Creating Active Device Materials for Nanoelectronics Using Block Copolymer Lithography
Abstract
:1. Introduction
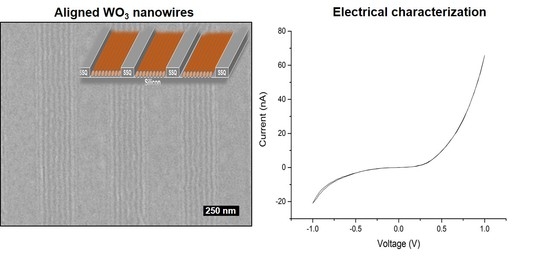
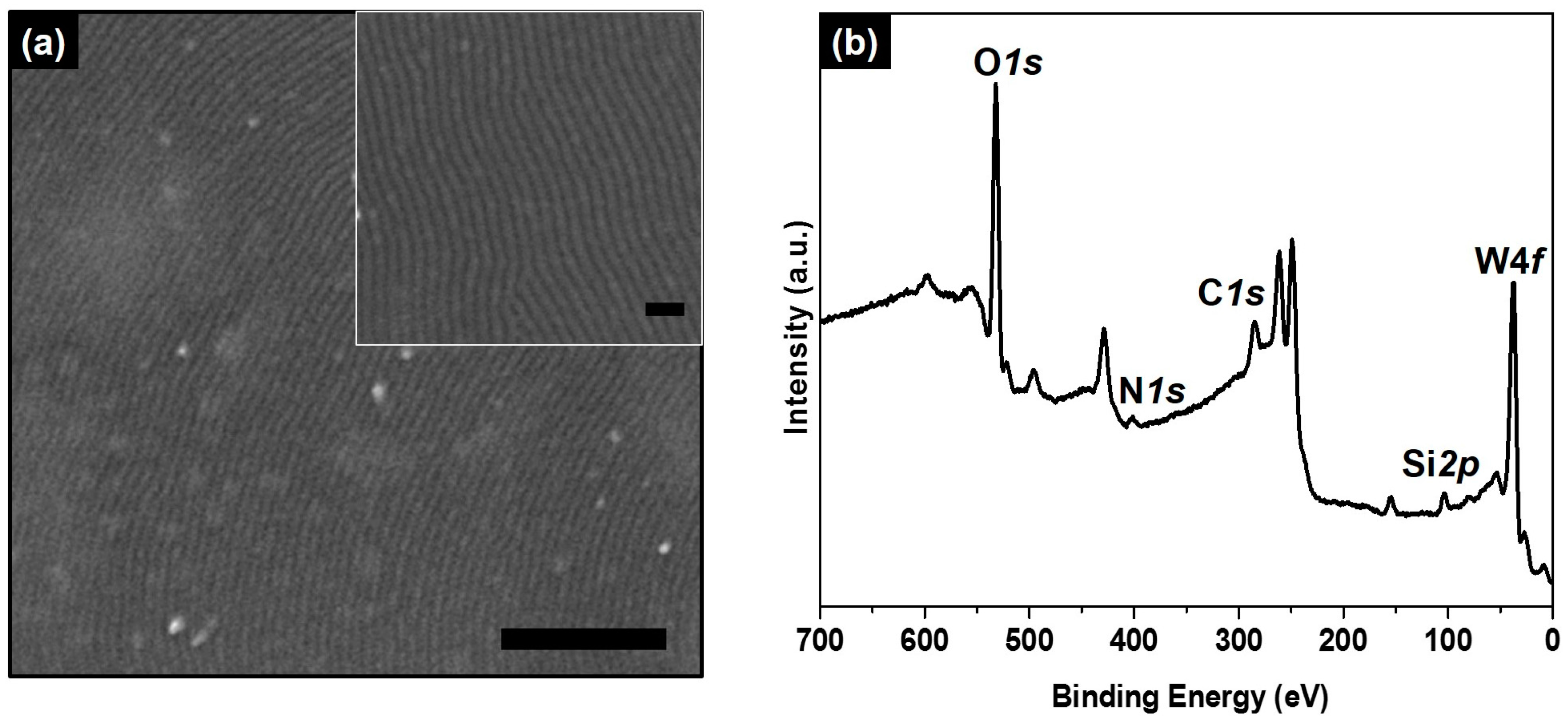
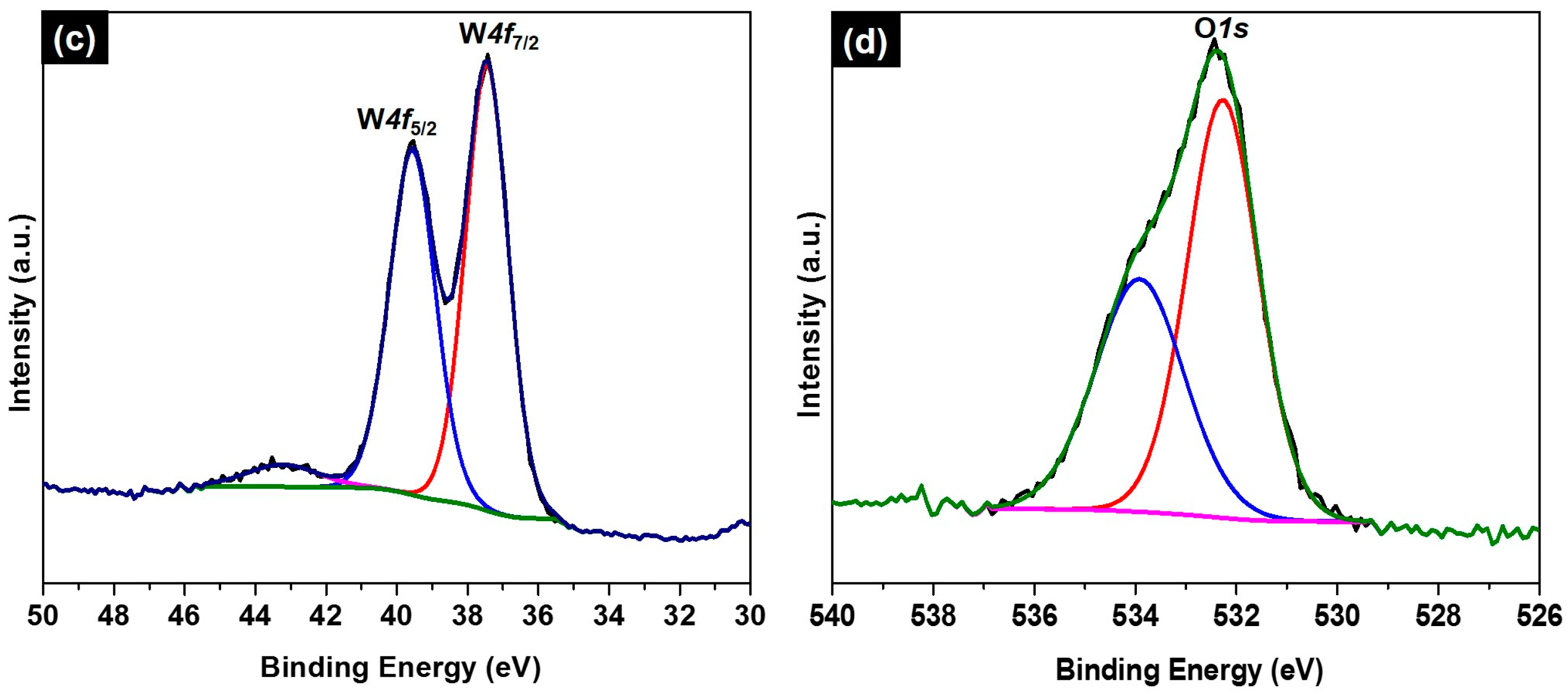
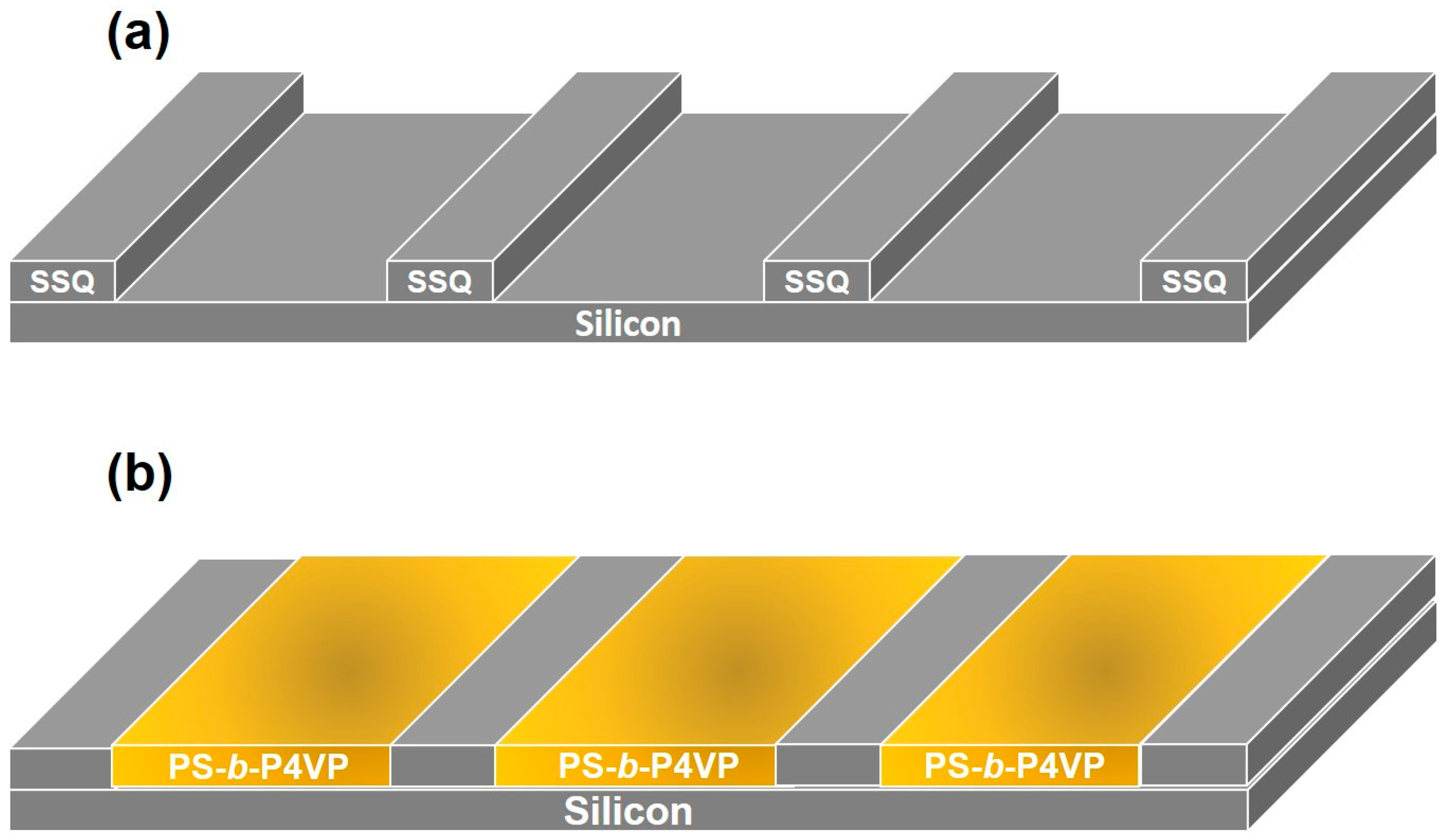
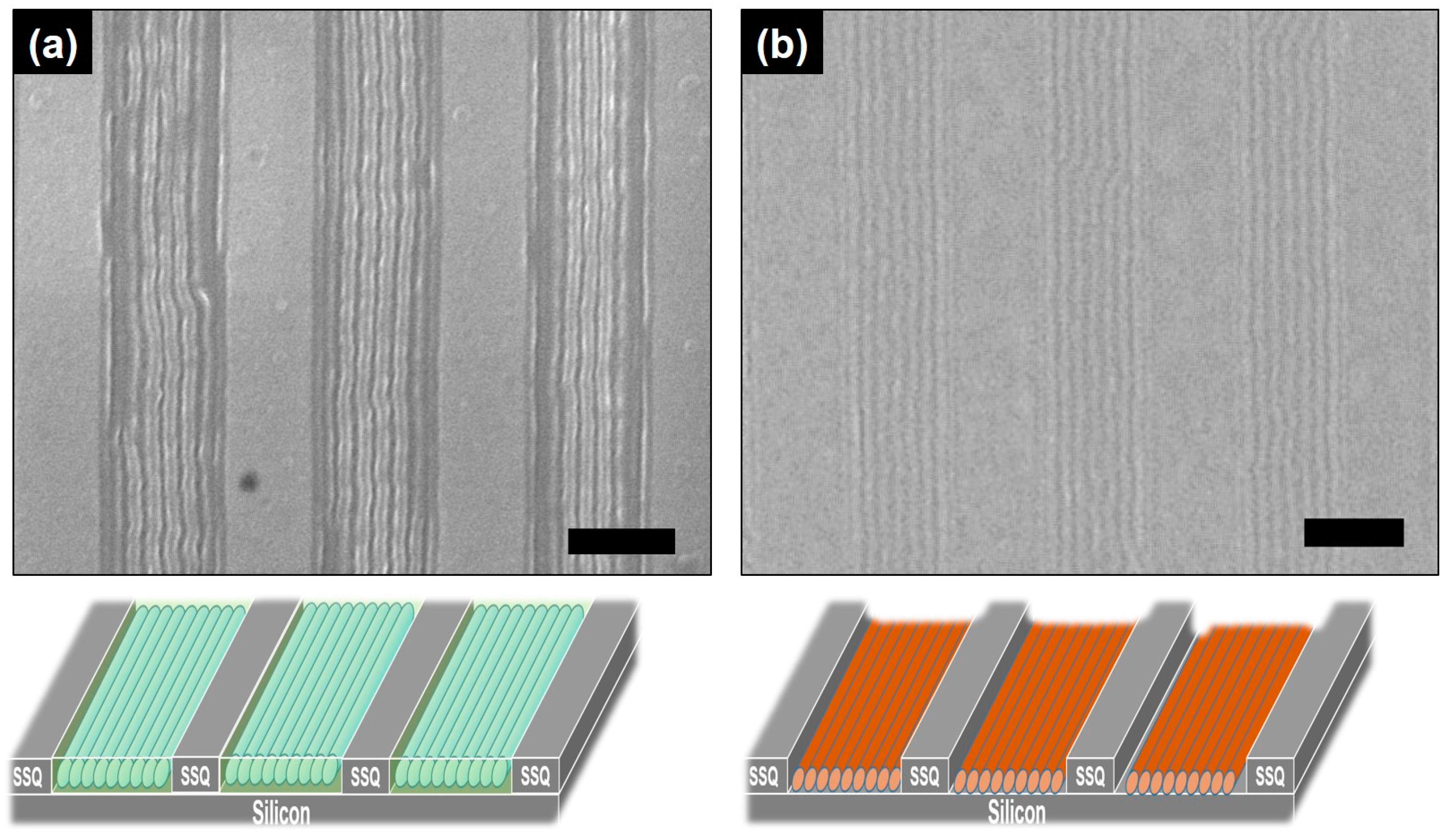
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Film Deposition and Nanowire Formation
3.3. Electrical Studies
3.4. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, L.; Liu, X.; Pal, S.; Wang, S.; Ober, C.K.; Giannelis, E.P. Extreme ultraviolet resist materials for sub-7 nm patterning. Chem. Soc. Rev. 2017, 46, 4855–4866. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Chen, P.-C.; Ryu, K.; Zhou, C. Devices and chemical sensing applications of metal oxide nanowires. J. Mater. Chem. 2009, 19, 828–839. [Google Scholar] [CrossRef]
- Lorenz, M.; Rao, M.S.R.; Venkatesan, T.; Fortunato, E.; Barquinha, P.; Branquinho, R.; Salgueiro, D.; Martins, R.; Carlos, E.; Liu, A.; et al. The 2016 oxide electronic materials and oxide interfaces roadmap. J. Phys. D Appl. Phys. 2016, 49, 433001. [Google Scholar] [CrossRef]
- Pal, N.; Bhaumik, A. Soft templating strategies for the synthesis of mesoporous materials: Inorganic, organic–inorganic hybrid and purely organic solids. Adv. Colloid Interface Sci. 2013, 189, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, H.-Y.; Yao, C.-T.; Ho, R.-M. Well-ordered nanohybrids and nanoporous materials from gyroid block copolymer templates. Chem. Soc. Rev. 2015, 44, 1974–2018. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.A. Directed self-assembly of block copolymers for nanocircuitry fabrication. Microelectron. Eng. 2015, 132, 207–217. [Google Scholar] [CrossRef]
- Black, C.T. Polymer self-assembly as a novel extension to optical lithography. ACS Nano 2007, 1, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Yang, S.Y.; Lee, Y.; Kim, Y. Functional nanomaterials based on block copolymer self-assembly. Prog. Polym. Sci. 2010, 35, 1325–1349. [Google Scholar] [CrossRef]
- Sinturel, C.; Bates, F.S.; Hillmyer, M.A. High X–low N block polymers: How far can we go? ACS Macro Lett. 2015, 4, 1044–1050. [Google Scholar] [CrossRef]
- Xiao, S.G.; Yang, X.M.; Edwards, E.W.; La, Y.H.; Nealey, P.F. Graphoepitaxy of cylinder-forming block copolymers for use as templates to pattern magnetic metal dot arrays. Nanotechnology 2005, 16, S324–S329. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo, P.A.R.; Gronheid, R.; Thode, C.J.; Wu, H.; Cao, Y.; Neisser, M.; Somervell, M.; Nafus, K.; Nealey, P.F. Implementation of a chemo-epitaxy flow for directed self-assembly on 300-mm wafer processing equipment. MOEMS 2012, 11, 031302. [Google Scholar] [CrossRef]
- Bates, C.M.; Maher, M.J.; Janes, D.W.; Ellison, C.J.; Willson, C.G. Block copolymer lithography. Macromolecules 2013, 47, 2–12. [Google Scholar] [CrossRef]
- Hu, H.; Gopinadhan, M.; Osuji, C.O. Directed self-assembly of block copolymers: A tutorial review of strategies for enabling nanotechnology with soft matter. Soft Matter 2014, 10, 3867–3889. [Google Scholar] [CrossRef] [PubMed]
- Koo, K.; Ahn, H.; Kim, S.-W.; Ryu, D.Y.; Russell, T.P. Directed self-assembly of block copolymers in the extreme: Guiding microdomains from the small to the large. Soft Matter 2013, 9, 9059–9071. [Google Scholar] [CrossRef]
- Gu, X.; Gunkel, I.; Russell, T.P. Pattern transfer using block copolymers. In Philosophical Transactions of the Royal Society A: Mathematical; Physical and Engineering; Royal Society: Terrace, London, UK, 2013. [Google Scholar]
- Lopes, W.A.; Jaeger, H.M. Hierarchical self-assembly of metal nanostructures on diblock copolymer scaffolds. Nature 2001, 414, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Wang, D.; Fan, X.; Buriak, J.M. Assembly of aligned linear metallic patterns on silicon. Nat. Nanotechnol. 2007, 2, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Lee, J.H.; Lee, J.Y.; Ross, C.A. Fabrication of diverse metallic nanowire arrays based on block copolymer self-assembly. Nano Lett. 2010, 10, 3722–3726. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.G.; Byun, M.; Jeong, C.K.; Lee, K.J. Performance enhancement of electronic and energy devices via block copolymer self-assembly. Adv. Mater. 2015, 27, 3982–3998. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.O.; Mun, J.H.; Hwang, G.-T.; Yoon, J.M.; Kim, J.Y.; Yun, J.M.; Yang, Y.-B.; Oh, Y.; Lee, J.Y.; Shin, J.; et al. Multicomponent nanopatterns by directed block copolymer self-assembly. ACS Nano 2013, 7, 8899–8907. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Ou, J.Z.; Strano, M.S.; Kaner, R.B.; Mitchell, A.; Kalantar-zadeh, K. Nanostructured tungsten oxide—Properties, synthesis, and applications. Adv. Funct. Mater. 2011, 21, 2175–2196. [Google Scholar] [CrossRef]
- Cummins, C.; Gangnaik, A.; Kelly, R.A.; Hydes, A.J.; O’Connell, J.; Petkov, N.; Georgiev, Y.M.; Borah, D.; Holmes, J.D.; Morris, M.A. Parallel arrays of sub-10 nm aligned germanium nanofins from an in situ metal oxide hardmask using directed self-assembly of block copolymers. Chem. Mater. 2015, 27, 6091–6096. [Google Scholar] [CrossRef]
- Barreca, D.; Carta, G.; Gasparotto, A.; Rossetto, G.; Tondello, E.; Zanella, P. A study of nanophase tungsten oxides thin films by XPS. Surf. Sci. Spec. 2001, 8, 258–267. [Google Scholar] [CrossRef]
- Karuppanan, S.; Kijung, Y. Growth and characterization of stoichiometric tungsten oxide nanorods by thermal evaporation and subsequent annealing. Nanotechnology 2007, 18, 395604. [Google Scholar]
- Shim, H.-S.; Kim, J.W.; Sung, Y.-E.; Kim, W.B. Electrochromic properties of tungsten oxide nanowires fabricated by electrospinning method. Sol. Energy Mater. Sol. Cells 2009, 93, 2062–2068. [Google Scholar] [CrossRef]
- Cummins, C.; Kelly, R.A.; Gangnaik, A.; Georgiev, Y.M.; Petkov, N.; Holmes, J.D.; Morris, M.A. Solvent vapor annealing of block copolymers in confined topographies: Commensurability considerations for nanolithography. Macromol. Rapid Commun. 2015, 36, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wilczek, M.; Hirtz, M.; Hao, J.; Wang, W.; Fuchs, H.; Gurevich, S.V.; Chi, L. Branch suppression and orientation control of langmuir–blodgett patterning on prestructured surfaces. Adv. Mater. Interfaces 2016, 3. [Google Scholar] [CrossRef]
- Welander, A.M.; Nealey, P.F.; Cao, H.; Bristol, R. Impact of trench width roughness on the graphoepitaxial assembly of block copolymers. J. Vac. Sci. Technol. B 2008, 26, 2484–2488. [Google Scholar] [CrossRef]
- Cummins, C.; Gangnaik, A.; Kelly, R.A.; Borah, D.; O’Connell, J.; Petkov, N.; Georgiev, Y.M.; Holmes, J.D.; Morris, M.A. Aligned silicon nanofins via the directed self-assembly of PS-b-P4VP block copolymer and metal oxide enhanced pattern transfer. Nanoscale 2015, 7, 6712–6721. [Google Scholar] [CrossRef] [PubMed]
- Cummins, C.; Borah, D.; Rasappa, S.; Chaudhari, A.; Ghoshal, T.; O’Driscoll, B.M.D.; Carolan, P.; Petkov, N.; Holmes, J.D.; Morris, M.A. Self-assembly of polystyrene-block-poly(4-vinylpyridine) block copolymer on molecularly functionalized silicon substrates: Fabrication of inorganic nanostructured etchmask for lithographic use. J. Mater. Chem. C 2013, 1, 7941–7951. [Google Scholar] [CrossRef]
- Ghoshal, T.; Senthamaraikannan, R.; Shaw, M.T.; Holmes, J.D.; Morris, M.A. Fabrication of ordered, large scale, horizontally-aligned si nanowire arrays based on an in situ hard mask block copolymer approach. Adv. Mater. 2014, 26, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Cummins, C.; Ghoshal, T.; Holmes, J.D.; Morris, M.A. Strategies for inorganic incorporation using neat block copolymer thin films for etch mask function and nanotechnological application. Adv. Mater. 2016, 28, 5586–5618. [Google Scholar] [CrossRef] [PubMed]
- Farrell, R.A.; Kinahan, N.T.; Hansel, S.; Stuen, K.O.; Petkov, N.; Shaw, M.T.; West, L.E.; Djara, V.; Dunne, R.J.; Varona, O.G.; et al. Large-scale parallel arrays of silicon nanowires via block copolymer directed self-assembly. Nanoscale 2012, 4, 3228–3236. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhu, J.; Yu, R. Synthesis and electrical characterization of tungsten oxide nanowires. Chin. Phys. B 2009, 18, 3024. [Google Scholar]
- Dobos, L.; Pécz, B.; Tóth, L.; Horváth, Z.J.; Horváth, Z.E.; Beaumont, B.; Bougrioua, Z. Structural and electrical properties of au and Ti/Au contacts to n-type gan. Vacuum 2008, 82, 794–798. [Google Scholar] [CrossRef]
- Hayden, O.; Agarwal, R.; Lu, W. Semiconductor nanowire devices. Nano Today 2008, 3, 12–22. [Google Scholar] [CrossRef]
- Borah, D.; Rasappa, S.; Salaun, M.; Zellsman, M.; Lorret, O.; Liontos, G.; Ntetsikas, K.; Avgeropoulos, A.; Morris, M.A. Soft graphoepitaxy for large area directed self-assembly of polystyrene-block-poly(dimethylsiloxane) block copolymer on nanopatterned poss substrates fabricated by nanoimprint lithography. Adv. Funct. Mater. 2015, 25, 3425–3432. [Google Scholar] [CrossRef]
- Sinturel, C.; Vayer, M.; Morris, M.; Hillmyer, M.A. Solvent vapor annealing of block polymer thin films. Macromolecules 2013, 46, 5399–5415. [Google Scholar] [CrossRef]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cummins, C.; Bell, A.P.; Morris, M.A. Creating Active Device Materials for Nanoelectronics Using Block Copolymer Lithography. Nanomaterials 2017, 7, 304. https://doi.org/10.3390/nano7100304
Cummins C, Bell AP, Morris MA. Creating Active Device Materials for Nanoelectronics Using Block Copolymer Lithography. Nanomaterials. 2017; 7(10):304. https://doi.org/10.3390/nano7100304
Chicago/Turabian StyleCummins, Cian, Alan P. Bell, and Michael A. Morris. 2017. "Creating Active Device Materials for Nanoelectronics Using Block Copolymer Lithography" Nanomaterials 7, no. 10: 304. https://doi.org/10.3390/nano7100304