Microwave-Hydrothermal Synthesis of SnO2-CNTs Hybrid Nanocomposites with Visible Light Photocatalytic Activity
Abstract
:1. Introduction
2. Materials and Methods
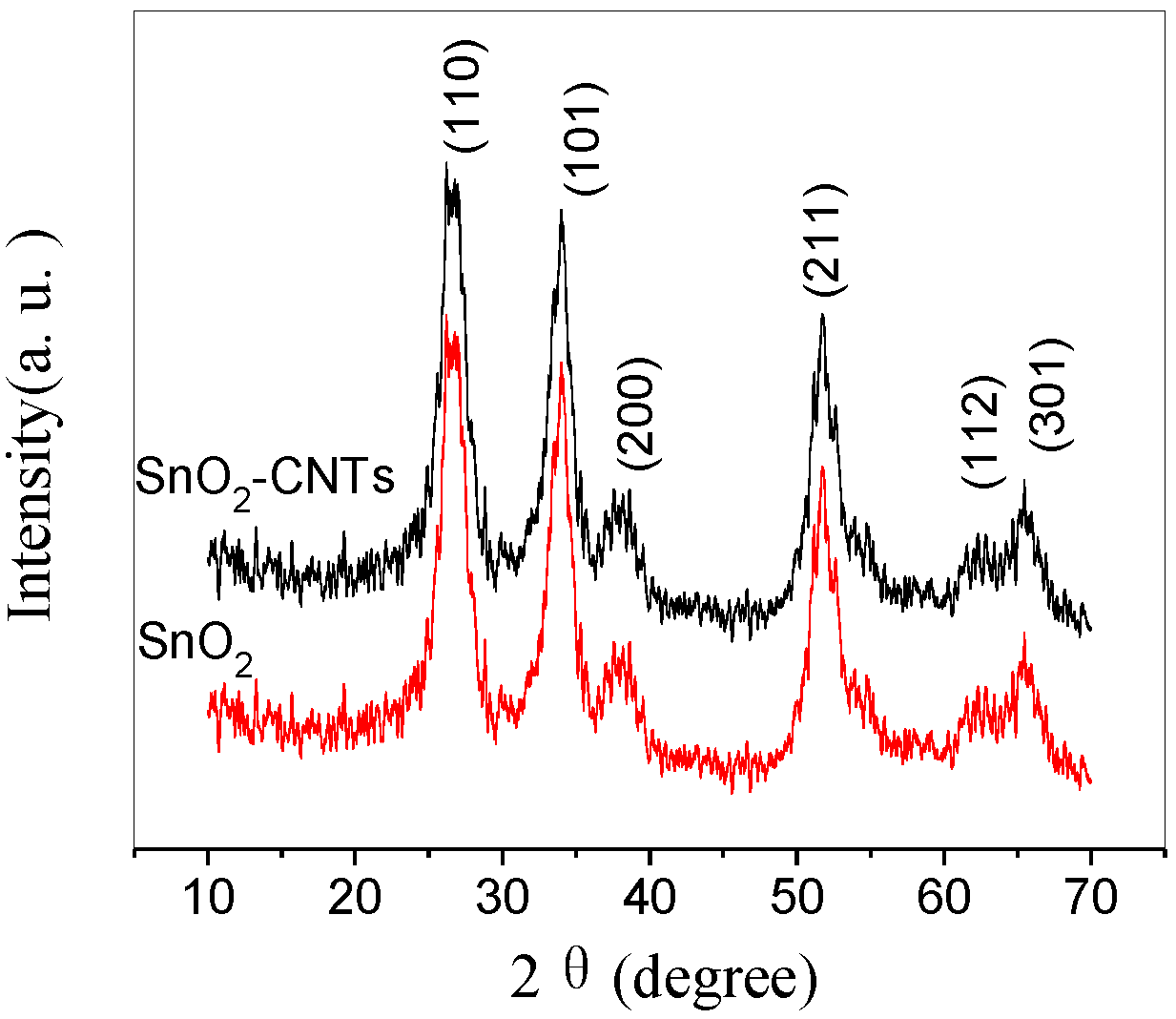
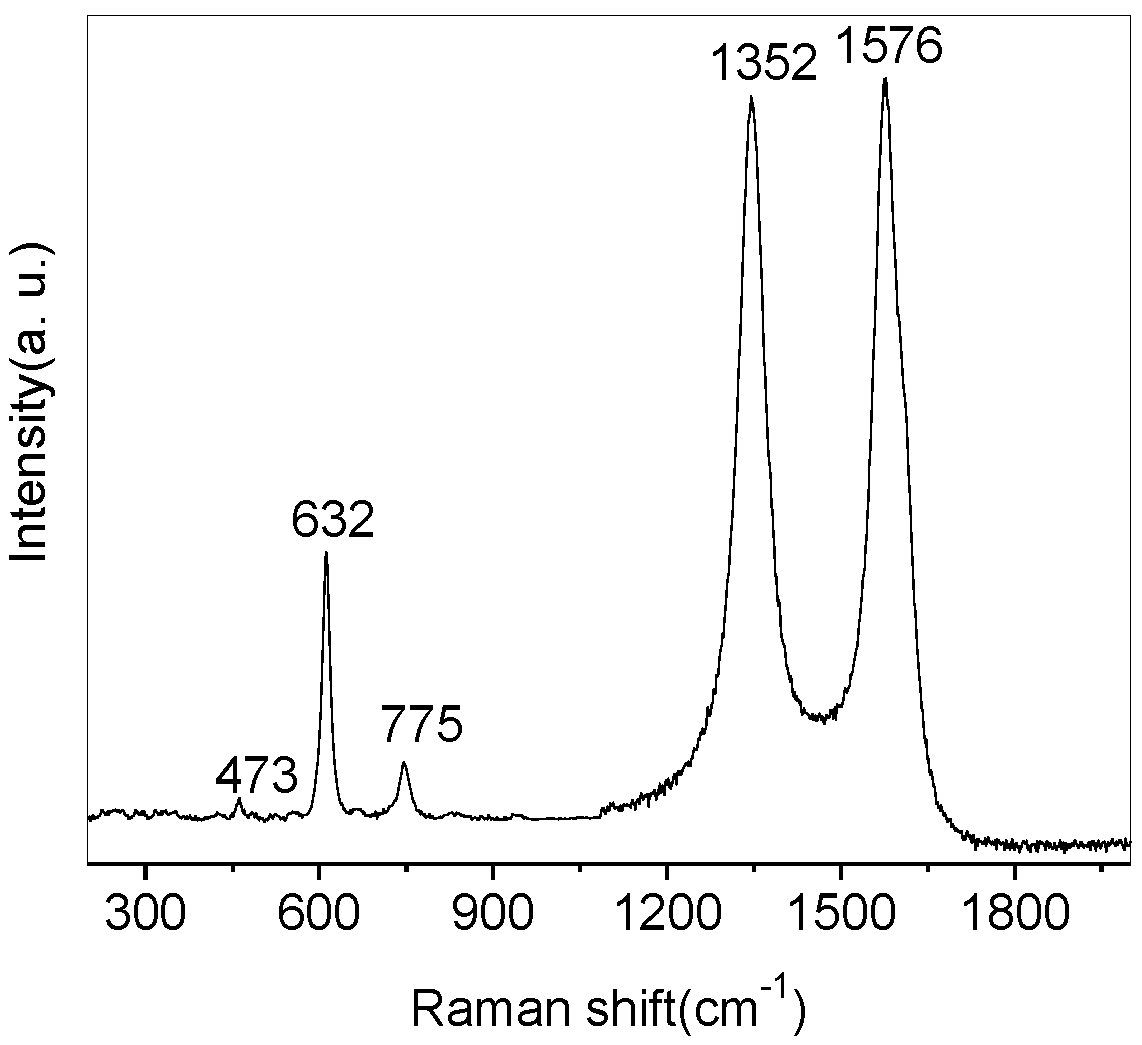
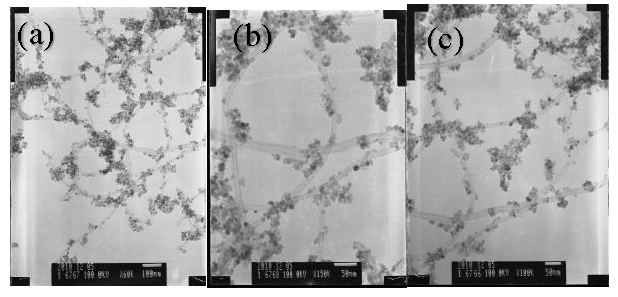
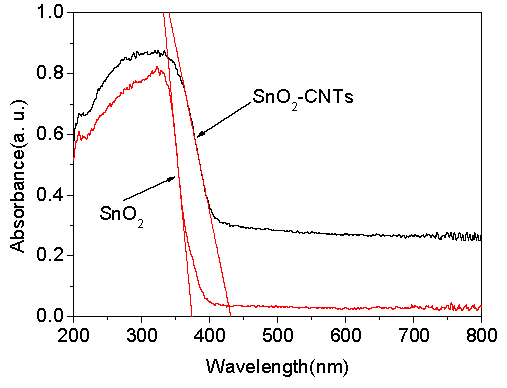
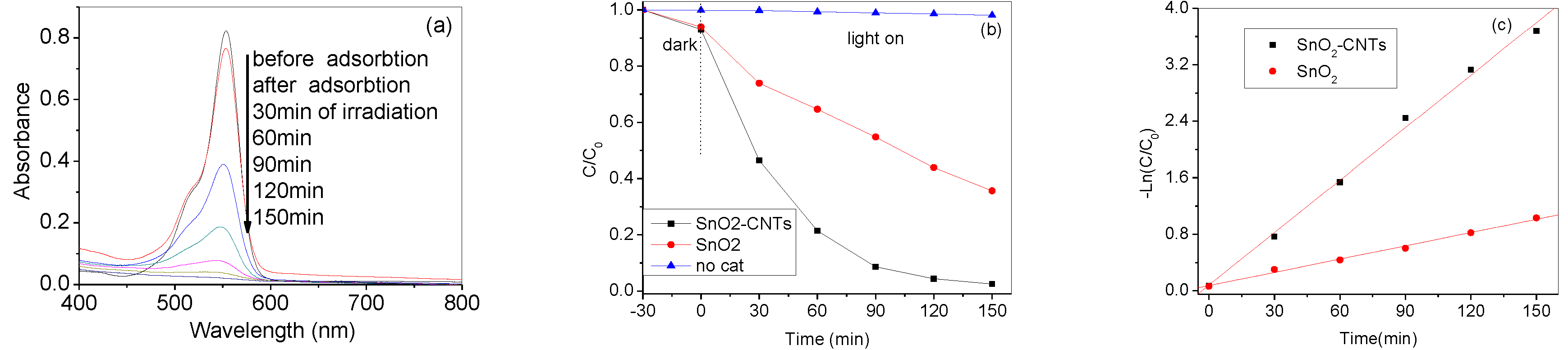
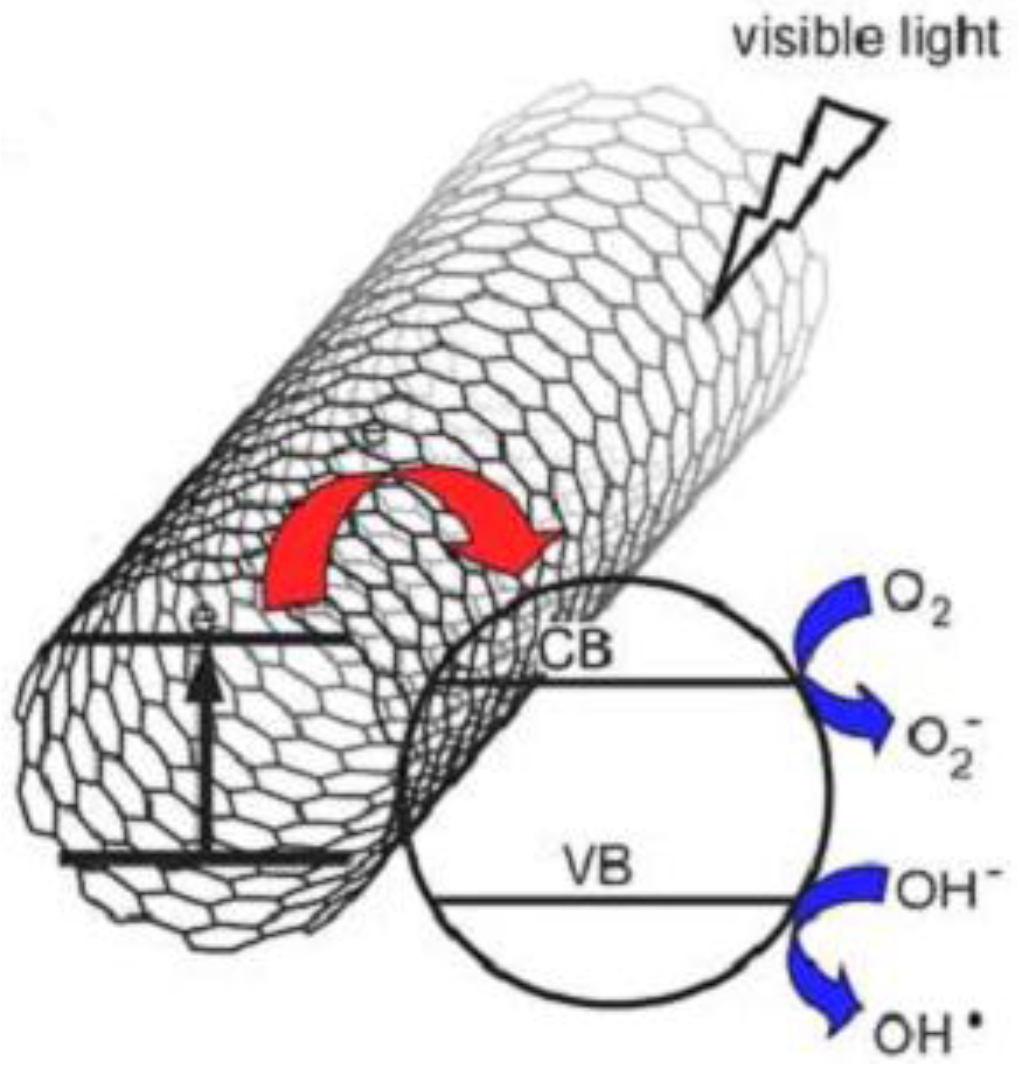
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lefebvre, O.; Moletta, R. Treatment of organic pollution in industrial saline wastewater: A literature review. Water Res. 2006, 40, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, C.; Lien, H. Treatment of chlorinated organic contaminants with nanoscale bimetallic particles. Catal. Today 1998, 40, 387–395. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.R.; Marin, S.T.; Choi, W.; Bahnemannt, D.W. Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Ng, Y.H.; Iwase, A.; Bell, N.J.; Kudo, A.; Amal, R. Semiconductor/reduced graphene oxide nanocomposites derived from photocatalytic reactions. Catal. Today 2011, 164, 353–357. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Q.; Yao, Y.; Qian, C.; Zhang, X.; Wei, X. Microwave-assisted synthesis and photocatalytic properties of carbon nanotube/zinc sulfide heterostructures. J. Phys. Chem. C 2008, 112, 16779–16783. [Google Scholar] [CrossRef]
- Cao, H.; Qiu, X.; Liang, Y.; Zhang, L.; Zhao, M.; Zhu, Q. Sol-gel template synthesis and photoluminescence of n- and p-type semiconductor oxide nanowires. ChemPhysChem 2006, 7, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Q.; Lao, C.; Wang, Z.L.; Xie, Z.; Zheng, L. High-sensitivity humidity sensor based on single SnO2 nanowire. J. Am. Chem. Soc. 2007, 129, 6070–6071. [Google Scholar] [CrossRef] [PubMed]
- Leite, E.R.; Weber, I.T.; Longo, E.; Varela, J. A New Method to Control Particle Size and Particle Size Distribution of SnO2 Nanoparticles for Gas Sensor Applications. Adv. Mater. 2000, 12, 965–968. [Google Scholar] [CrossRef]
- Moreno, M.S.; Varela, A.; Otero-Diaz, L.C. Cation nonstoichiometry in tin-monoxide-phase with tweed microstructure. Phys. Rev. B 1997, 56, 5186. [Google Scholar] [CrossRef]
- Park, M.-S.; Wang, G.-X.; Kang, Y.-M.; Wexler, D.; Dou, S.-X.; Liu, H.-K. Preparation and electrochemical properties of SnO2 nanowires for application in Lithium-Ion batteries. Angew. Chem. Int. Ed. 2007, 46, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.S.; Cao, H.Q.; Yin, S.F.; Liu, X.W.; Zhang, X.R. Amino acid-assisted hydrothermal synthesis and photocatalysis of SnO2 nanocrystals. J. Phys. Chem. C 2009, 113, 17893–17898. [Google Scholar] [CrossRef]
- Wang, N.; Xu, J.; Guan, L. Synthesis and enhanced photocatalytic activity of tin oxide nanoparticles coated on multiwalled carbon nanotube. Mater. Res. Bull. 2011, 46, 1372–1376. [Google Scholar] [CrossRef]
- Wang, L.; Shen, L.; Zhu, L.; Jin, H.; Bing, N.; Wang, L. Preparation and photocatalytic properties of SnO2 coated on nitrogen-doped carbon nanotubes. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]
- Tian, L.; Ye, L.; Deng, K.; Zan, L. TiO2/carbon nanotube hybrid nanostructures: Solvothermal synthesis and their visible light photocatalytic activity. J. Solid State Chem. 2011, 184, 1465–1471. [Google Scholar] [CrossRef]
- Woan, K.; Pyrgiotakis, G.; Sigmund, W. Photocatalytic Carbon-Nanotube-TiO2 Composites. Adv. Mater. 2009, 21, 1–7. [Google Scholar] [CrossRef]
- Xu, Y.; Zhuang, Y.; Fu, X. New Insight for Enhanced Photocatalytic Activity of TiO2 by Doping Carbon Nanotubes: A Case Study on Degradation of Benzene and Methyl Orange. J. Phys. Chem. C 2010, 114, 2669–2676. [Google Scholar] [CrossRef]
- Bouazza, N.; Ouzzine, M.; Ródenas, M.A.L.; Eder, D.; Linares-Solano, A. TiO2 nanotubes and CNT-TiO2 hybrid materials for the photocatalytic oxidation of propene at low concentration. Appl. Catal. B 2009, 92, 377–383. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, S.; Zhang, H.; Liu, S.; Nawaz, F.; Xiao, F. Nanoporous polymer monoliths as adsorptive supports for robust photocatalyst of Degussa P25. J. Colloid Interface Sci. 2009, 339, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Komarneni, S.; Rajhaa, R.K.; Katsuki, H. Microwave-hydrothermal processing of titanium dioxide. Mater. Chem. Phys. 1999, 61, 50–54. [Google Scholar] [CrossRef]
- Ponzoni, C.; Cannio, M.; Boccaccini, D.N.; Bahl, C.R.H.; Agersted, K.; Leonelli, C. Ultrafast microwave hydrothermal synthesis and characterization of Bi1−xLaxFeO3 micronized particles. Mater. Chem. Phys. 2015, 162, 69–75. [Google Scholar] [CrossRef]
- Yin, S.; Liu, B.; Sato, T. Microwave-assisted hydrothermal synthesis of nitrogen-doped titania nanoparticles. Funct. Mater. Lett. 2008, 1, 173–176. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, P.; Liu, B.; Liu, X.; Sato, T.; Xue, D.; Lee, S.W. Microwave-assisted hydrothermal synthesis of nitrogen-doped titania photocatalyst and its DeNOx ability under visible LED light irradiation. Res. Chem. Intermed. 2010, 36, 69–75. [Google Scholar] [CrossRef]
- Zhou, J.X.; Zhang, M.S.; Hong, J.M.; Yin, Z. Raman spectroscopic and photoluminescence study of single-crystalline SnO2 nanowire. Solid State Commun. 2006, 138, 242–246. [Google Scholar] [CrossRef]
- Yang, L.; Dong, S.; Sun, J.; Feng, J.; Wu, Q.; Sun, S. Microwave-assisted preparation, characterization and photocatalytic properties of a dumbbell-shaped ZnO photocatalyst. J. Hazard. Mater. 2010, 179, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Lettmann, C.; Hildenbrand, K.; Kisch, H.; Macyk, W.; Maier, W.F. Visible light photodegradation of 4-chlorophenol with a coke-containing titanium dioxide photocatalyst. Appl. Catal. B 2001, 32, 215–227. [Google Scholar] [CrossRef]
- Jung, K.H.; Hong, J.S.; Vittal, R.; Kim, K.J. Enhanced photocurrent of dye-sensitized solar cells by modification of TiO2 with carbon nanotubes. Chem. Lett. 2002, 8, 864–865. [Google Scholar] [CrossRef]
- Banerjee, S.; Wong, S.S. Synthesis and characterization of carbon nanotube-nanocrystal heterostructures. Nano Lett. 2002, 2, 195–200. [Google Scholar] [CrossRef]
- Li, B.J.; Cao, H.Q. ZnO@graphene composite with enhanced performance for the removal of dye from water. J. Mater. Chem. 2011, 21, 3346–3349. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Dai, W. Microwave-Hydrothermal Synthesis of SnO2-CNTs Hybrid Nanocomposites with Visible Light Photocatalytic Activity. Nanomaterials 2017, 7, 54. https://doi.org/10.3390/nano7030054
Wu S, Dai W. Microwave-Hydrothermal Synthesis of SnO2-CNTs Hybrid Nanocomposites with Visible Light Photocatalytic Activity. Nanomaterials. 2017; 7(3):54. https://doi.org/10.3390/nano7030054
Chicago/Turabian StyleWu, Shuisheng, and Weili Dai. 2017. "Microwave-Hydrothermal Synthesis of SnO2-CNTs Hybrid Nanocomposites with Visible Light Photocatalytic Activity" Nanomaterials 7, no. 3: 54. https://doi.org/10.3390/nano7030054





