Ti-Based Biomedical Material Modified with TiOx/TiNx Duplex Bioactivity Film via Micro-Arc Oxidation and Nitrogen Ion Implantation
Abstract
:1. Introduction
2. Experimental Procedure
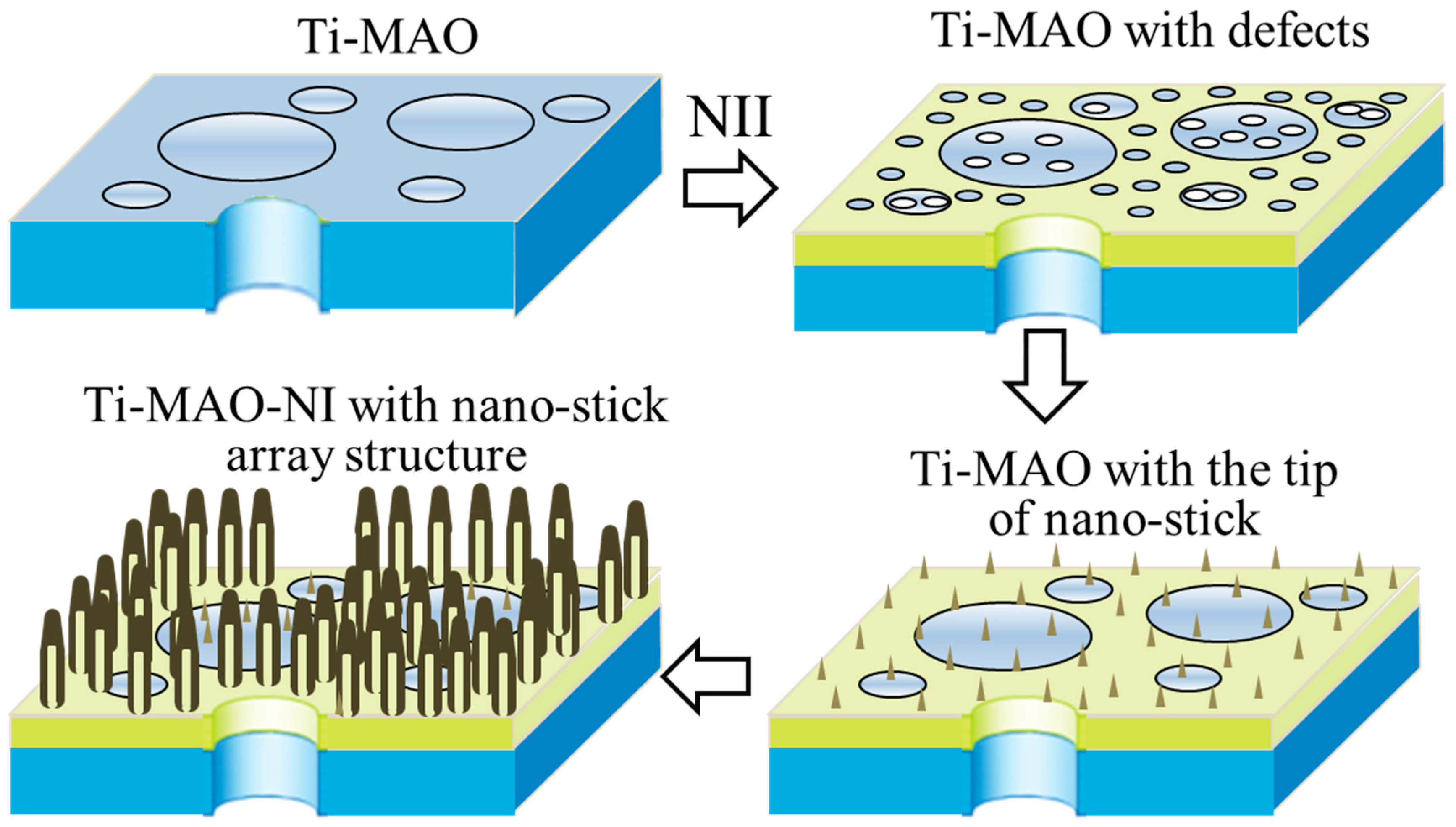
2.1. Preparation (MAO and NII Treatments)
2.2. Structure Characterization
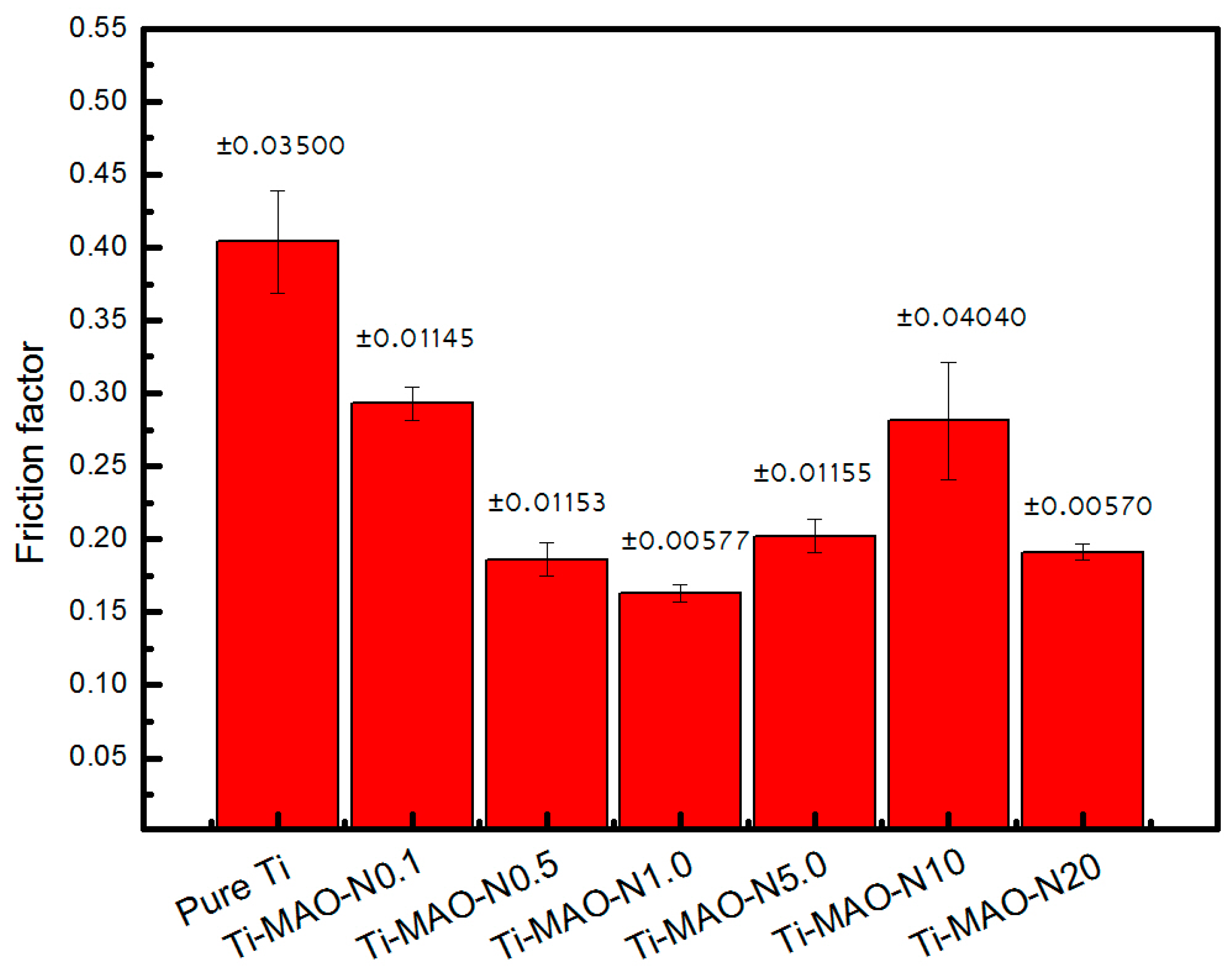
2.3. Friction and Wear Test Bioactivity Evaluation
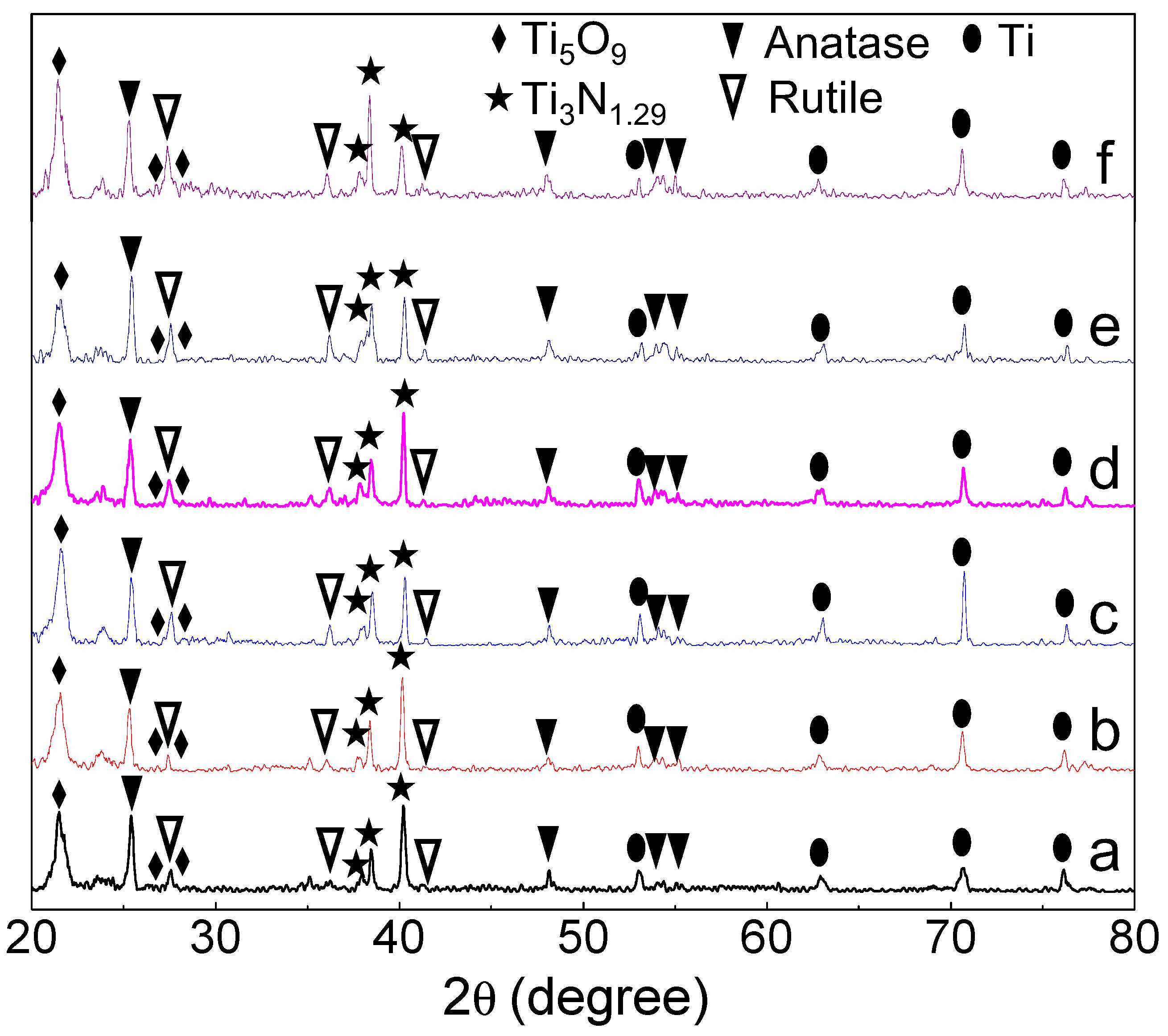
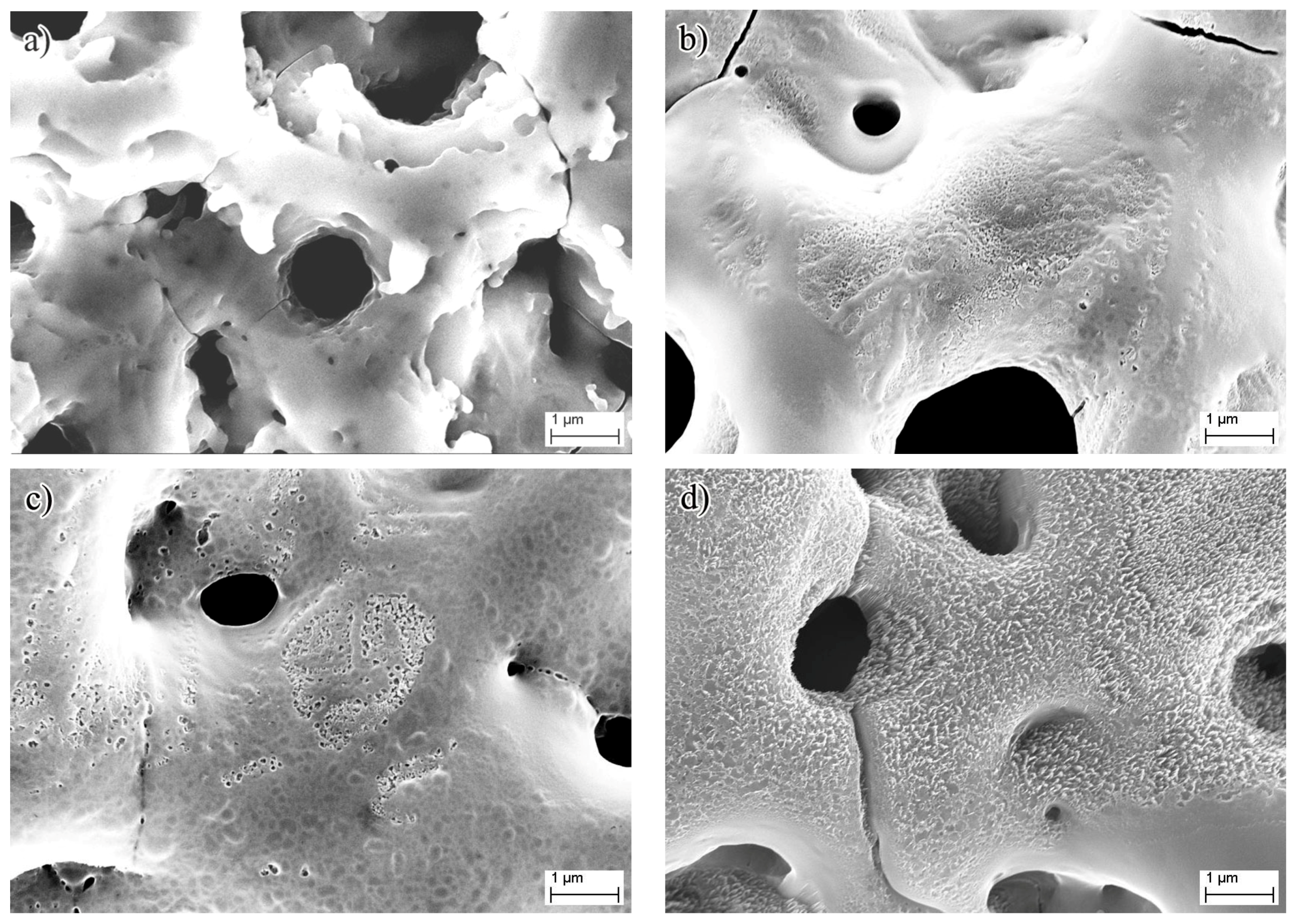
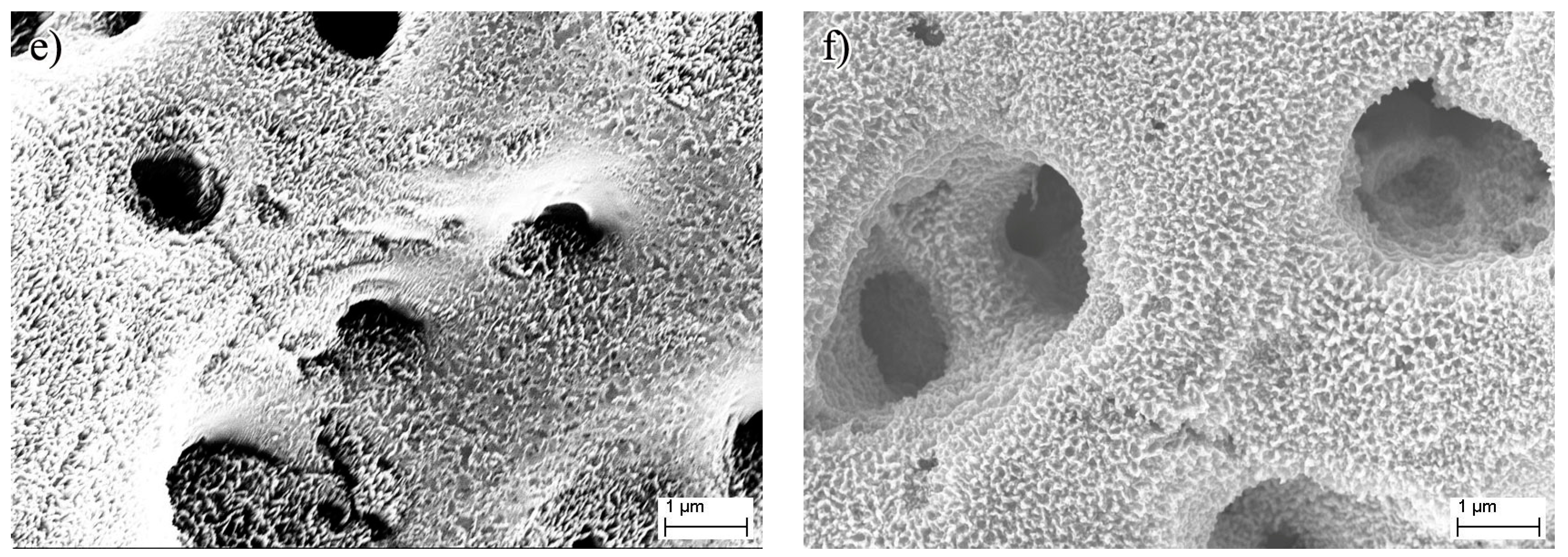
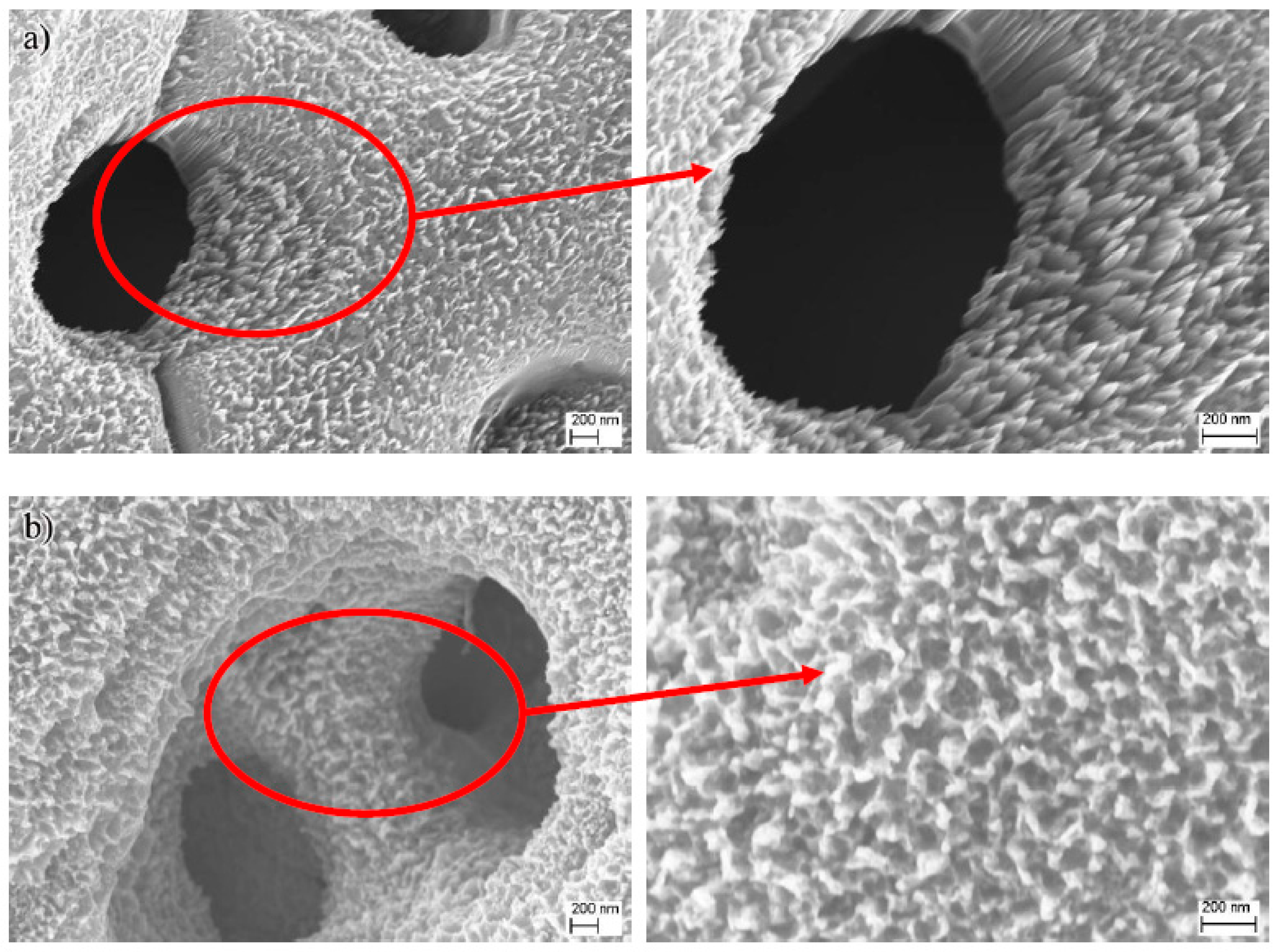
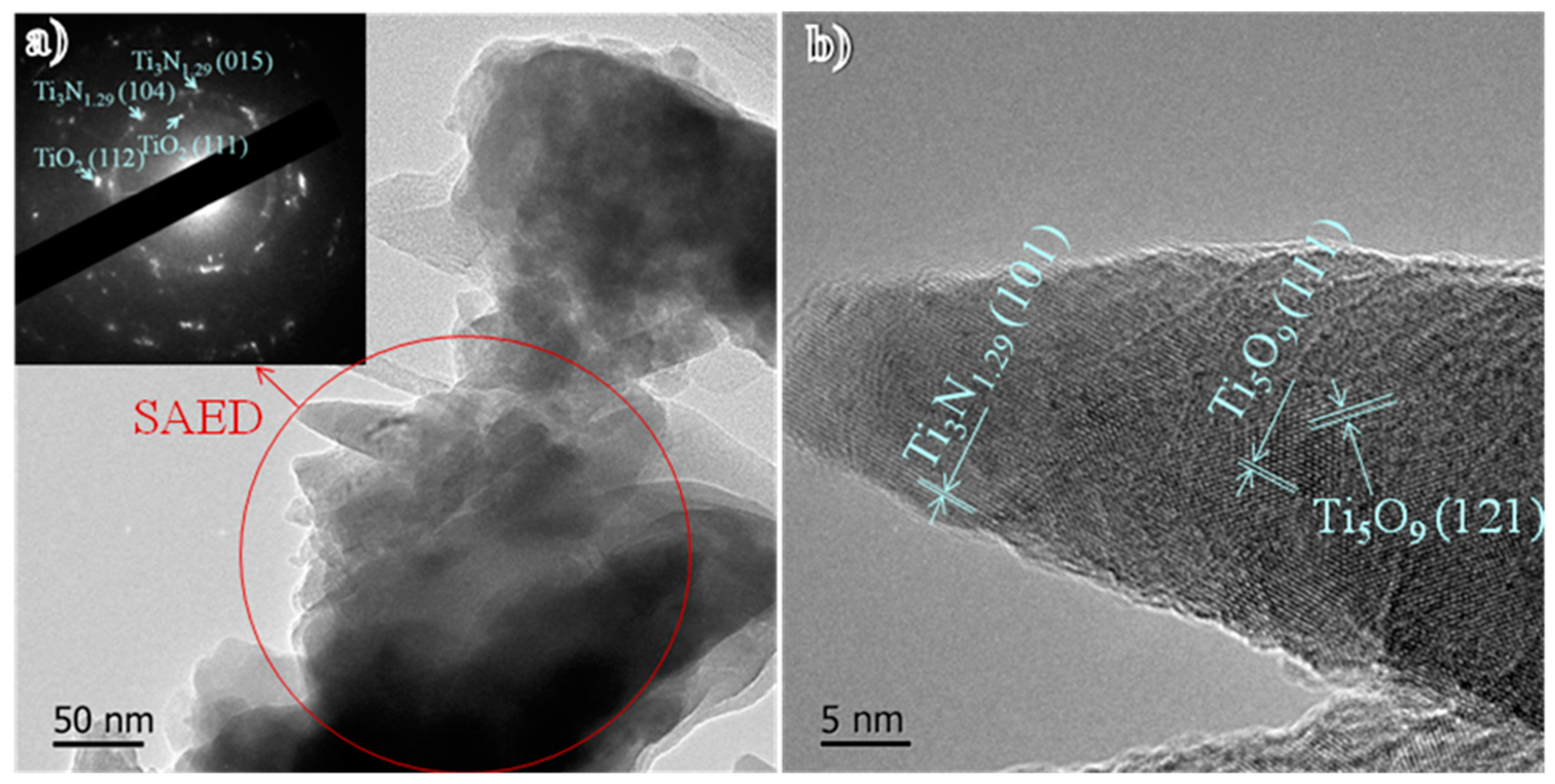
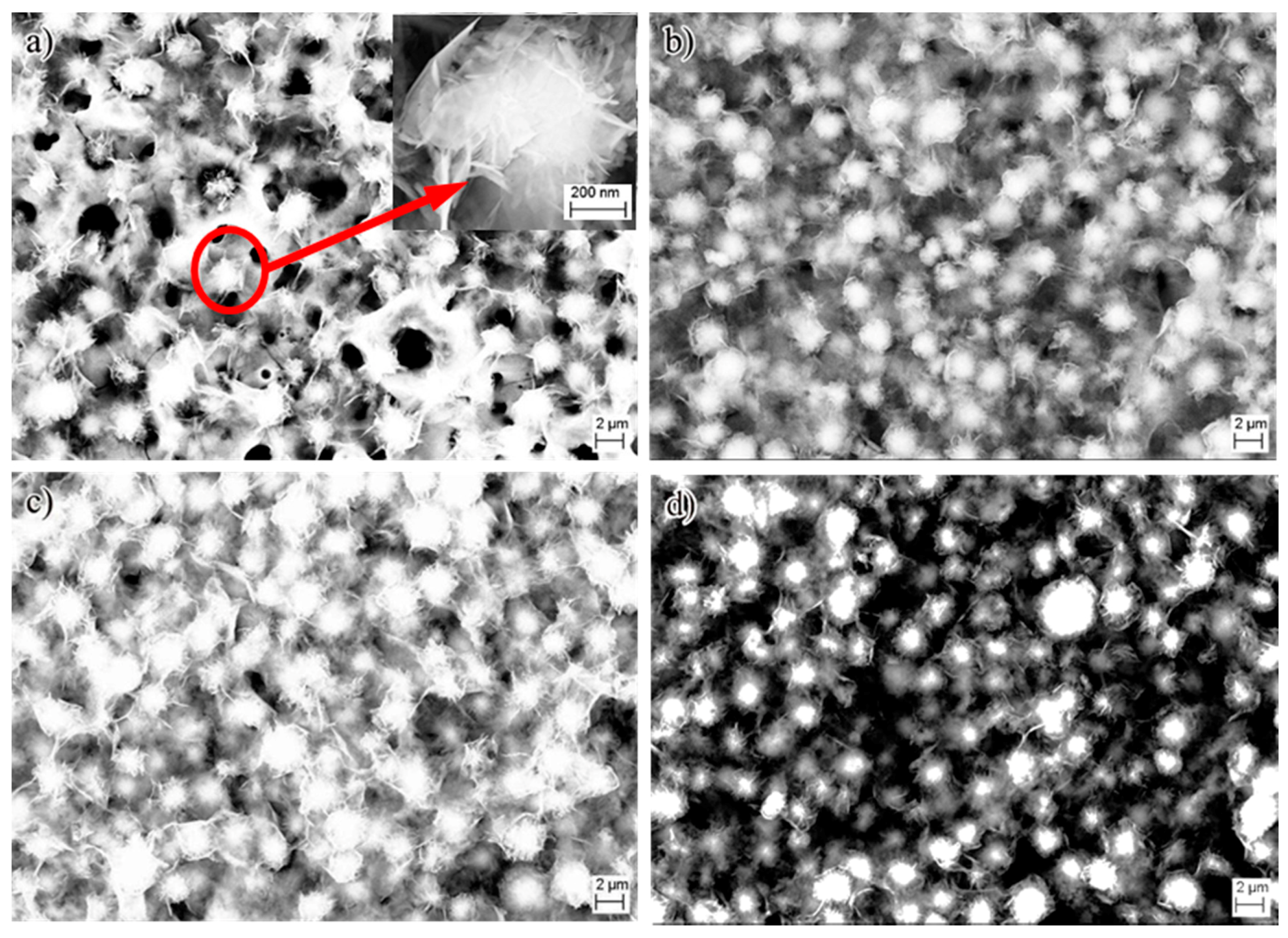
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rack, H.J.; Qazi, J.I. Titanium alloys for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2006, 26, 1269–1277. [Google Scholar] [CrossRef]
- Kokubo, T.; Kim, H.M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Chen, J.Y.; Qi, S.K.; He, L.; Zhao, J.Z.; Zhang, X.D. Characterization of surface oxide films on titanium and bioactivity. J. Mater. Sci. Mater. Med. 2002, 13, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.D.; Jang, T.S.; Wang, L.; Kim, H.E.; Koh, Y.H.; Song, J. Progress in Research on the Surface/Interface of Materials for Hard issue Implant. Biomaterials 2015, 37, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lee, P.D.; Dashwood, R.J.; Lindley, T.C. Titanium foams for biomedical applications: A review. Mater. Technol. 2010, 25, 127–136. [Google Scholar] [CrossRef]
- Niinomi, M.; Akahori, T. Improvement of the fatigue life of titanium alloys for biomedical devices through microstructural control. Expert Rev. Med. Devices 2010, 7, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Metallic biomaterials. J. Artif. Organs 2008, 11, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Oshida, Y.; Tuna, E.B.; Aktören, O.; Gençay, K. Dental Implant Systems. Int. J. Mol. Sci. 2010, 11, 1580–1678. [Google Scholar] [CrossRef] [PubMed]
- Subramani, K.; Jung, R.E.; Molenberg, A. Biofilm on Dental Implants—A Review of the Literature. Int. J. Oral Maxillofac. Implants 2009, 24, 616–626. [Google Scholar] [PubMed]
- Graham, W.G.; Stalder, K.R. Plasmas in liquids and some of their applications in nanoscience. J. Phys. D Appl. Phys. 2011, 44, 17. [Google Scholar] [CrossRef]
- Dzhurinskiy, D.; Gao, Y.; Yeung, W.K.; Strumban, E.; Leshchinsky, V.; Chu, P.J.; Matthews, A.; Yerokhin, A.; Maev, R.G. Characterization and corrosion evaluation of TiO2:n-HA coatings on titanium alloy formed by plasma electrolytic oxidation. Surf. Coat. Technol. 2015, 269, 258–265. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, M.Q.; He, G.; Zhang, X.L. Surface mdification of porous titanium with microarc oxidation and its effects on osteogenesis activity in vitro. J. Nanomater. 2015, 16, 408634. [Google Scholar]
- Walsh, F.C.; Low, C.T.J.; Wood, R.J.K.; Stevens, K.T.; Archer, J.; Poeton, A.R.; Ryder, A. Plasma electrolytic oxidation (PEO) for production of anodised coatings on lightweight metal (Al, Mg, Ti) alloys. Trans. Inst. Met. Finish. 2009, 87, 122–135. [Google Scholar] [CrossRef]
- Beline, T.; Marques, I.D.V.; Matos, A.O.; Ogawa, E.S.; Ricomini, A.P.; Rangel, E.C.; da Cruz, N.C.; Sukotjo, C.; Mathew, M.T.; Landers, R. Production of a biofunctional titanium surface using plasma electrolytic oxidation and glow-discharge plasma for biomedical applications. Biointerphases 2016, 11, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Marin, E.; Diamanti, M.V.; Boffelli, M.; Sendoh, M.; Pedeferri, M.P.; Mazinani, A.; Moscatelli, M.; Del Curto, B.; Zhu, W.; Pezzotti, G. Effect of etching on the composition and structure of anodic spark deposition films on titanium. Mater. Des. 2016, 108, 77–85. [Google Scholar] [CrossRef]
- Paduanoa, F.; Marrelli, M.; Alom, N.; Amer, M.; White, J.L.; Shakesheff, M.K.; Tatulloa, M. Decellularized bone extracellular matrix and human dental pulp stem cells as a construct for bone regeneration. J. Biomater. Sci. Polym. Ed. 2017, 28, 730–748. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Tatullo, M. Influence of PRF in the healing of bone and gingival tissues. Clinical and histological evaluations. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1958–1962. [Google Scholar] [PubMed]
- Marrelli, M.; Falisi, G.; Apicella, A.; Apicella, D.; Amantea, M.; Cielo, A.; Bonanome, L.; Palmieri, F.; Santacroce, L.; Giannini, S.; et al. Behaviour of dental pulp stem cells on different types of innovative mesoporous and nanoporous silicon scaffolds with different functionalizations of the surfaces. J. Biol. Regul. Homeost. Agents 2015, 29, 217–223. [Google Scholar]
- Lai, Y.K.; Huang, J.Y.; Cui, Z.Q.; Ge, M.Z.; Zhang, K.Q.; Chen, Z.; Chi, L.F. Recent advances in TiO2-based nanostructured surfaces with controllable wettability and adhesion. Small 2016, 12, 2203–2224. [Google Scholar] [CrossRef] [PubMed]
- Krasimir, V.; Melanie, M.R. Plasma Nanoengineering and Nanofabrication. Nanomaterials 2016, 6, 122. [Google Scholar]
- Nabavi, H.F.; Aliofkhazraei, M.; Rouhaghdam, A.S. Morphology and corrosion resistance of hybrid plasma electrolytic oxidation on CP-Ti. Surf. Coat. Technol. 2017, 322, 59–69. [Google Scholar] [CrossRef]
- Aleksandra, R.; Adrian, T.; Tomasz, J.; Wiesław, K.; Beata, S.; Marzena, W.S.; Magdalena, S.; Ewa, T.; Lars, P.N.; Piotr, P. The Bioactivity and Photocatalytic Properties of Titania Nanotube Coatings Produced with the Use of the Low-Potential Anodization of Ti6Al4V Alloy Surface. Nanomaterials 2017, 7, 197. [Google Scholar]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Liu, J.D.; Zhao, S.P.; Wang, H.L.; Cui, Y.X.; Jiang, W.W.; Liu, S.M.; Wang, N.; Liu, C.Q.; Chai, W.P.; Ding, W.Y. Study on the chemical bond structure and chemical stability of N doped into TiO2 film by N ion beam implantation. Micro Nano Lett. 2016, 11, 758–761. [Google Scholar] [CrossRef]
- Piscanec, S.; Colombi Ciacchi, L.; Vesselli, E.; Comelli, G.; Sbaizero, O.; Meriani, S.; De Vita, A. Bioactivity of TiN-coated titanium implants. Acta Mater. 2004, 52, 1237–1245. [Google Scholar] [CrossRef]
- Hamidia, M.F.F.A.; Harunb, W.S.W.; Samykanoc, M.; Ghanib, S.A.C.; Ghazallib, Z.; Ahmadd, F.; Sulonge, A.B. A review of biocompatible metal injection moulding process parameters for biomedical applications. Mater. Sci. Eng. C 2017, 78, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Tadashi, K.; Hiroaki, T. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar]
- Shirai, T.; Shimizu, T.; Ohtani, K.; Zen, Y.; Takaya, M.; Tsuchiya, H. Antibacterial iodine-supported titanium implants. Acta Biomater. 2011, 7, 1928–1933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, Z.G.; Li, W.; Zhu, M. Effect of Ti-OH groups on microstructure and bioactivity of TiO2 coating prepared by micro-arc oxidation. Appl. Surf. Sci. 2013, 268, 381–386. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Marrelli, M.; Falisi, G.; Rastelli, C.; Palmieri, F.; Gargari, M.; Zavan, B.; Paduano, F.; Benagiano, V. Mechanical influence of tissue culture plates and extracellular matrix on mesenchymal stem cell behavior: A topical review. Int. J. Immunopathol. Pharmacol. 2016, 29, 3–8. [Google Scholar] [CrossRef] [PubMed]

| Sample Name | Element Content (wt %) | ||||
|---|---|---|---|---|---|
| Ti | O | Ca | P | N | |
| Ti-MAO-N0.1 | 41.12 | 43.39 | 10.30 | 3.47 | 1.72 |
| Ti-MAO-N1.0 | 42.30 | 41.19 | 11.01 | 3.48 | 2.02 |
| Ti-MAO-N5.0 | 36.79 | 40.52 | 14.43 | 4.94 | 3.32 |
| Ti-MAO-N20 | 51.89 | 36.05 | 5.87 | 4.14 | 2.05 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Wang, X.; Lin, Z.; Lin, H.; Zhang, Z.; Li, W.; Yang, X.; Cui, J. Ti-Based Biomedical Material Modified with TiOx/TiNx Duplex Bioactivity Film via Micro-Arc Oxidation and Nitrogen Ion Implantation. Nanomaterials 2017, 7, 343. https://doi.org/10.3390/nano7100343
Zhang P, Wang X, Lin Z, Lin H, Zhang Z, Li W, Yang X, Cui J. Ti-Based Biomedical Material Modified with TiOx/TiNx Duplex Bioactivity Film via Micro-Arc Oxidation and Nitrogen Ion Implantation. Nanomaterials. 2017; 7(10):343. https://doi.org/10.3390/nano7100343
Chicago/Turabian StyleZhang, Peng, Xiaojian Wang, Zhidan Lin, Huaijun Lin, Zhiguo Zhang, Wei Li, Xianfeng Yang, and Jie Cui. 2017. "Ti-Based Biomedical Material Modified with TiOx/TiNx Duplex Bioactivity Film via Micro-Arc Oxidation and Nitrogen Ion Implantation" Nanomaterials 7, no. 10: 343. https://doi.org/10.3390/nano7100343