Self‐propagating Combustion Triggered Synthesis of 3D Lamellar Graphene/BaFe12O19 Composite and Its Electromagnetic Wave Absorption Properties
Abstract
:1. Introduction
2. Experimental
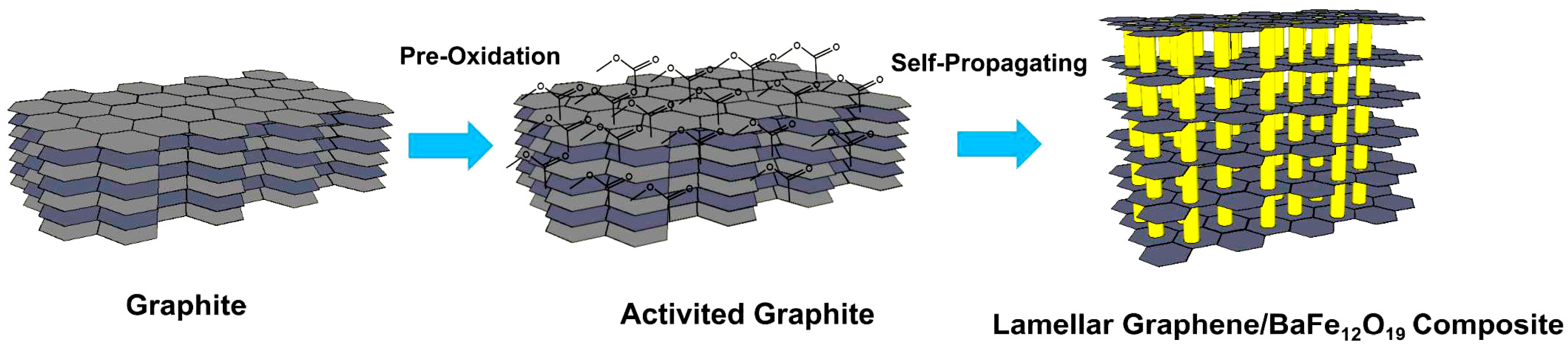
2.1 Activation of Graphite Sheets
2.2 In Situ Synthesis of Lamellar Graphene/BaFe12O19 Composite
2.3. Characterizations
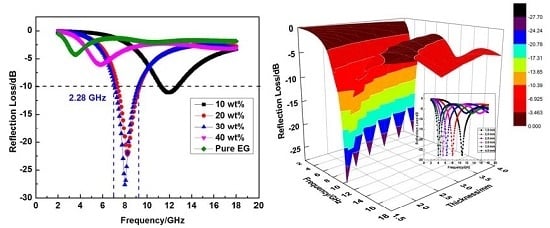
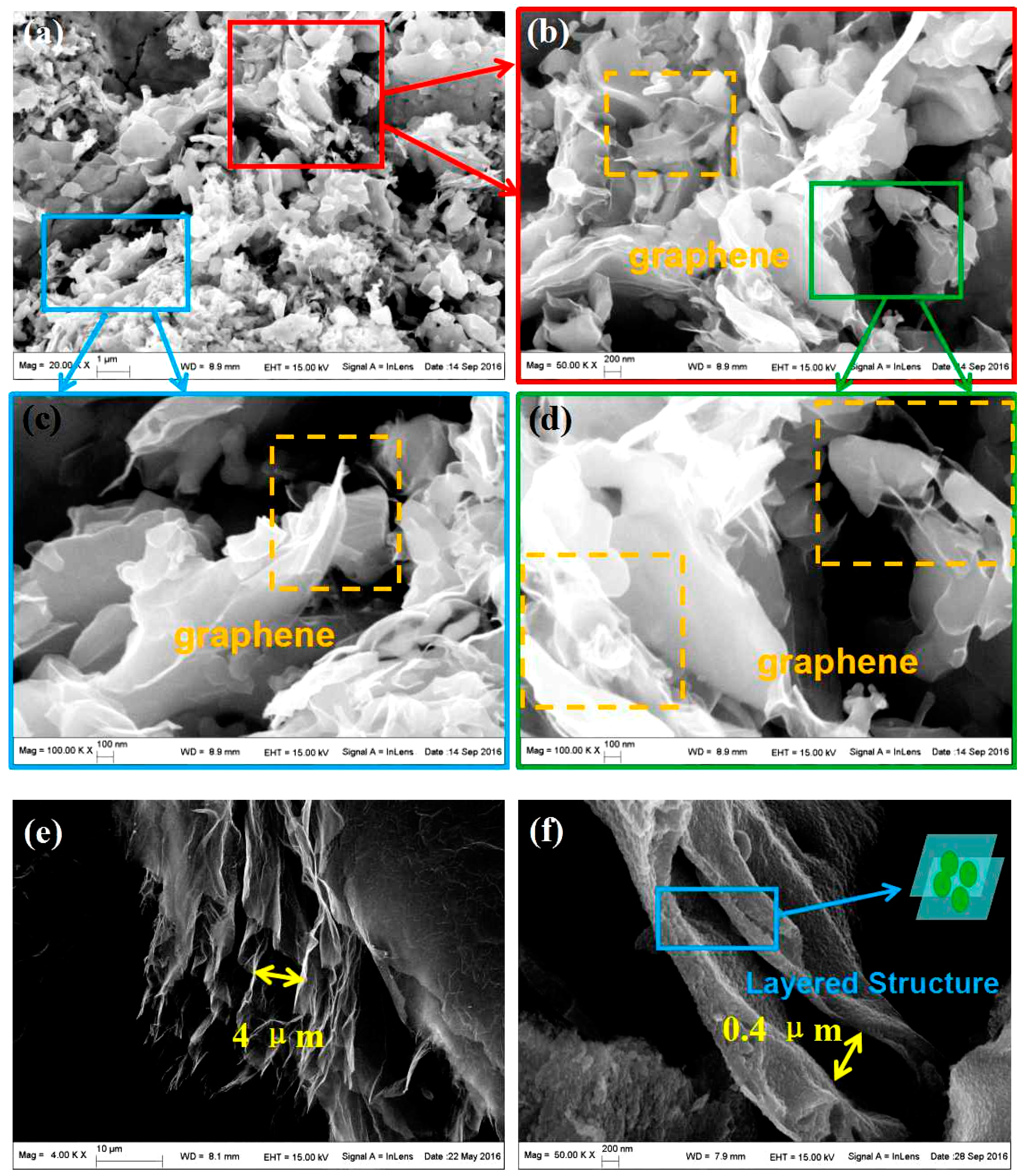
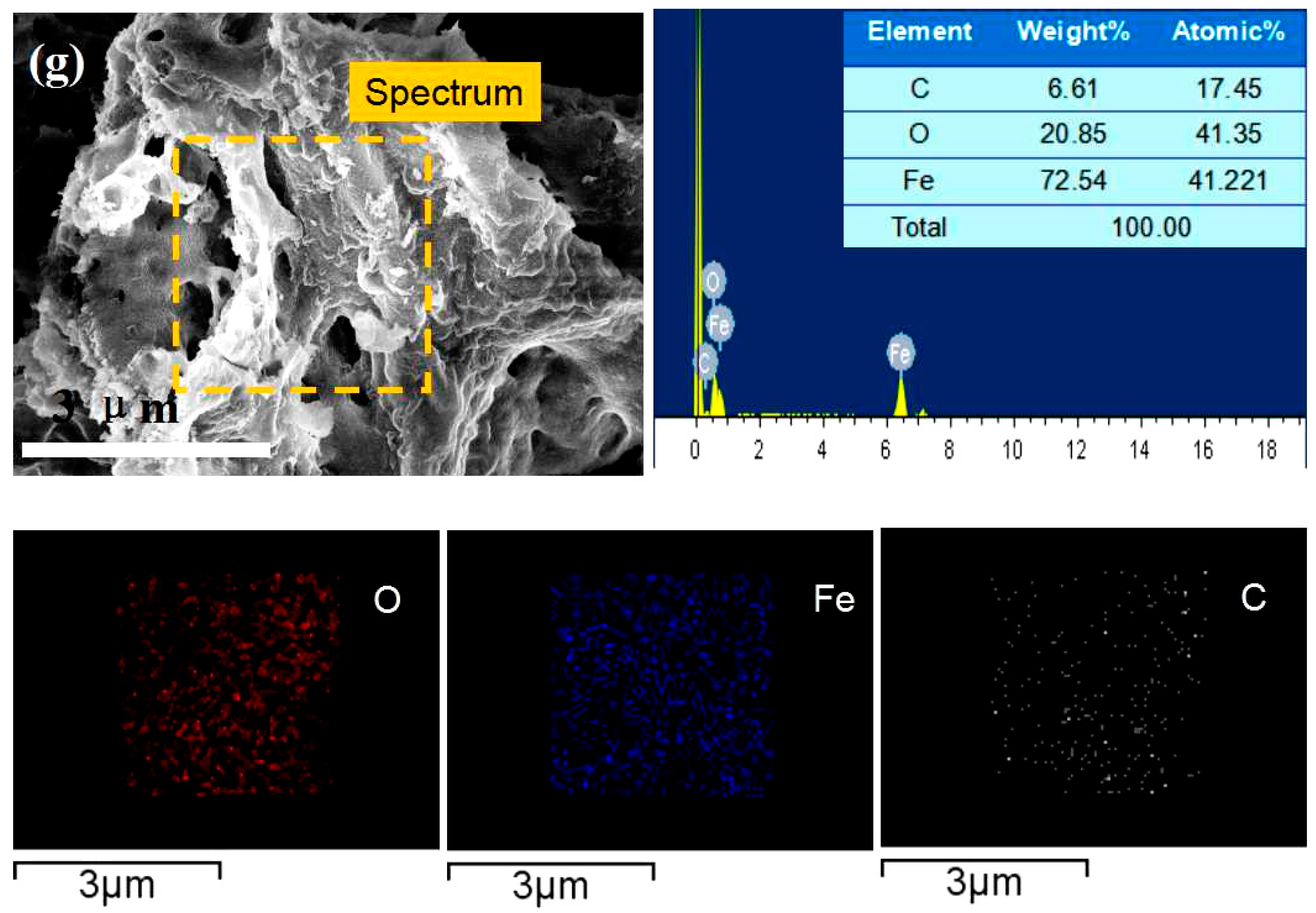
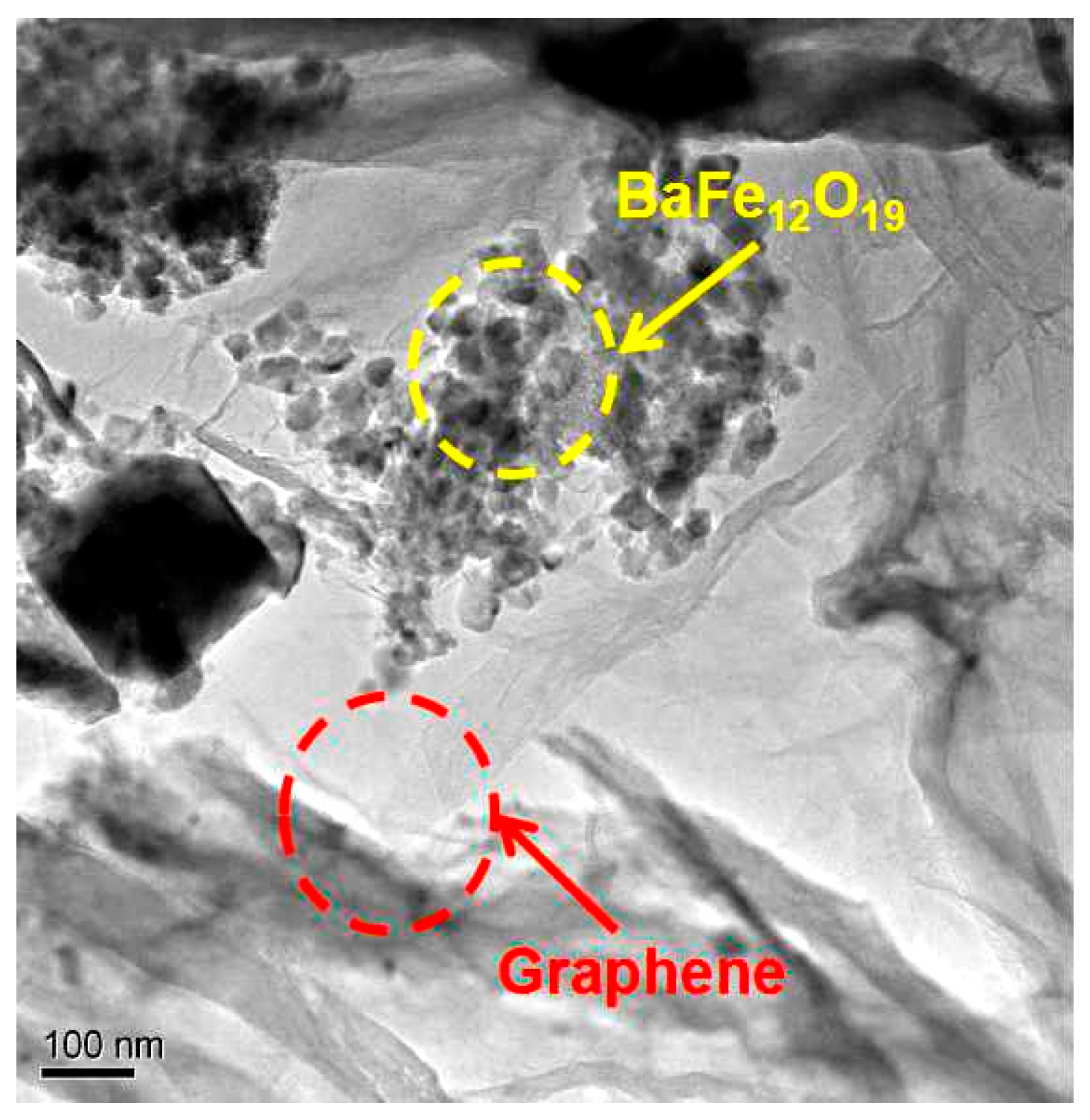
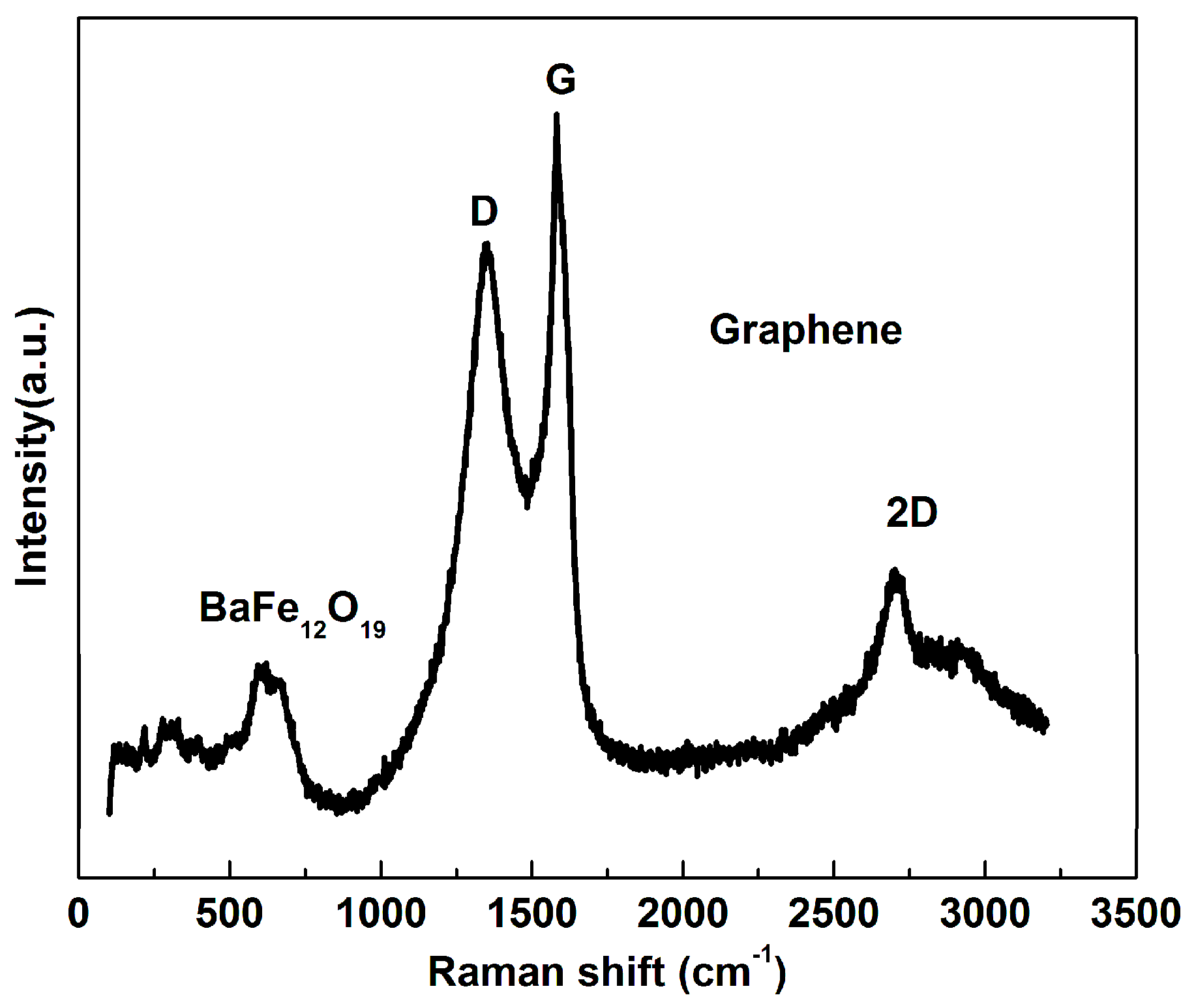
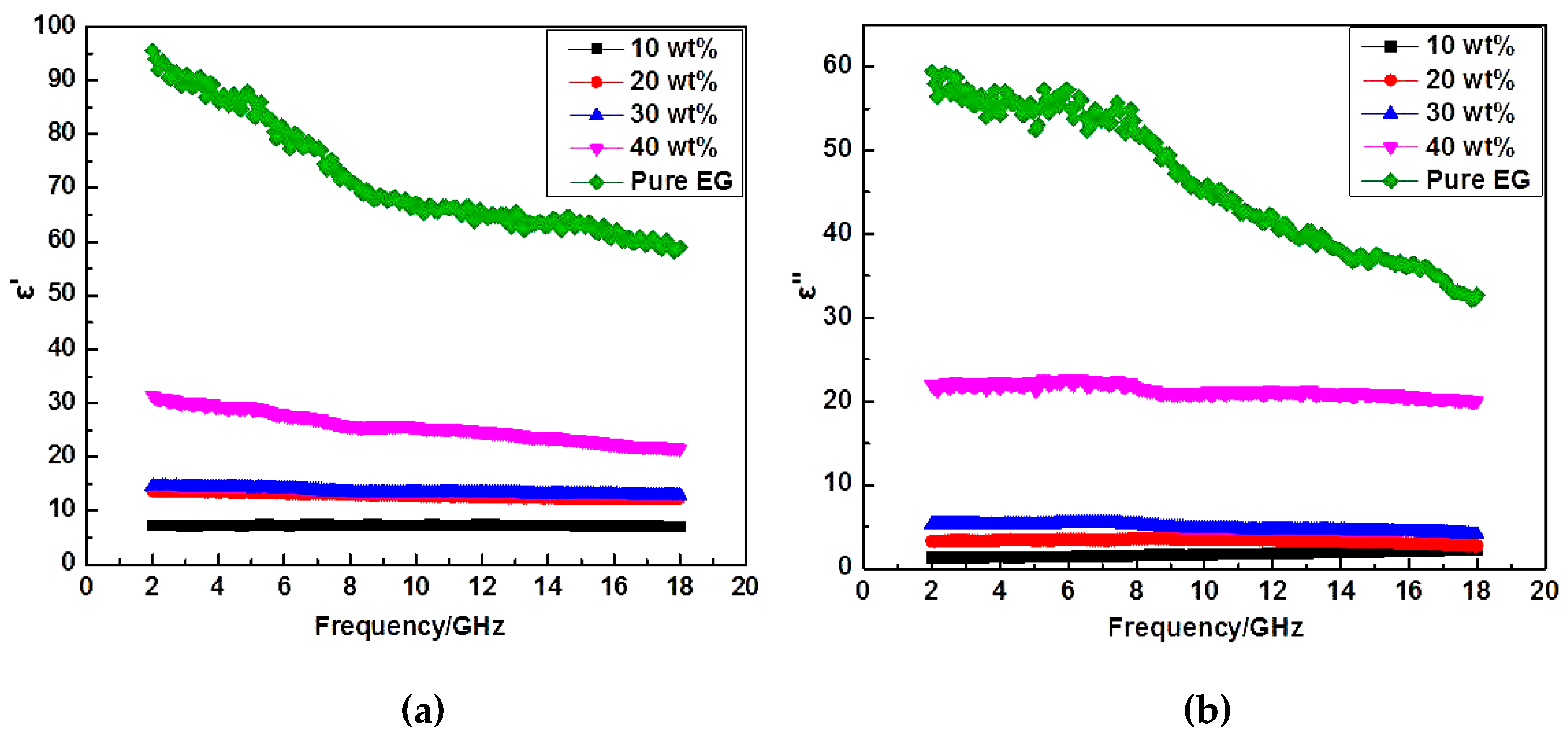
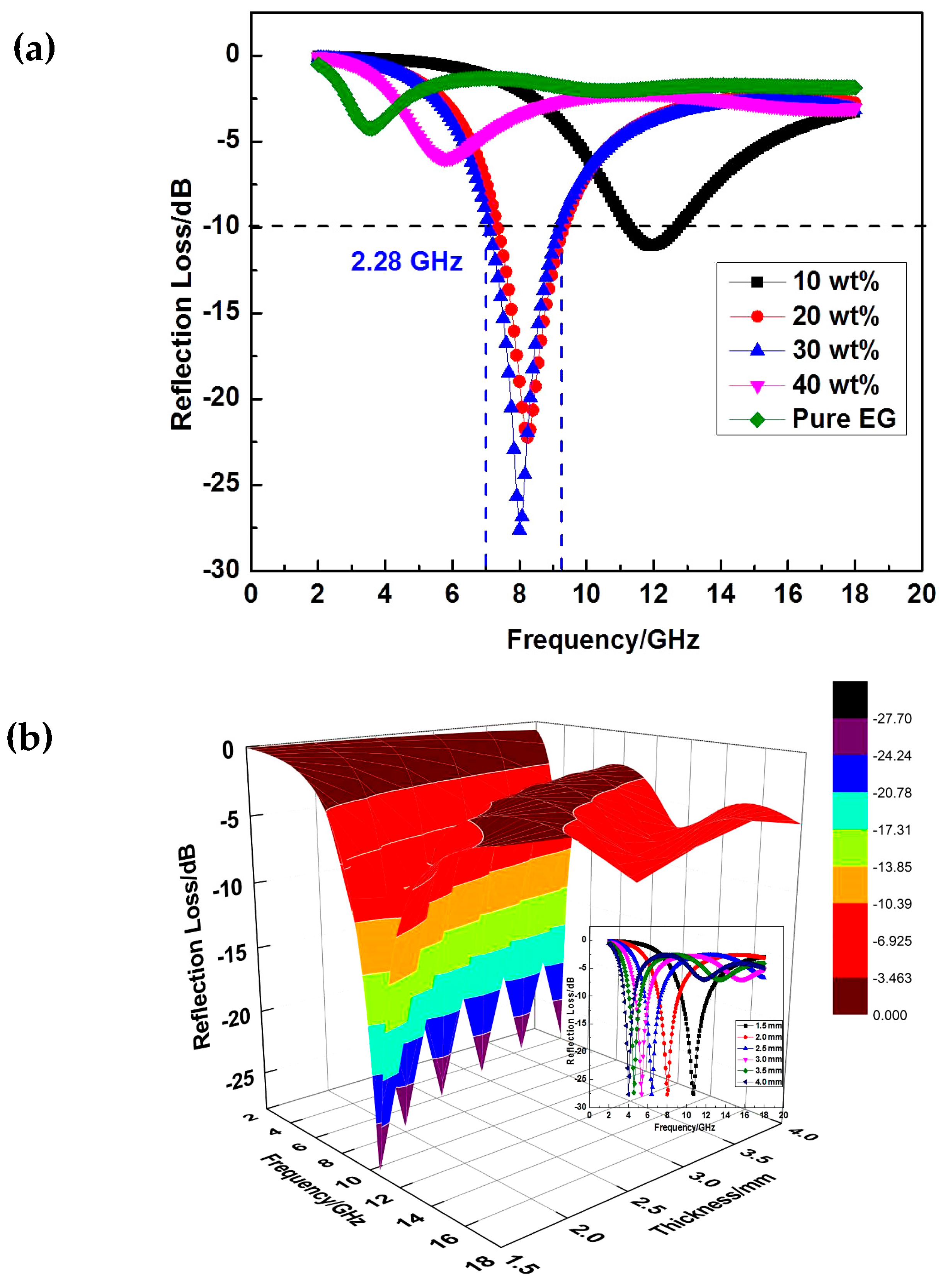
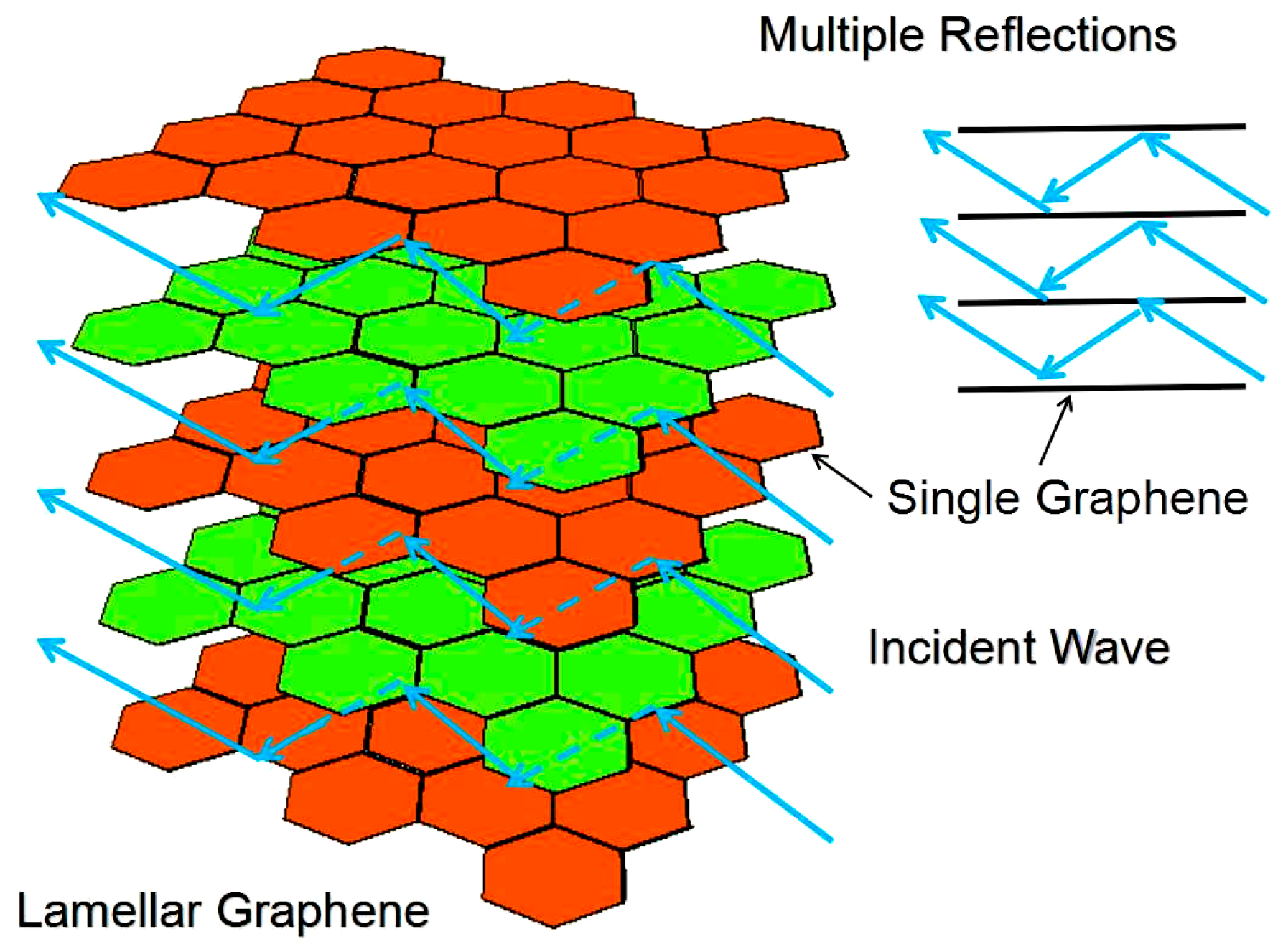
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Valentini, M.; Piana, F.; Pionteck, J.; Lamastrad, F.R.; Nannia, F. Electromagnetic properties and performance of exfoliated graphite (EG)-Thermoplastic polyurethane (TPU) nanocomposites at microwaves. Compos. Sci. Technol. 2015, 114, 26–33. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Zhang, T.; Chang, H.; Xiao, P.; Chen, H.; Huang, Z.; Chen, Y. Broadband and tunable high-performance microwave absorption of an ultralight and highly compressible graphene foam. Adv. Mater. 2015, 27, 2049–2053. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Pang, Y.; Zhu, M.; Kobayashi, S. Microporous Co@CoO nanoparticles with superior microwave absorption properties. Nanoscale 2014, 6, 2447–2454. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, Y.; Sun, X.; Huang, H.; Liu, P.; Zong, M.; Wang, Y. Synthesis and microwave absorption enhancement of graphene@Fe3O4@SiO2@NiO nanosheet hierarchical structures. Nanoscale 2014, 6, 3157–3164. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Bai, G.; Huang, Y.; Li, F.; Ma, Y.; Guo, T.; He, X.; Lin, X.; Gao, H.; Chen, Y. Microwave absorption of single-walled carbon nanotubes/soluble cross-linked polyurethane composites. J. Phys. Chem. C 2007, 111, 13696–13700. [Google Scholar] [CrossRef]
- Liu, H.; Yang, W.; He, F.; Li, Y.; Yang, X.; Zhang, K. Graphene-based composite with microwave absorption property prepared by in situ reduction. Polym. Compos. 2014, 35, 461–467. [Google Scholar] [CrossRef]
- Ohlan, A.; Singh, K.; Chandra, A.; Dhawan, S.K. Microwave absorption behavior of core-shell structured poly(3,4-ethylenedioxy thiophene)-barium ferrite nanocomposites. ACS Appl. Mater. Interfaces 2010, 2, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Wang, W.N.; Iwaki, T.; Yabuki, A.; Okuyama, K. Low-temperature crystallization of barium ferrite nanoparticles by a sodium citrate-aided synthetic process. J. Phys. Chem. C 2007, 111, 10175–10180. [Google Scholar] [CrossRef]
- Li, L.; Chen, K.; Liu, H.; Tong, G.; Qian, H.; Hao, B. Attractive microwave-absorbing properties of M-BaFe12O19, ferrite. J. Alloys Compd. 2013, 557, 11–17. [Google Scholar] [CrossRef]
- Ting, T.H.; Wu, K.H. Synthesis, characterization of polyaniline/BaFe12O19, composites with microwave-absorbing properties. J. Magn. Magn. Mater. 2010, 322, 2160–2166. [Google Scholar] [CrossRef]
- Hong, X.; Xie, Y.; Wang, X.; Li, M.; Le, Z.; Gao, Y.; Huang, Y.; Qina, Y.; Ling, Y. A novel ternary hybrid electromagnetic wave-absorbing composite based on BaFe11.92(LaNd)0.04O19-titanium dioxide/multiwalled carbon nanotubes/polythiophene. Compos. Sci. Technol. 2015, 117, 215–224. [Google Scholar] [CrossRef]
- Liu, J.; Xu, J.; Che, R.; Chen, H.; Liu, Z.; Xia, F. Hierarchical magnetic yolk-shell microspheres with mixed barium silicate and barium titanium oxide shells for microwave absorption enhancement. J. Mater. Chem. 2012, 22, 9277–9284. [Google Scholar] [CrossRef]
- Li, C.J.; Wang, B.; Wang, J.N. Magnetic and microwave absorbing properties of electrospun Ba(1−x)LaxFe12O19 nanofibers. J. Magn. Magn. Mater. 2012, 324, 1305–1311. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, C.; Ma, C.; Ren, W.; Cheng, H.-M. Lightweight and flexible graphene foam composites for high-performance electromagnetic interference shielding. Adv. Mater. 2013, 25, 1296–1300. [Google Scholar] [CrossRef] [PubMed]
- Moglie, F.; Micheli, D.; Laurenzi, S.; Marchetti, M.; Primiani, V.M. Electromagnetic shielding performance of carbon foams. Carbon 2012, 50, 1972–1980. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, X.; Bai, X.; Zhou, S.; Sun, C.; Yan, J.; Chen, P. Synthesis and electromagnetic, microwave absorbing properties of core-shell Fe3O4-poly(3,4-ethylenedioxythiophene) microspheres. ACS Appl. Mater. Interfaces 2011, 3, 3839–3845. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Abdulla, W.A.; Wang, D.; Zhao, X.S. Three-dimensional porous LiFePO4 cathode material modified with nitrogen-doped graphene aerogel for high-power lithium ion batteries. Energy Environ. Sci. 2015, 8, 869–875. [Google Scholar] [CrossRef]
- Yin, L.; Chen, T.; Liu, S.; Gao, Y.; Wu, B.; Wei, Y.; Li, G.; Jian, X.; Zhang, X. Preparation and microwave-absorbing property of BaFe12O19 nanoparticles and BaFe12O19/Fe3C/CNTs composites. RSC Adv. 2015, 5, 91665–91669. [Google Scholar] [CrossRef]
- Wang, H.; Gao, H.; Chen, M.; Xua, X.; Wang, X.; Pan, C.; Gao, J. Microwave-assisted synthesis of reduced graphene oxide/titania nanocomposites as an adsorbent for methylene blue adsorption. Appl. Surf. Sci. 2015, 360, 840–848. [Google Scholar] [CrossRef]
- Han, W.; Ren, L.; Gong, L.; Qi, X.; Liu, Y.; Yang, L.; Wei, X.; Zhong, J. Self-assembled three-dimensional graphene-based aerogel with embedded multifarious functional nanoparticles and its excellent photoelectrochemical activities. ACS Sustain. Chem. Eng. 2014, 2, 2189–2196. [Google Scholar] [CrossRef]
- Yao, X.; Guo, G.; Ma, X.; Zhao, Y.; Ang, C.; Luo, Z.; Nguyen, K.; Li, P.; Yan, Q.; Zhao, Y. In-situ integration of anisotropic SnO2 heterostructures inside three-dimensional graphene aerogel for enhanced lithium storage. ACS Appl. Mater. Interfaces 2015, 7, 26085–26093. [Google Scholar] [CrossRef] [PubMed]
- Mahpishanian, S.; Sereshti, H. Three-dimensional graphene aerogel-supported iron oxide nanoparticles as an efficient adsorbent for magnetic solid phase extraction of organophosphorus pesticide residues in fruit juices followed by gas chromatographic determination. J. Chromatogr. A 2016, 1443, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, G.; Cao, W.; Wei, Y.; Liang, J.; Guo, L.; Cao, M. Enhanced microwave absorption property of reduced graphene oxide (RGO)-MnFe2O4 nanocomposites and polyvinylidene fluoride. ACS Appl. Mater. Interfaces 2014, 6, 7471–7478. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, T.; Ji, X.; Jin, W.; Yang, W.; Peng, X.; Duan, S.; Dang, A.; Li, H.; Li, T. Self‐propagating Combustion Triggered Synthesis of 3D Lamellar Graphene/BaFe12O19 Composite and Its Electromagnetic Wave Absorption Properties. Nanomaterials 2017, 7, 55. https://doi.org/10.3390/nano7030055
Zhao T, Ji X, Jin W, Yang W, Peng X, Duan S, Dang A, Li H, Li T. Self‐propagating Combustion Triggered Synthesis of 3D Lamellar Graphene/BaFe12O19 Composite and Its Electromagnetic Wave Absorption Properties. Nanomaterials. 2017; 7(3):55. https://doi.org/10.3390/nano7030055
Chicago/Turabian StyleZhao, Tingkai, Xianglin Ji, Wenbo Jin, Wenbo Yang, Xiarong Peng, Shichang Duan, Alei Dang, Hao Li, and Tiehu Li. 2017. "Self‐propagating Combustion Triggered Synthesis of 3D Lamellar Graphene/BaFe12O19 Composite and Its Electromagnetic Wave Absorption Properties" Nanomaterials 7, no. 3: 55. https://doi.org/10.3390/nano7030055