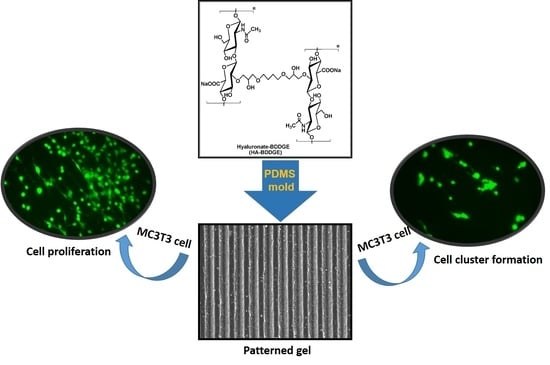
Evaluation of MC3T3 Cells Proliferation and Drug Release Study from Sodium Hyaluronate-1,4-butanediol Diglycidyl Ether Patterned Gel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
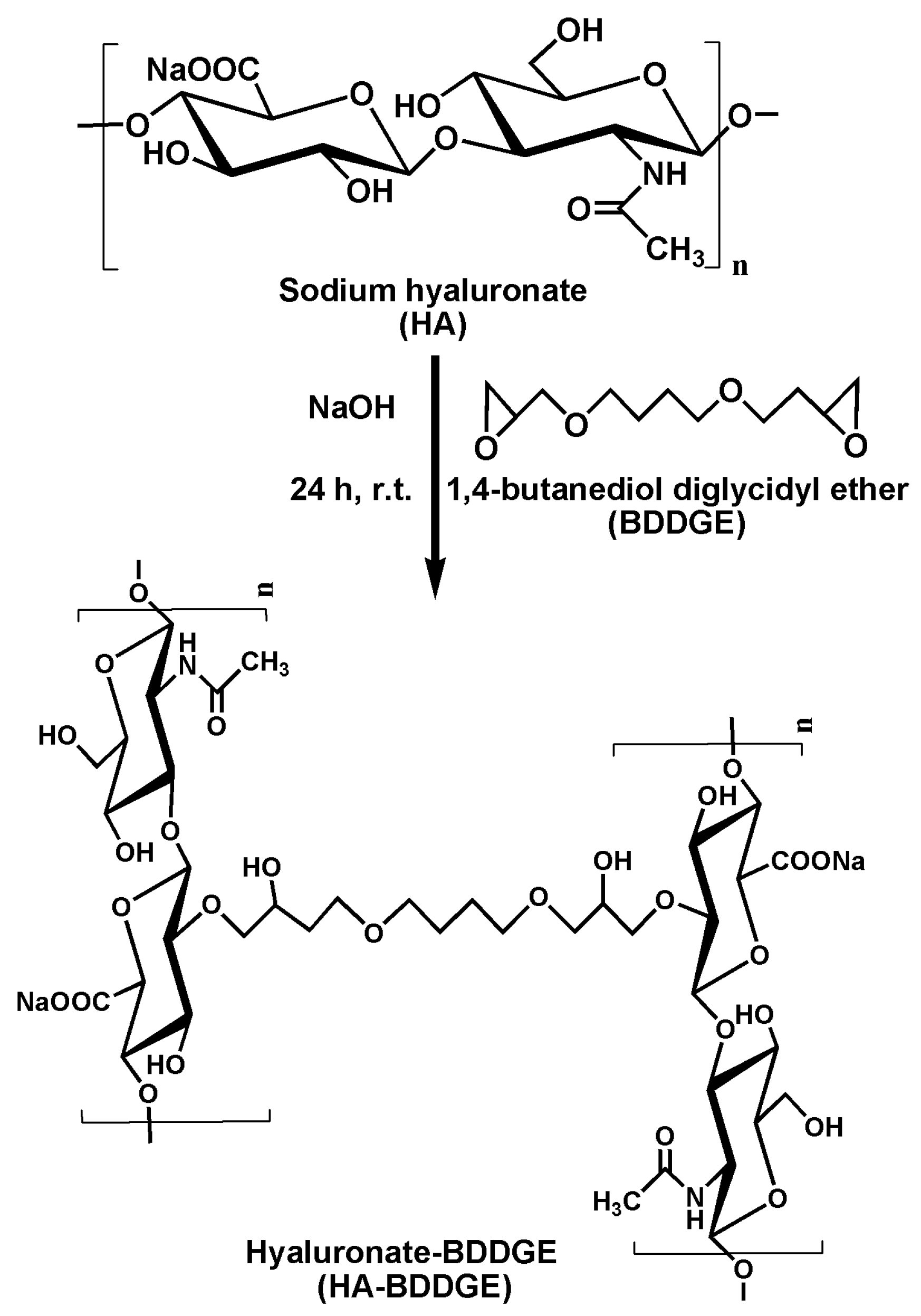
2.2. Fabrication of Sodium Hyaluronate-BDDGE Patterned Gel (HA-BDDGE)
2.3. Characterizations
2.4. Swelling Study
2.5. DMOG Loading in HA-BDDGE Patterned Gel and In Vitro Release Study
2.6. In Vitro MC3T3 Cell Culture on the Surface of HA-BDDGE Patterned Gel
2.7. Live & Dead Assay
2.8. Alkaline Phosphatase (ALP) Activity Assay
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results and Discussion
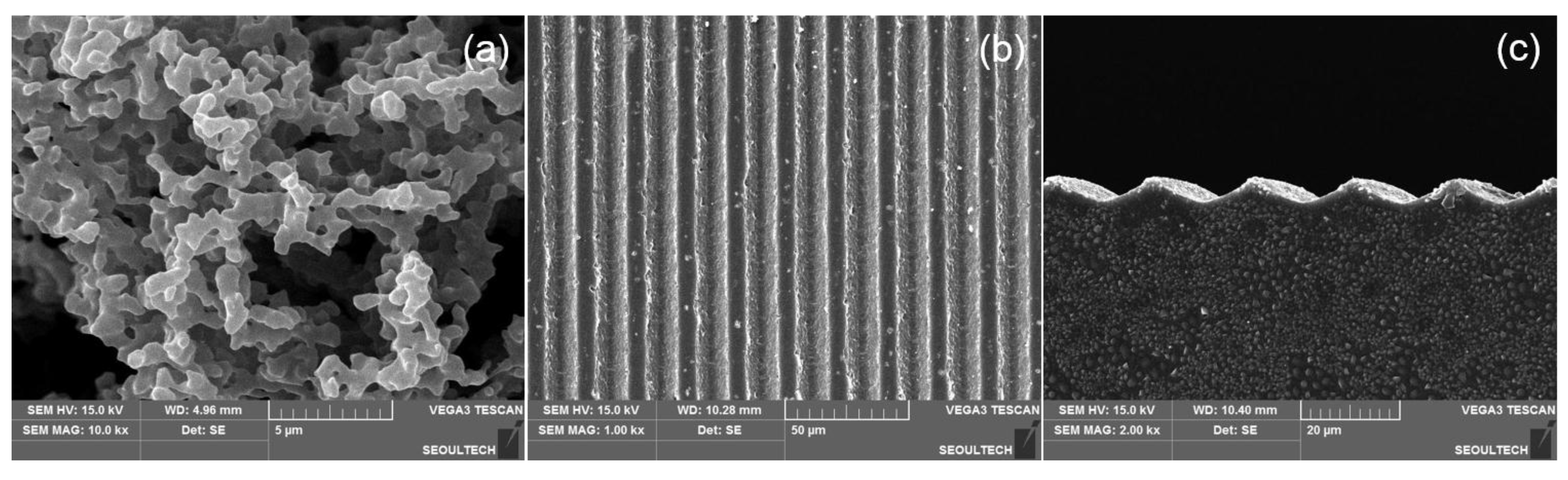
3.1. Fabrication of Sodium Hyaluronate-BDDGE Patterned Gel (HA-BDDGE)
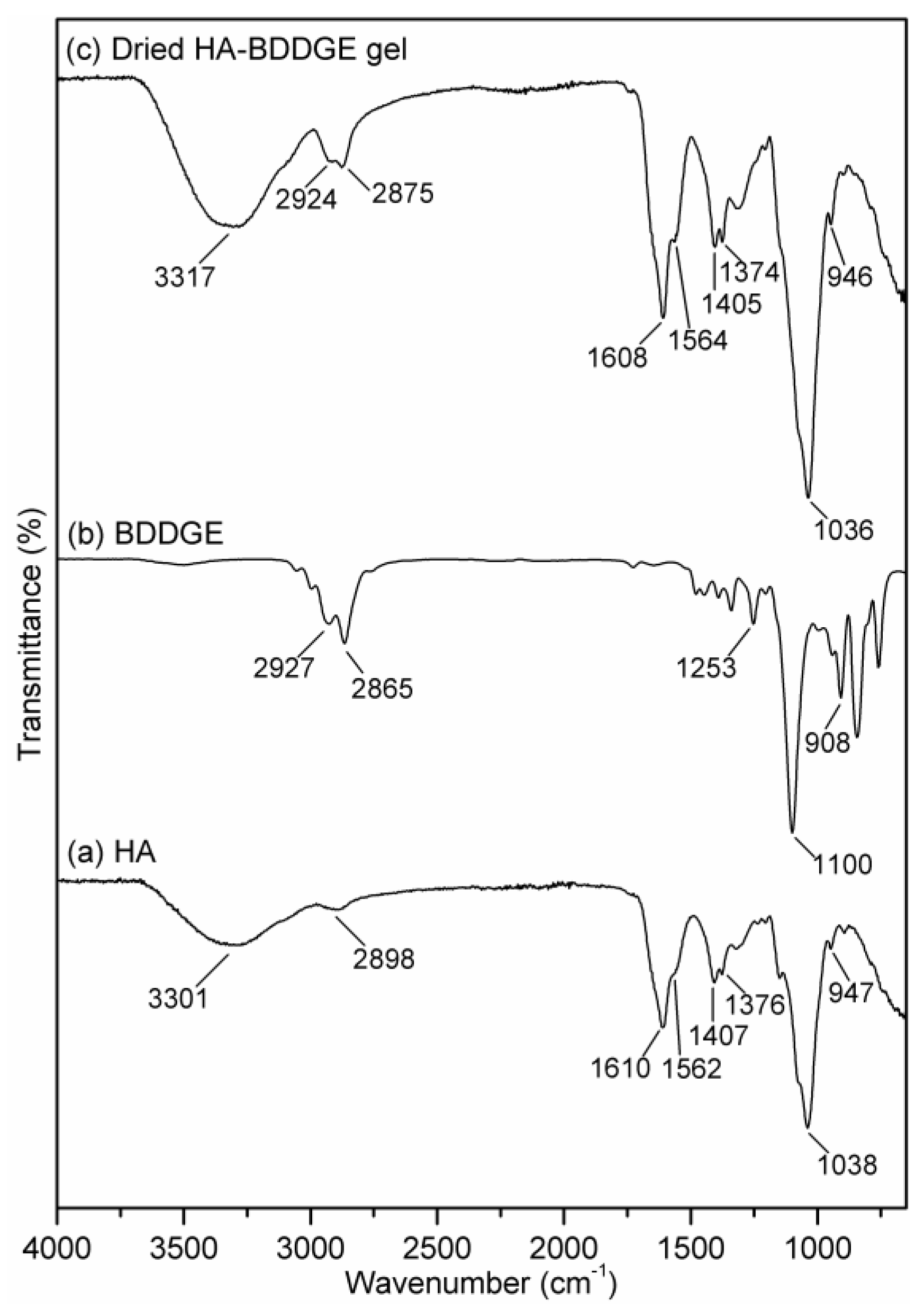
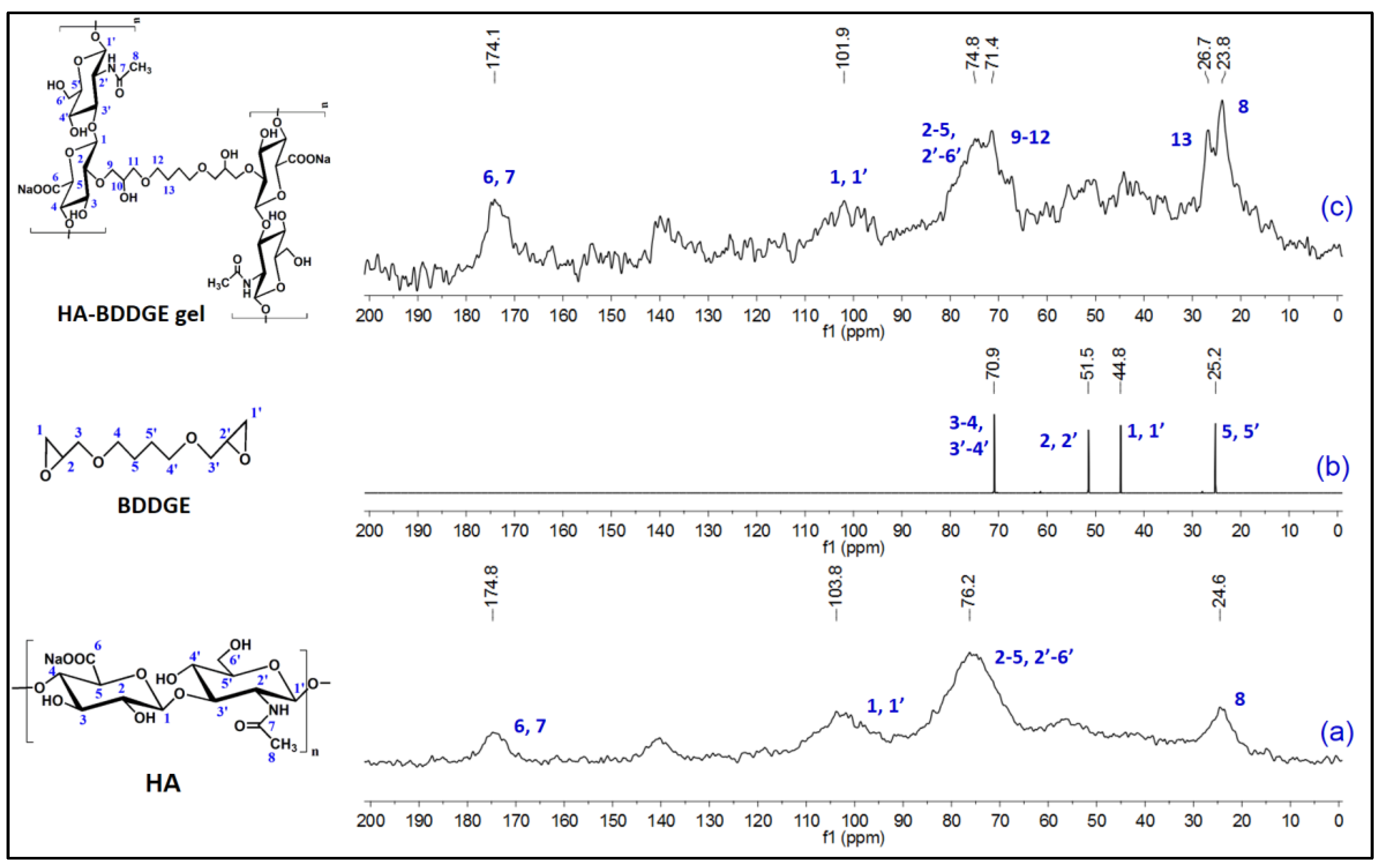
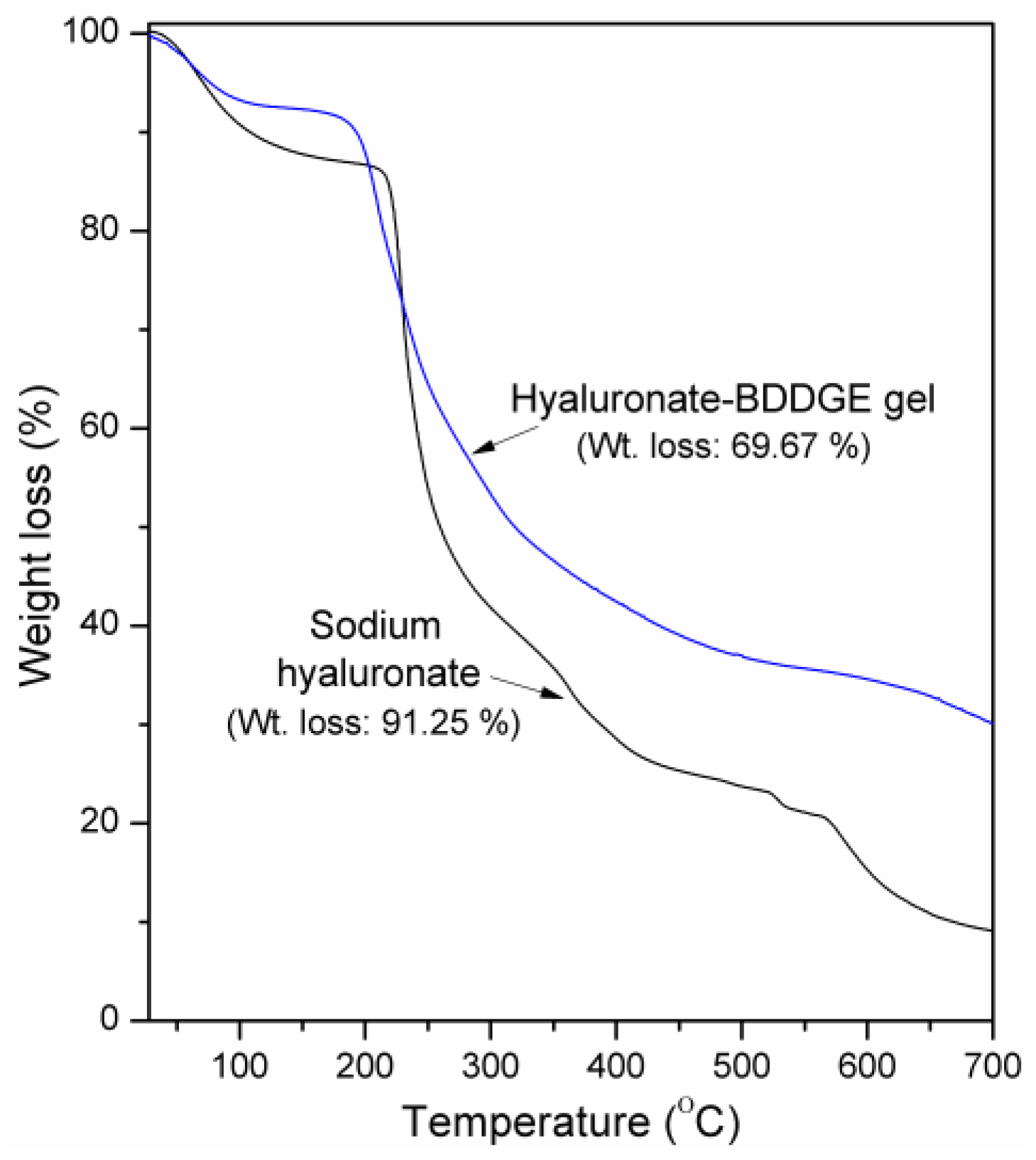
3.2. Characterizations
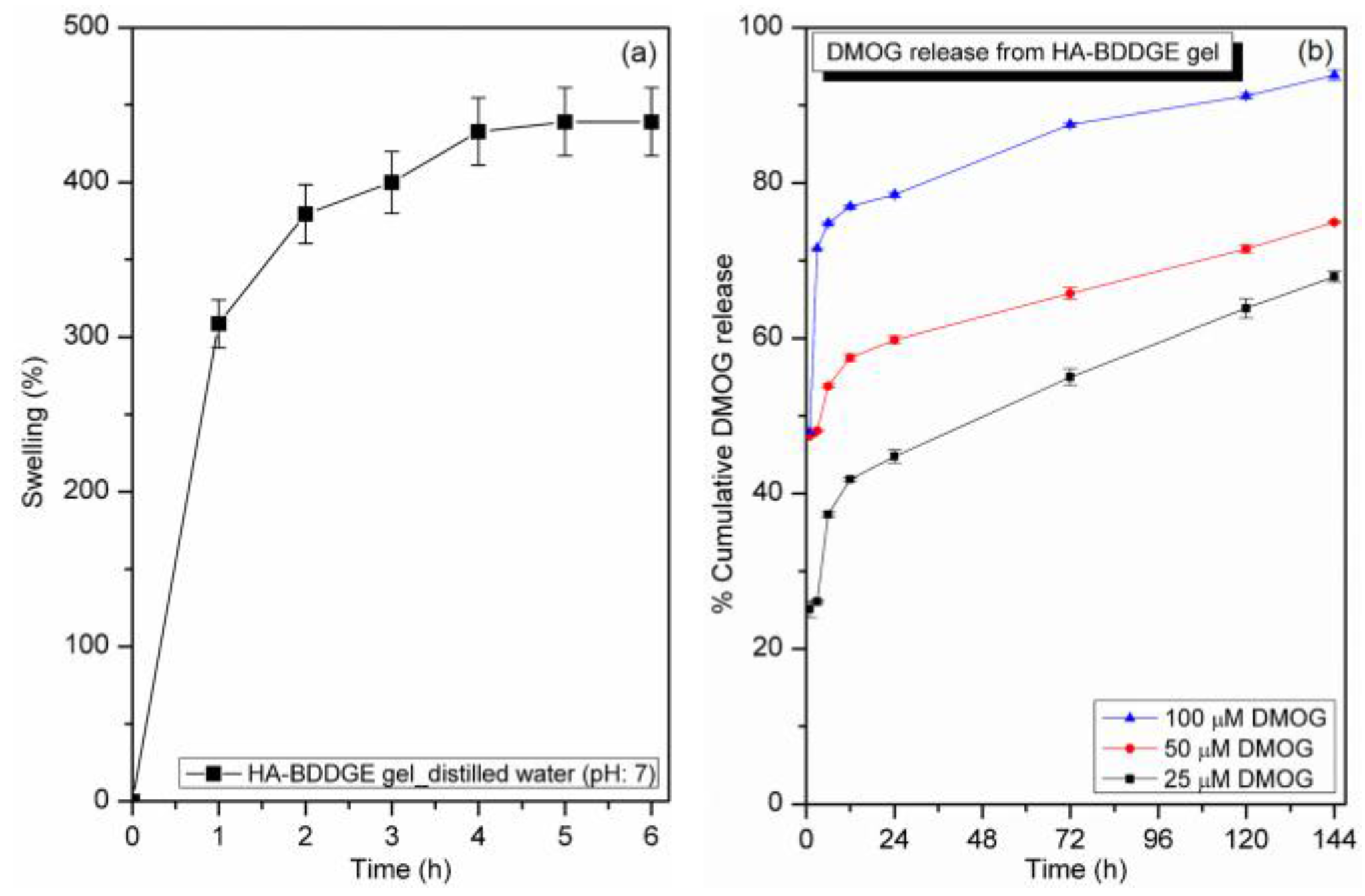
3.3. Swelling Study
3.4. In Vitro DMOG Release from Loaded HA-BDDGE Patterned Gel
3.5. In Vitro MC3T3 Cells Study
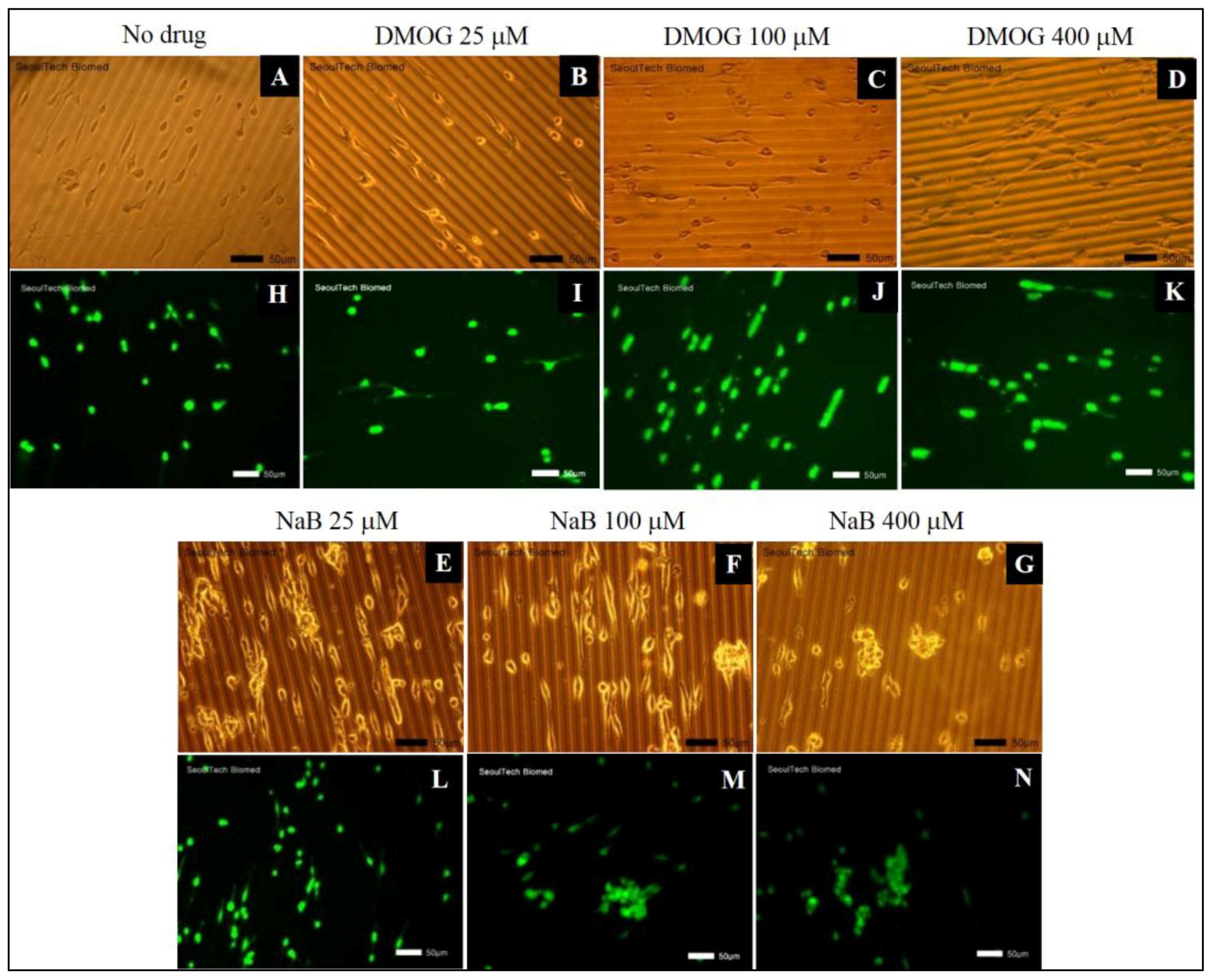
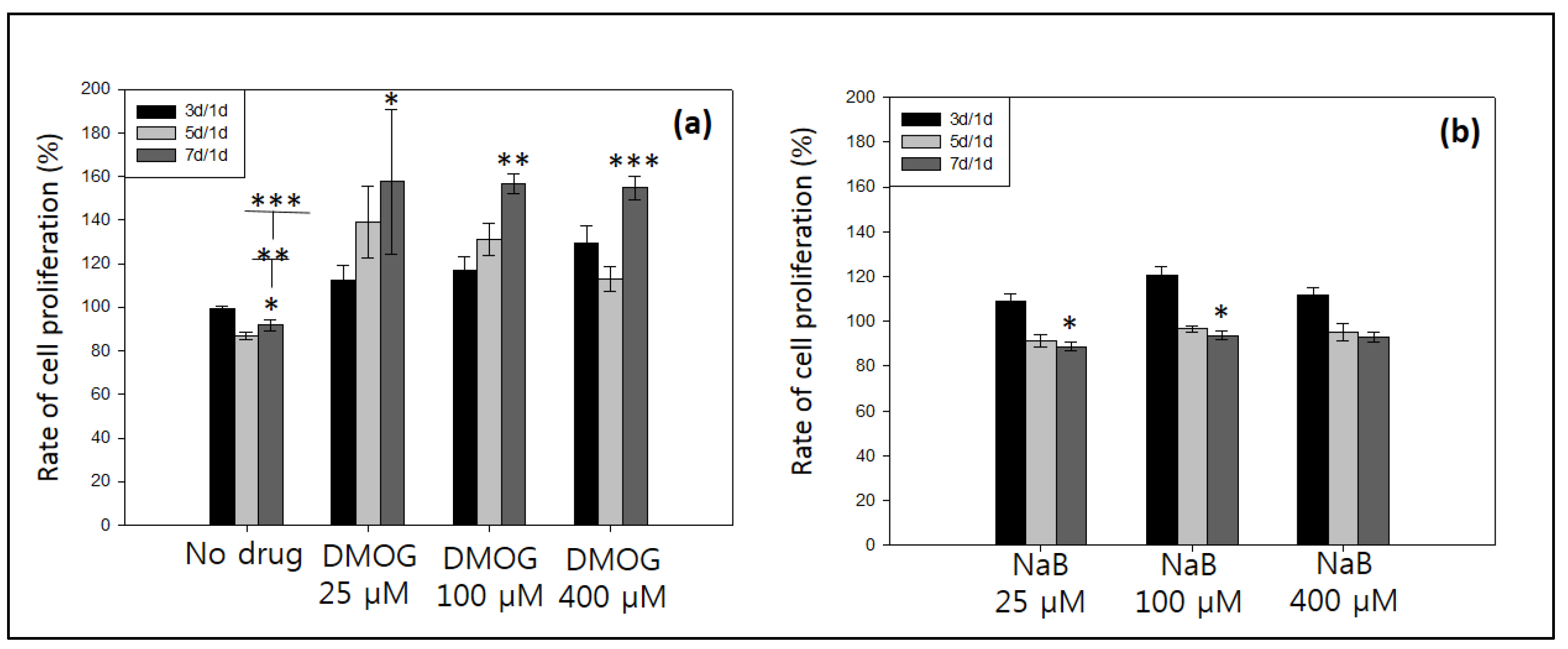
3.5.1. Effect of Drug Molecules of Cell Proliferation
3.5.2. Effect of DMOG or NaB to MC3T3 Cell Proliferation Cultured on the Surface of HA-BDDGE Patterned Gel
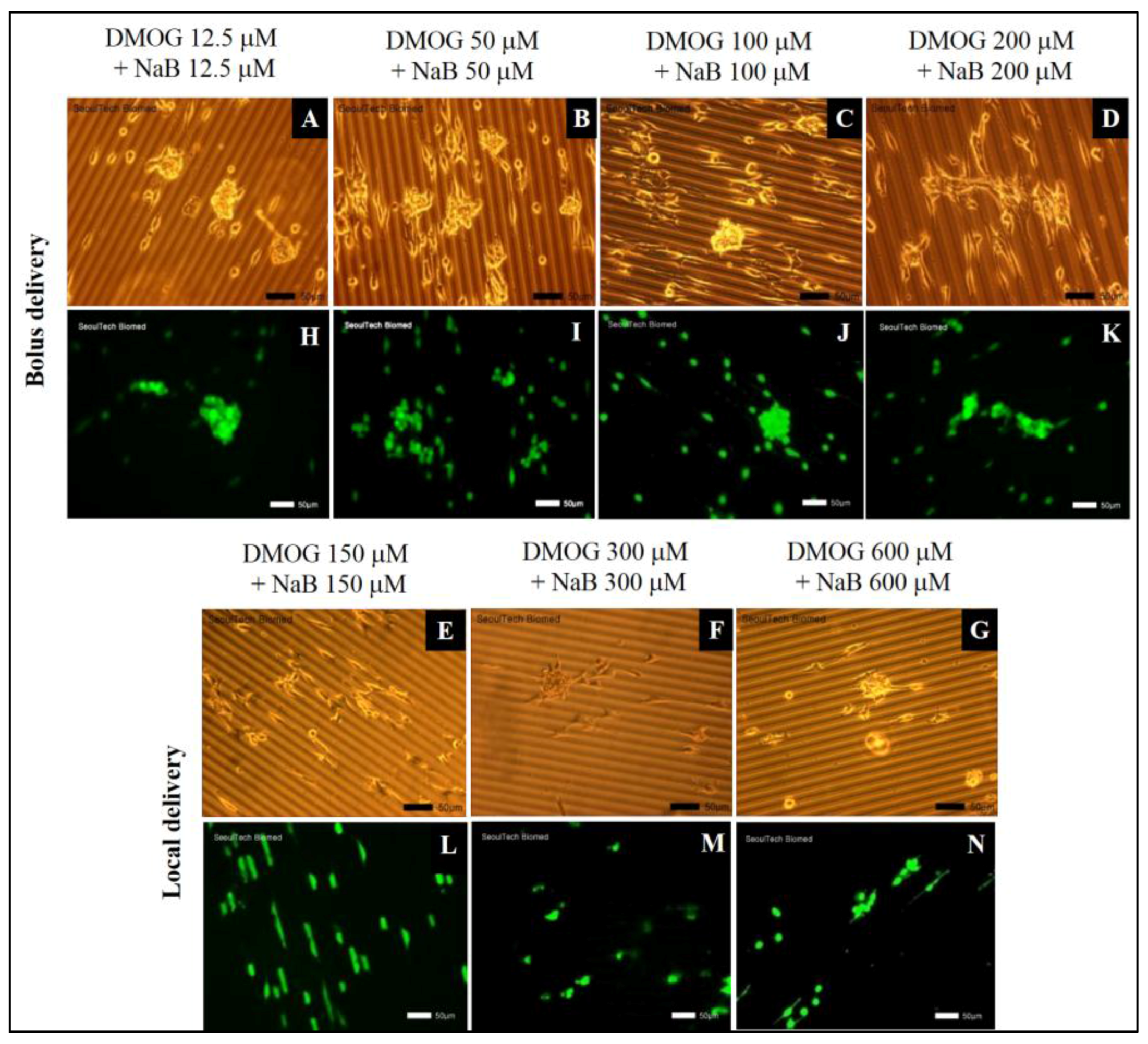
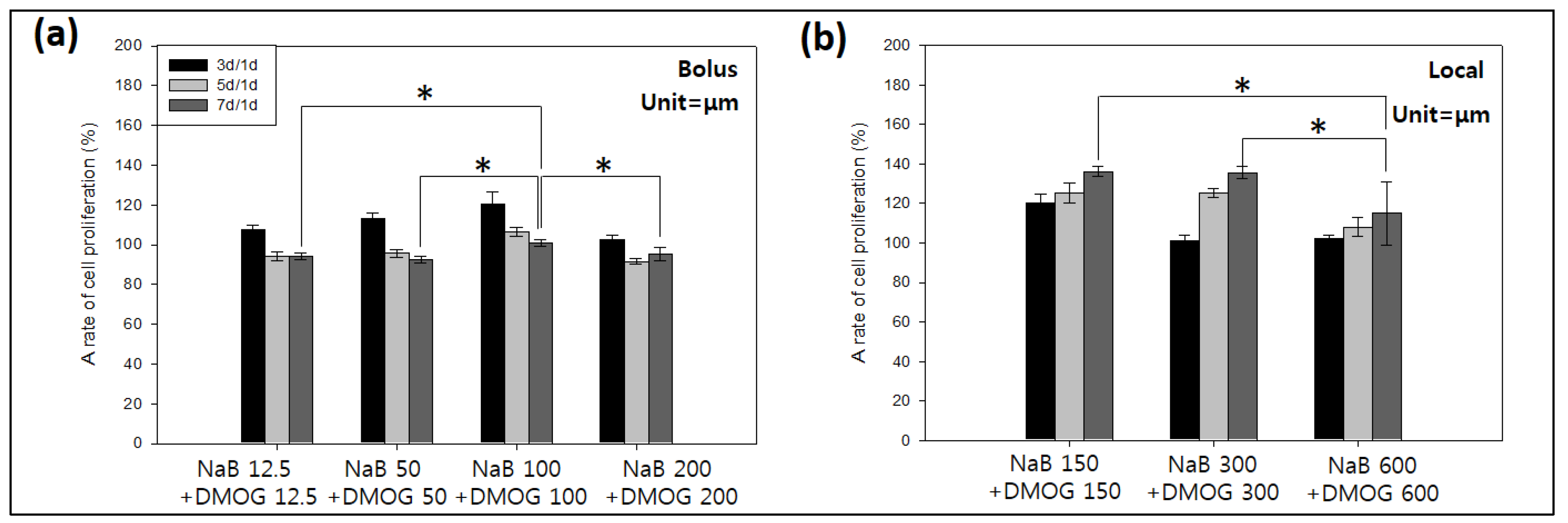
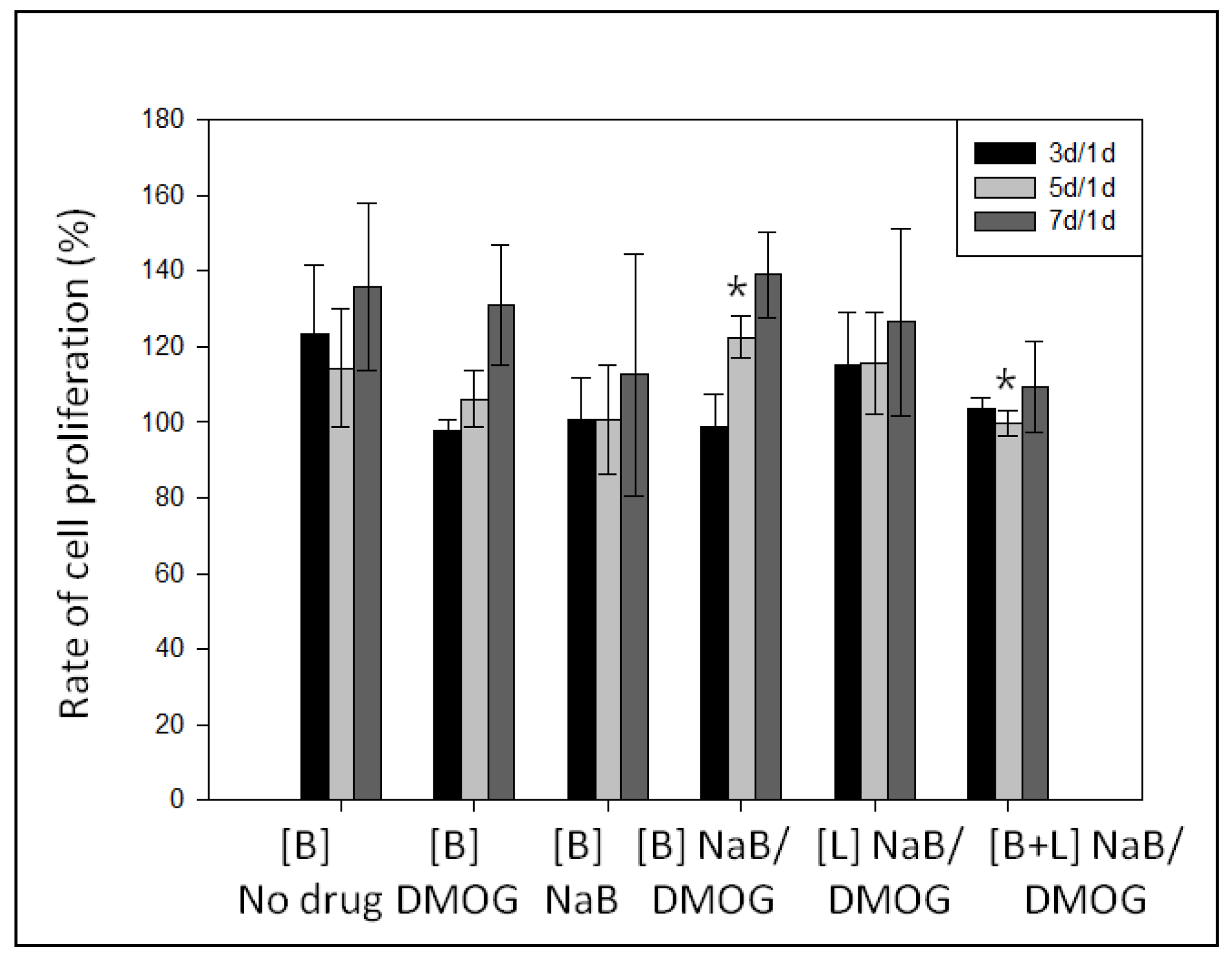
3.5.3. Effect of DMOG and NaB to MC3T3 Cell Proliferation on the Surface of HA-BDDGE Patterned Gel Depending on the Way of Delivery
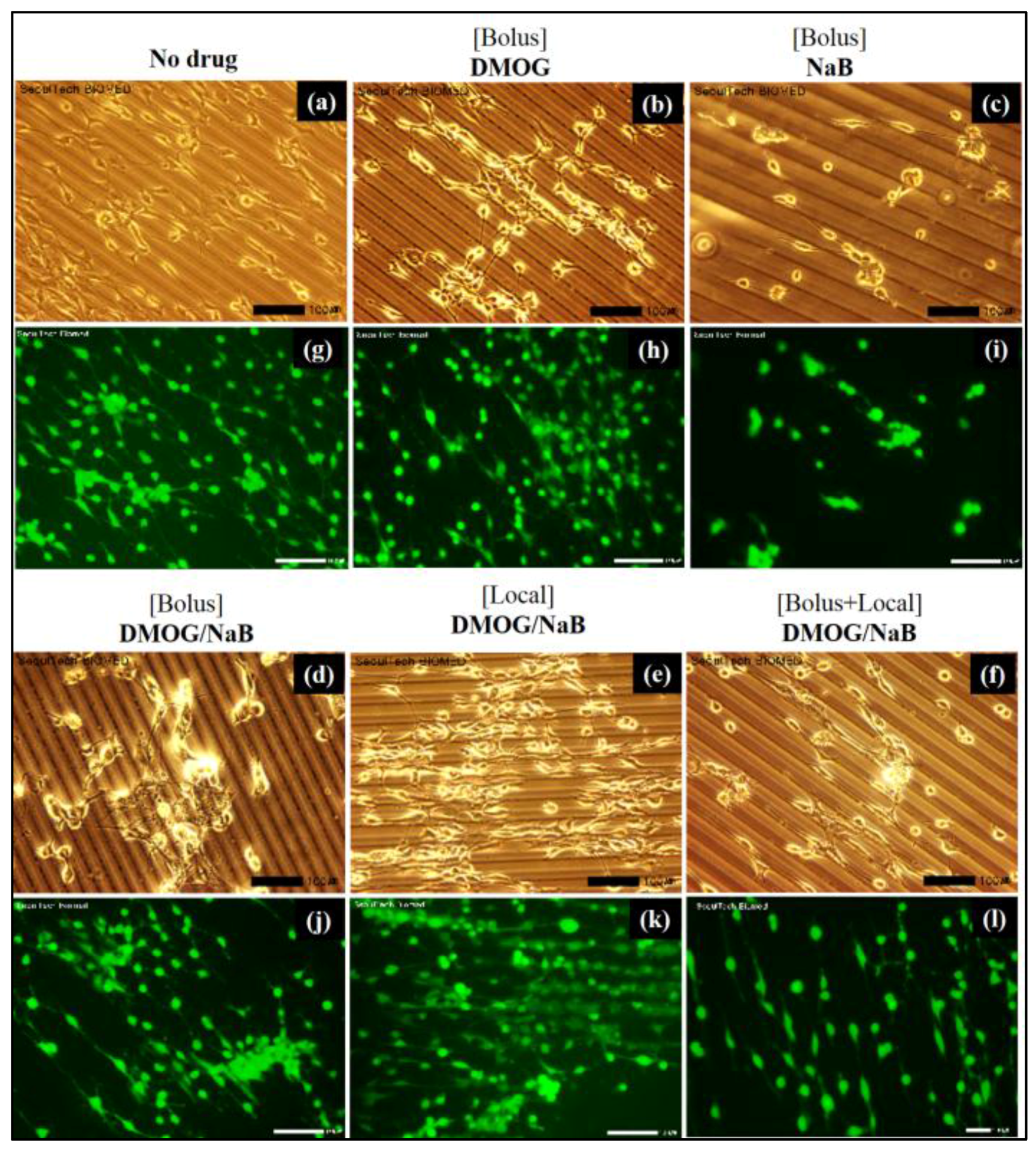
3.5.4. Cell Cluster Formation on the Surface of HA-BDDGE Patterned Gel by Bolus and Local Deliveries of DMOG and NaB
3.6. Effect of DMOG and NaB on Osteogenic or Angiogenic Responses of MC3T3 Cells Cultured on Pattern Gel
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cha, S.H.; Lee, H.J.; Koh, W.G. Study of myoblast differentiation using multi-dimensional scaffolds consisting of nano and micropatterns. Biomater. Res. 2017, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Khetan, S.; Burdick, J.A. Patterning hydrogels in three dimensions towards controlling cellular interactions. Soft Matter 2011, 7, 830–838. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.M.; Kim, K.S.; Lim, Y.M.; Nho, Y.C.; Shin, H. Modulation of Spreading, Proliferation, and Differentiation of Human Mesenchymal Stem Cells on Gelatin-Immobilized Poly(l-lactide-co-ϵ-caprolactone) Substrates. Biomacromolecules 2008, 9, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Ali, M.Y.; Saif, M.T.A. A novel technique for micro-patterning proteins and cells on polyacrylamide gels. Soft Matter 2012, 8, 7197–7206. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.; Lee, S.; Breen, A.; Shirazi, A.N.; Valtchev, P.; Dehghani, F. Enhancing the mechanical properties and physical stability of biomimetic polymer hydrogels for micro-patterning and tissue engineering applications. Eur. Polym. J. 2014, 59, 161–170. [Google Scholar] [CrossRef]
- Akintewe, O.O.; DuPont, S.J.; Elinen, K.K.; Cross, M.C.; Toomey, R.G.; Gallant, N.D. Microcontact printing of tissue precursors via geometrically patterned shape-changing hydrogel stamps preserves cell viability and organization. Bioprinting 2017. [Google Scholar] [CrossRef]
- Khademhosseini, A.; Eng, G.; Yeh, J.; Fukuda, J.; Blumling, J., III; Langer, R.; Burdick, J.A. Micromolding of photocrosslinkable hyaluronic acid for cell encapsulation and entrapment. J. Biomed. Mater. Res. A 2006, 79, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Alaribe, F.N.; Manoto, S.L.; Motaung, S.C.K.M. Scaffolds from biomaterials: Advantages and limitations in bone and tissue engineering. Biologia 2016, 71, 353–366. [Google Scholar] [CrossRef]
- Dorsey, T.B.; Grath, A.; Wang, A.; Xu, C.; Dai, G.; Hong, Y. Evaluation of photochemistry reaction kinetics to pattern bioactive proteins on hydrogels for biological applications. Bioact. Mater. 2017. [Google Scholar] [CrossRef]
- Lovett, M.; Lee, K.; Edwards, A.; Kaplan, D.L. Vascularization Strategies for Tissue Engineering. Tissue Eng. Part B Rev. 2009, 15, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, K.; Shimizu, T.; Horaguchi, S.; Sekine, H.; Yamato, M.; Umezu, M.; Okano, T. In Vitro Engineering of Vascularized Tissue Surrogates. Sci. Rep. 2013, 3, 1316. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue Engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zeng, Q.; Li, H. The calcium silicate/alginate composite: Preparation and evaluation of its behavior as bioactive injectable hydrogels. Acta Biomater. 2013, 9, 9107–9117. [Google Scholar] [CrossRef] [PubMed]
- Kook, Y.J.; Lee, D.H.; Song, J.E.; Tripathy, N.; Jeon, Y.S.; Jeon, H.Y.; Oliveira, J.M.; Reis, R.L.; Khang, G. Osteogenesis evaluation of duck’s feet-derived collagen/hydroxyapatite sponges immersed in dexamethasone. Biomater. Res. 2017, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.N.; Shah, S.R.; Lu, S. Injectable dual-gelling cell-laden composite hydrogels for bone tissue engineering. Biomaterials 2016, 83, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Ni, P.; Wang, B. Injectable and thermo-sensitive PEG-PCL-PEG copolymer/collagen/n-HA hydrogel composite for guided bone regeneration. Biomaterials 2012, 33, 4801–4809. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Saravanan, S.; Sastry, T.P. Nanohydroxyapatite-reinforced chitosan composite hydrogel for bone tissue repair in vitro and in vivo. J. Nanobiotechnol. 2015, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Vishnu Priya, M.; Sivshanmugam, A.; Boccaccini, A.R. Injectable osteogenic and angiogenic nanocomposite hydrogels for irregular bone defects. Biomed. Mater. 2016, 11, 035017. [Google Scholar] [CrossRef] [PubMed]
- Gothard, D.; Smith, E.L.; Kanczler, J.M.; Black, C.R.; Wells, J.A.; Roberts, C.A.; White, L.J.; Qutachi, O.; Peto, H.; Rashidi, H.; et al. In Vivo Assessment of Bone Regeneration in Alginate/Bone ECM Hydrogels with Incorporated Skeletal Stem Cells and Single Growth Factors. PLoS ONE 2015, 10, e0145080. [Google Scholar] [CrossRef] [PubMed]
- Laino, L.; Iezzi, G.; Piattelli, A.; Muzio, L.L.; Cicciu, M. Vertical Ridge Augmentation of the Atrophic Posterior Mandible with Sandwich Technique: Bone Block from the Chin Area versus Corticocancellous Bone Block Allograft—Clinical and Histological Prospective Randomized Controlled Study. BioMed Res. Int. 2014, 2014, 982104. [Google Scholar] [CrossRef] [PubMed]
- Petrauskaite, O.; Gomes, P.S.; Fernandes, M.H.; Juodzbalys, G.; Stumbras, A.; Maminskas, J.; Liesiene, J.; Cicciu, M. Biomimetic Mineralization on a Macroporous Cellulose-Based Matrix for Bone Regeneration. BioMed Res. Int. 2013, 2013, 452750. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Lee, S.Y.; Yoon, H.; Noh, I. Biological evaluation of micro-patterned hyaluronic acid hydrogel for bone tissue engineering. Pure Appl. Chem. 2014, 86, 1911–1922. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Sidwell, R.U.; Dhillon, A.P.; Butler, P.E.M.; Rustin, M.H.A. Localized granulomatous reaction to a semi-permanent hyaluronic acid and acrylic hydrogel cosmetic filler. Clin. Exp. Dermatol. 2004, 29, 630–632. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.J.; Maxwell, C.A.; Lowe, P.; Duickb, M.G.; Shah, K. Hyaluronic acid skin fillers: Adverse reactions and skin testing. J. Am. Acad. Dermatol. 2011, 45, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Mohabatpour, F.; Karkhaneh, A.; Sharifi, A.M. A hydrogel/fiber composite scaffold for chondrocyte encapsulation in cartilage tissue regeneration. RSC Adv. 2016, 6, 83135–83145. [Google Scholar] [CrossRef]
- Park, H.; Lee, H.J.; Ana, H.; Lee, K.Y. Alginate hydrogels modified with low molecular weight hyaluronate for cartilage regeneration. Carbohydr. Polym. 2017, 162, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tan, J.; Zhu, H.; Lin, G.; Yin, F.; Wang, L.; Song, K.; Wang, Y.; Zhou, G.; Yi, W. Development of kartogenin-conjugated chitosan–hyaluronic acid hydrogel for nucleus pulposus regeneration. Biomater. Sci. 2017, 5, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Hubbell, J.A. Hydrogel systems for barriers and local drug delivery in the control of wound healing. J. Control. Release 1996, 39, 305–313. [Google Scholar] [CrossRef]
- Bose, R.; Lee, S.H.; Park, H.S. Lipid-based surface engineering of PLGA nanoparticles for drug and gene delivery applications. Biomater. Res. 2016, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, M.; Takoh, K.; Matsue, T. Micropatterning of HeLa Cells on Glass Substrates and Evaluation of Respiratory Activity Using Microelectrodes. Langmuir 2002, 18, 3645–3649. [Google Scholar] [CrossRef]
- Falconnet, D.; Csucs, G.; Grandin, H.M.; Textor, M. Surface engineering approaches to micropattern surfaces for cell-based assays. Biomaterials 2006, 27, 3044–3063. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.W.; Wolfram, T.; Goldyn, A.M.; Bruellhoff, K.; Rioj, B.A.; Moller, M.; Spatz, J.P.; Saif, T.A.; Groll, J.; Kemkemer, R. Myoblast morphology and organization on biochemically micro-patterned hydrogel coatings under cyclic mechanical strain. Biomaterials 2009, 31, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Jiang, X.; Whiteside, G.M. Microengineering the Environment of Mammalian Cells in Culture. MRS Bull. 2005, 30, 194–201. [Google Scholar] [CrossRef]
- Poellmann, M.J.; Harrell, P.A.; King, W.P.; Johnson, A.J.W. Geometric microenvironment directs cell morphology on topographically patterned hydrogel substrates. Acta Biomater. 2010, 6, 3514–3523. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.M.; Jean, R.P.; Tan, J.L.; Liu, W.F.; Sniadecki, N.J.; Spector, A.A.; Chen, C.S. Emergent patterns of growth controlled by multicellular form and mechanics. Proc. Natl. Acad. Sci. USA 2005, 102, 11594–11599. [Google Scholar] [CrossRef] [PubMed]
- James, J.; Goluch, E.D.; Hu, H.; Liu, C.; Mrksich, M. Subcellular curvature at the perimeter of micropatterned cells influences lamellipodial distribution and cell polarity. Cell. Motil. Cytoskelet. 2008, 65, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, G.; Campbell, C.J.; Bishop, K.J.M.; Komarova, Y.A.; Chaga, O.; Soh, S.; Huda, S.; Kandere-Grzybowska, K.; Grzybowski, B.A. Directing cell motions on micropatterned ratchets. Nat. Phys. 2009, 5, 606–612. [Google Scholar] [CrossRef]
- Gilli, R.; Kacurakova, M.; Mathlouthi, M.; Navarini, L.; Paoletti, S. FTIR studies of sodium hyaluronate and its oligomers in the amorphous solid phase and in aqueous solution. Carbohydr. Res. 1994, 263, 315–326. [Google Scholar] [CrossRef]
- Kim, D.; Park, H.J.; Lee, K.Y. Study on Curing Behaviors of Epoxy Acrylates by UV with and without Aromatic Component. Macromol. Res. 2015, 23, 944–951. [Google Scholar] [CrossRef]
- Cowman, M.K.; Hittner, D.M.; Feder-Davis, J. 13C-NMR Studies of Hyaluronan: Conformational Sensitivity to Varied Environments. Macromolecules 1996, 29, 2894–2902. [Google Scholar] [CrossRef]
- Sheu, C.; Shalumon, K.T.; Chen, C.H.; Kuo, C.Y.; Fong, Y.T.; Chen, J.P. Dual crosslinked hyaluronic acid nanofibrous membranes for prolonged prevention of post-surgical peritoneal adhesion. J. Mater. Chem. B 2016, 4, 6680–6693. [Google Scholar] [CrossRef]
- Iwami, K.; Moriyama, T. Effects of short chain fatty acid, sodium butyrate, on osteoblastic cells and osteoclastic cells. Int. J. Biochem. 1993, 25, 1631–1635. [Google Scholar] [CrossRef]
- Woo, K.M.; Jung, H.M.; Oh, J.H.; Rahman, S.; Kim, S.M.; Baek, J.H.; Ryoo, H.M. Synergistic effects of dimethyloxalylglycine and butyrate incorporated into α-calcium sulfate on bone regeneration. Biomaterials 2015, 39, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Chen, S.; Song, W.Q.; Gao, Y.S.; Guan, J.J.; Wang, Y.; Sun, Y.; Zhang, C.Q. Dimethyloxaloylglycine Improves Angiogenic Activity of Bone Marrow Stromal Cells in the Tissue Engineered Bone. Int. J. Biol. Sci. 2014, 10, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Tarkkonen, K.; Hieta, R.; Kytola, V.; Nykter, M.; Kiviranta, R. Comparative analysis of osteoblast gene expression profiles and Runx2 genomic occupancy of mouse and human osteoblasts in vitro. Gene 2017, 626, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Herford, A.S.; Cicciu, M.; Eftimie, L.F.; Miller, M.; Signorino, F.; Fama, F.; Cervino, G.; Giudice, G.L.; Bramanti, E.; Lauritano, F.; et al. rhBMP-2 applied as support of distraction osteogenesis: A split-mouth histological study over nonhuman primates mandibles. Int. J. Clin. Exp. Med. 2016, 9, 17187–17194. [Google Scholar]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bang, S.; Das, D.; Yu, J.; Noh, I. Evaluation of MC3T3 Cells Proliferation and Drug Release Study from Sodium Hyaluronate-1,4-butanediol Diglycidyl Ether Patterned Gel. Nanomaterials 2017, 7, 328. https://doi.org/10.3390/nano7100328
Bang S, Das D, Yu J, Noh I. Evaluation of MC3T3 Cells Proliferation and Drug Release Study from Sodium Hyaluronate-1,4-butanediol Diglycidyl Ether Patterned Gel. Nanomaterials. 2017; 7(10):328. https://doi.org/10.3390/nano7100328
Chicago/Turabian StyleBang, Sumi, Dipankar Das, Jiyun Yu, and Insup Noh. 2017. "Evaluation of MC3T3 Cells Proliferation and Drug Release Study from Sodium Hyaluronate-1,4-butanediol Diglycidyl Ether Patterned Gel" Nanomaterials 7, no. 10: 328. https://doi.org/10.3390/nano7100328