome: Effect on CD4+ lymphocyte depletion and human immunodeficiency virus replication. Blood 1997, 89, 1357–1363. [Google Scholar]
- Park, I.; Kim, M.; Park, O.J.; Park, M.G.; Choe, W.; Kang, I.; Kim, S.; Ha, J. Cryptotanshinone sensitizes DU145 prostate cancer cells to Fas(APO1/CD95)-mediated apoptosis through Bcl-2 and MAPK regulation. Cancer Lett. 2010, 298, 88–98. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Cheng, Y.; Zhao, Z.; Tashiro, S.; Onodera, S.; Ikejima, T. Fas-mediated autophagy requires JNK activation in HeLa cells. Biochem. Biophys. Res. Commun. 2008, 377, 1205–1210. [Google Scholar] [CrossRef]
- Palzer, R.J.; Heidelberger, C. Studies of the quantitative biology of hyperthermic killing of HeLa cells. Cancer Res. 1973, 33, 415–421. [Google Scholar]
- Kluck, R.M.; Bossy-Wetzel, E.; Green, D.R.; Newmeyer, D.D. The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science 1997, 33, 415–421. [Google Scholar]
- Yang, J.; Liu, X.; Bhalla, K.; Kim, C.N.; Ibrado, A.M.; Cai, J.; Peng, T.; Jones, D.P.; Wang, X. Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science 1997, 275, 1129–1132. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ota, S.; Yamazaki, N.; Tomitaka, A.; Yamada, T.; Takemura, Y. Hyperthermia Using Antibody-Conjugated Magnetic Nanoparticles and Its Enhanced Effect with Cryptotanshinone. Nanomaterials 2014, 4, 319-330. https://doi.org/10.3390/nano4020319
Ota S, Yamazaki N, Tomitaka A, Yamada T, Takemura Y. Hyperthermia Using Antibody-Conjugated Magnetic Nanoparticles and Its Enhanced Effect with Cryptotanshinone. Nanomaterials. 2014; 4(2):319-330. https://doi.org/10.3390/nano4020319
Chicago/Turabian StyleOta, Satoshi, Naoya Yamazaki, Asahi Tomitaka, Tsutomu Yamada, and Yasushi Takemura. 2014. "Hyperthermia Using Antibody-Conjugated Magnetic Nanoparticles and Its Enhanced Effect with Cryptotanshinone" Nanomaterials 4, no. 2: 319-330. https://doi.org/10.3390/nano4020319




