Fabrication of Functional Polyurethane/Rare Earth Nanocomposite Membranes by Electrospinning and Its VOCs Absorption Capacity from Air
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
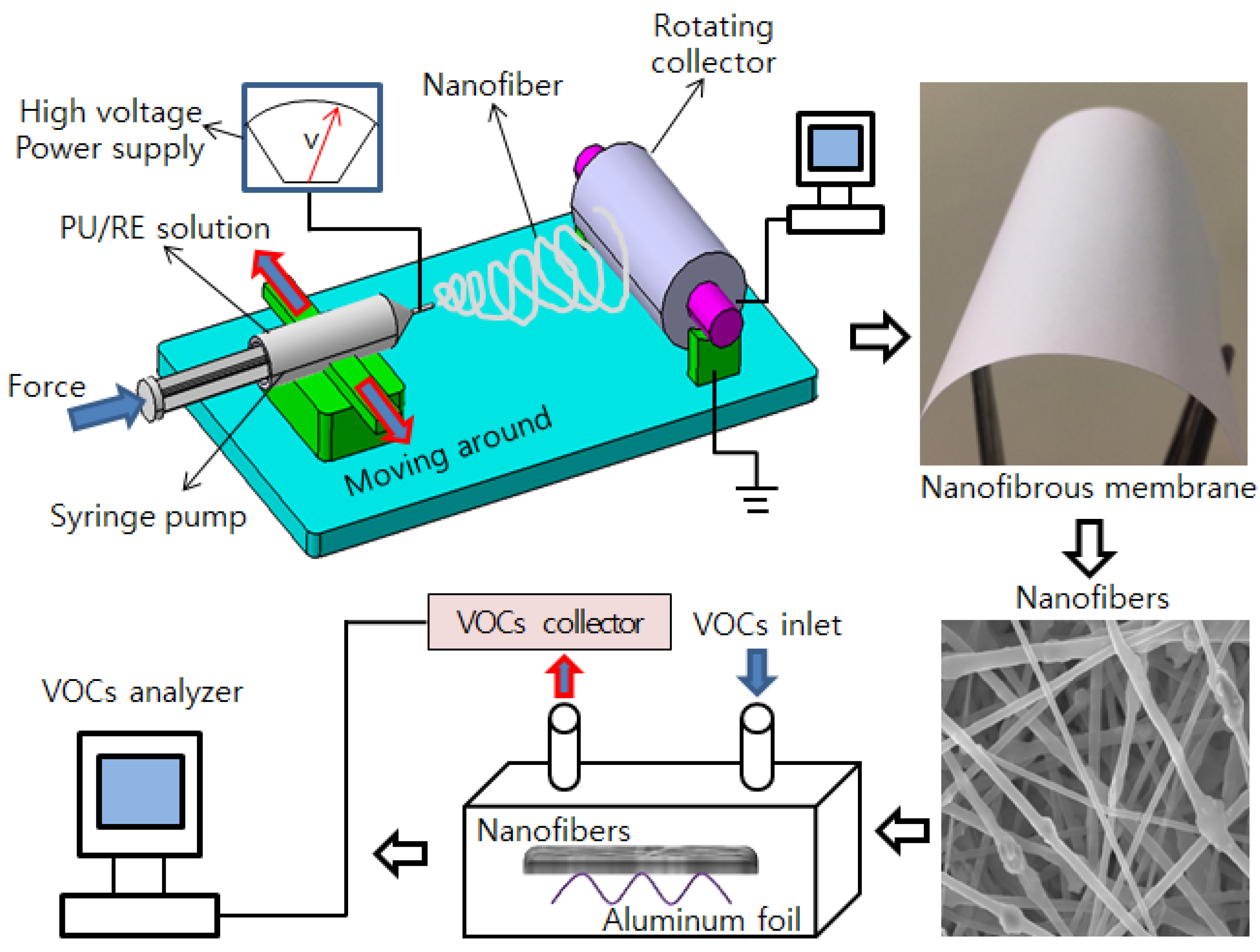
2.2. Fabrication of Composite Nanofibrous Membranes
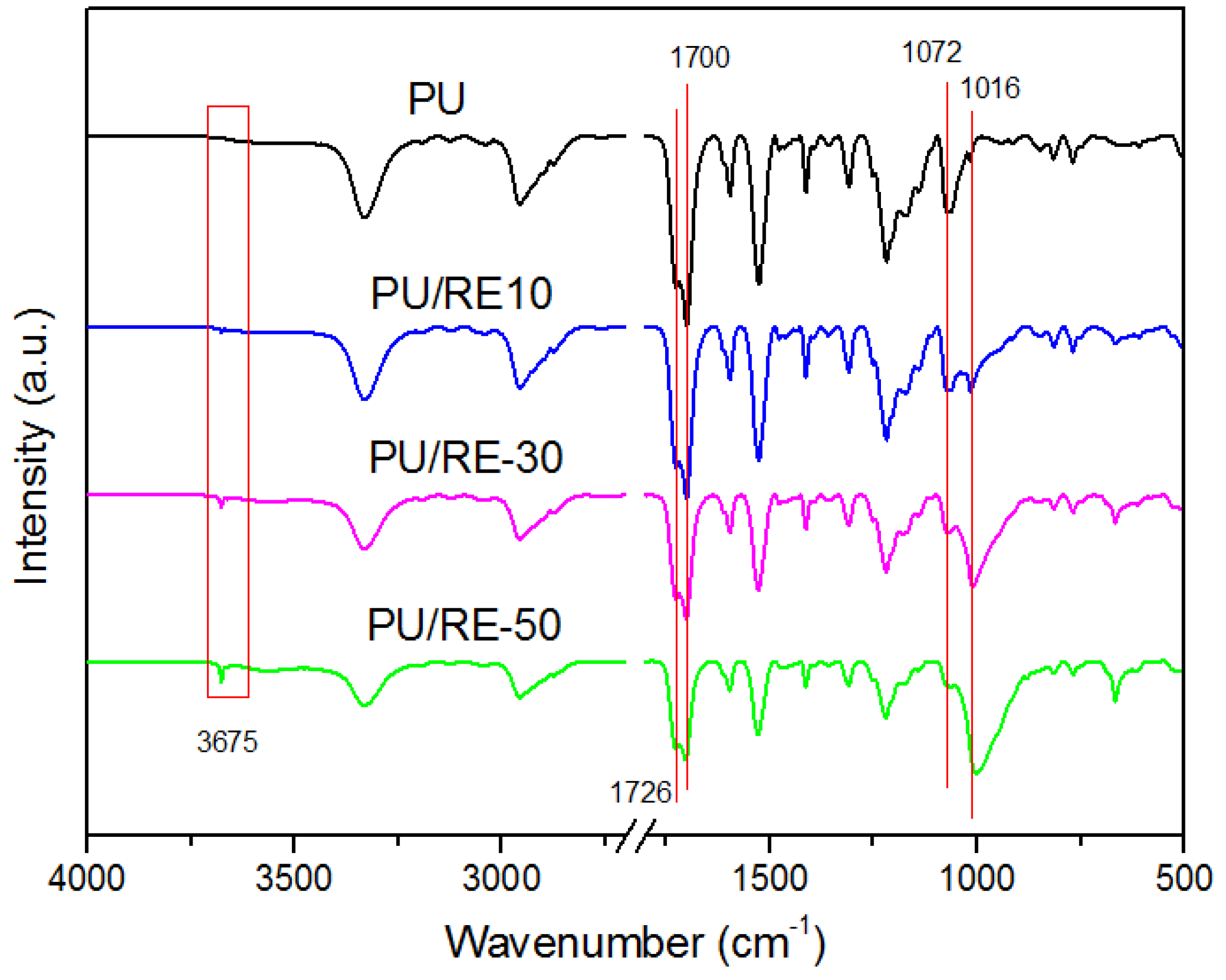
2.3. Characterization
2.4. VOCs Absorption Experiment
3. Results and Discussion
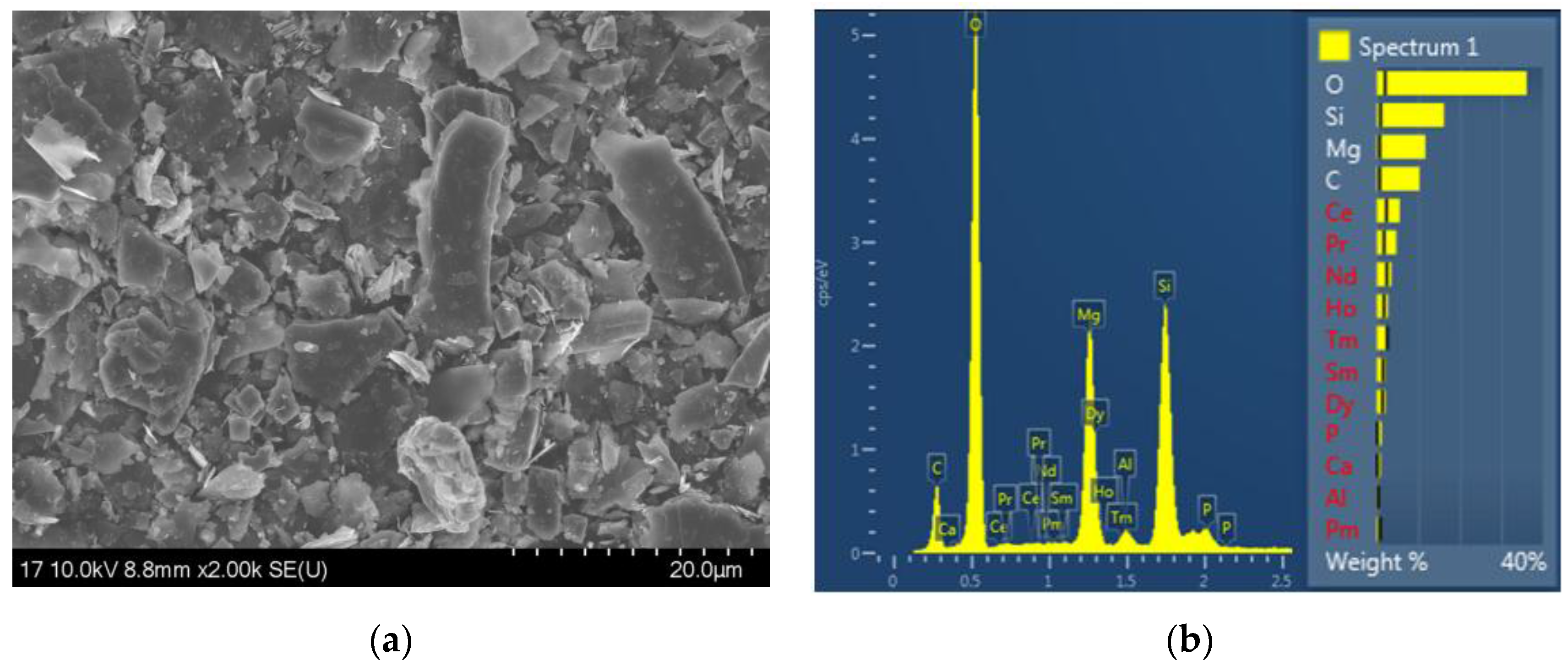
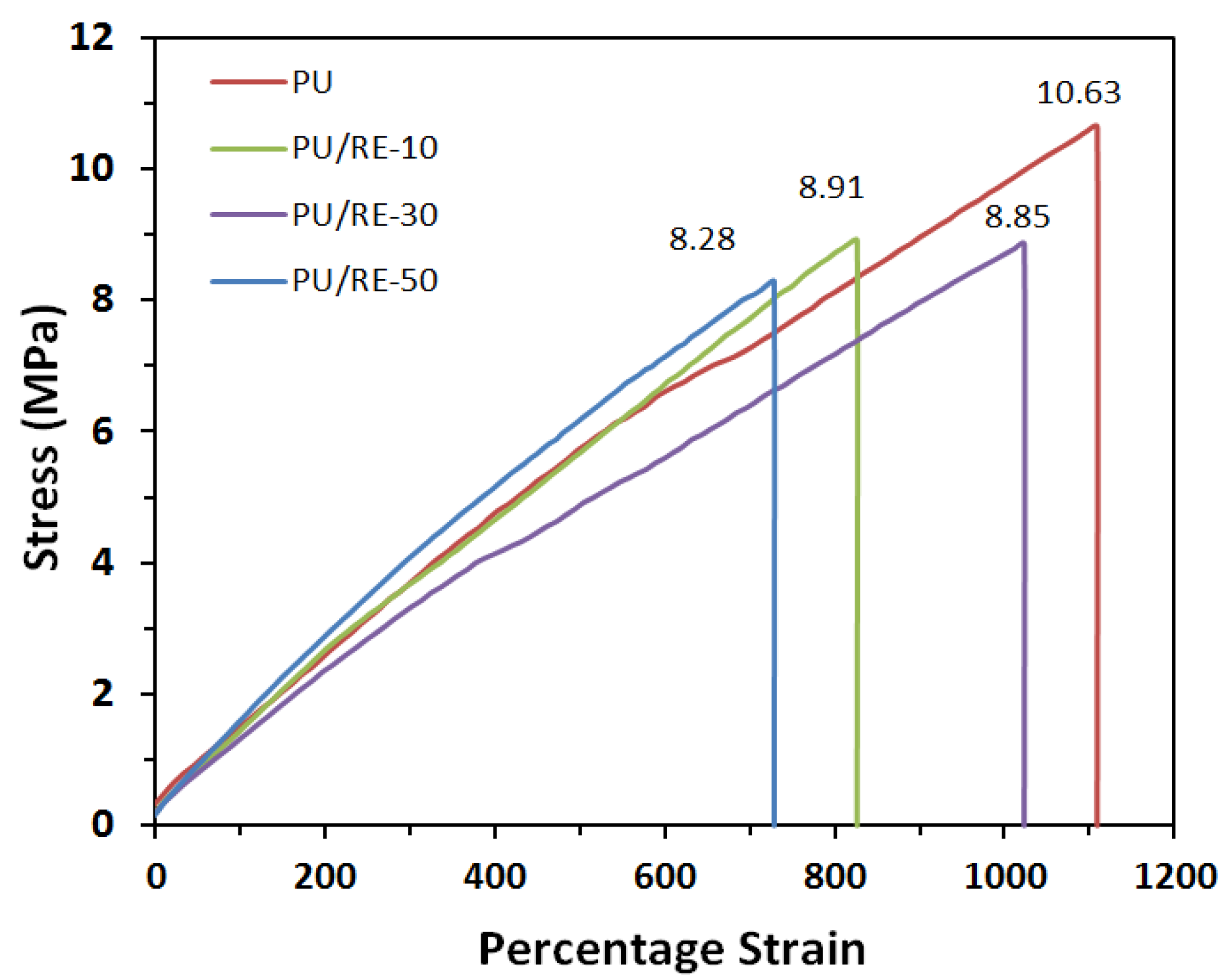
3.1. Physical Properties of Spinning Solutions and Properties of PU/RE Fiber
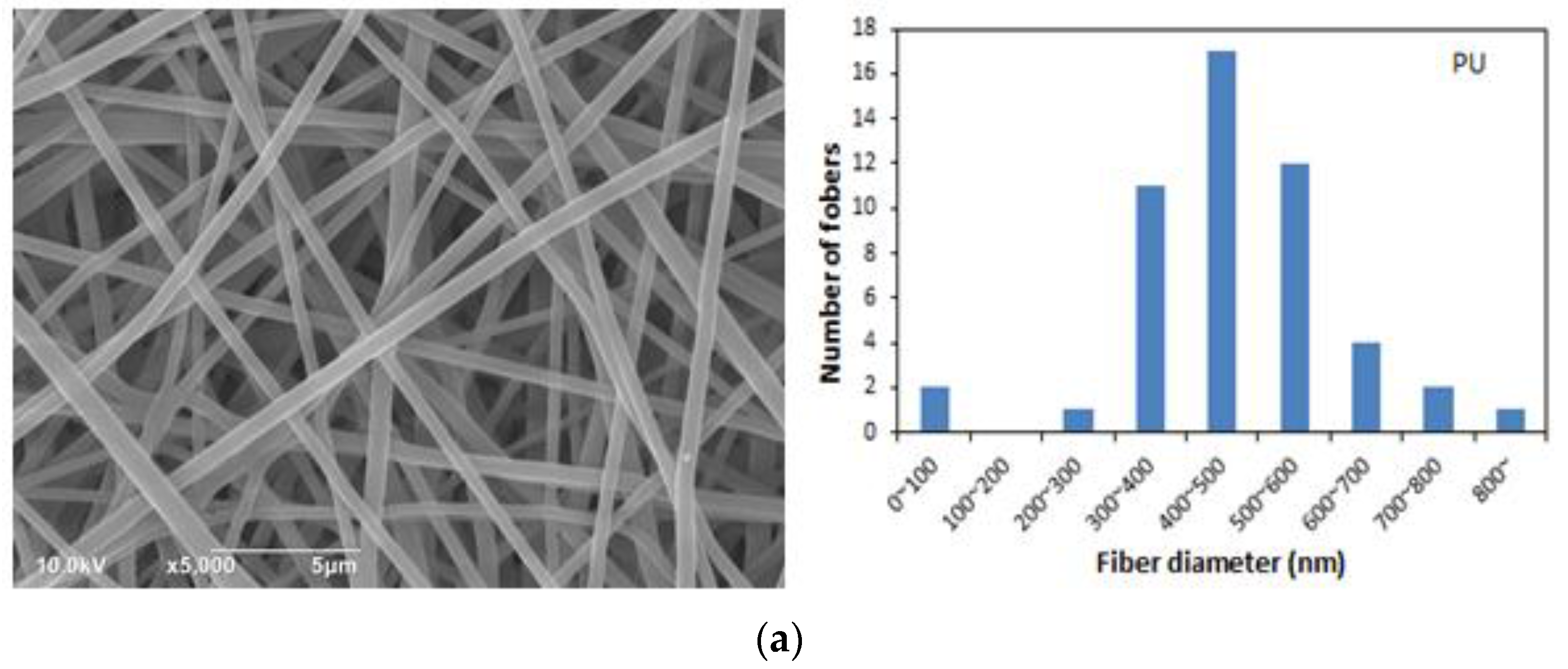
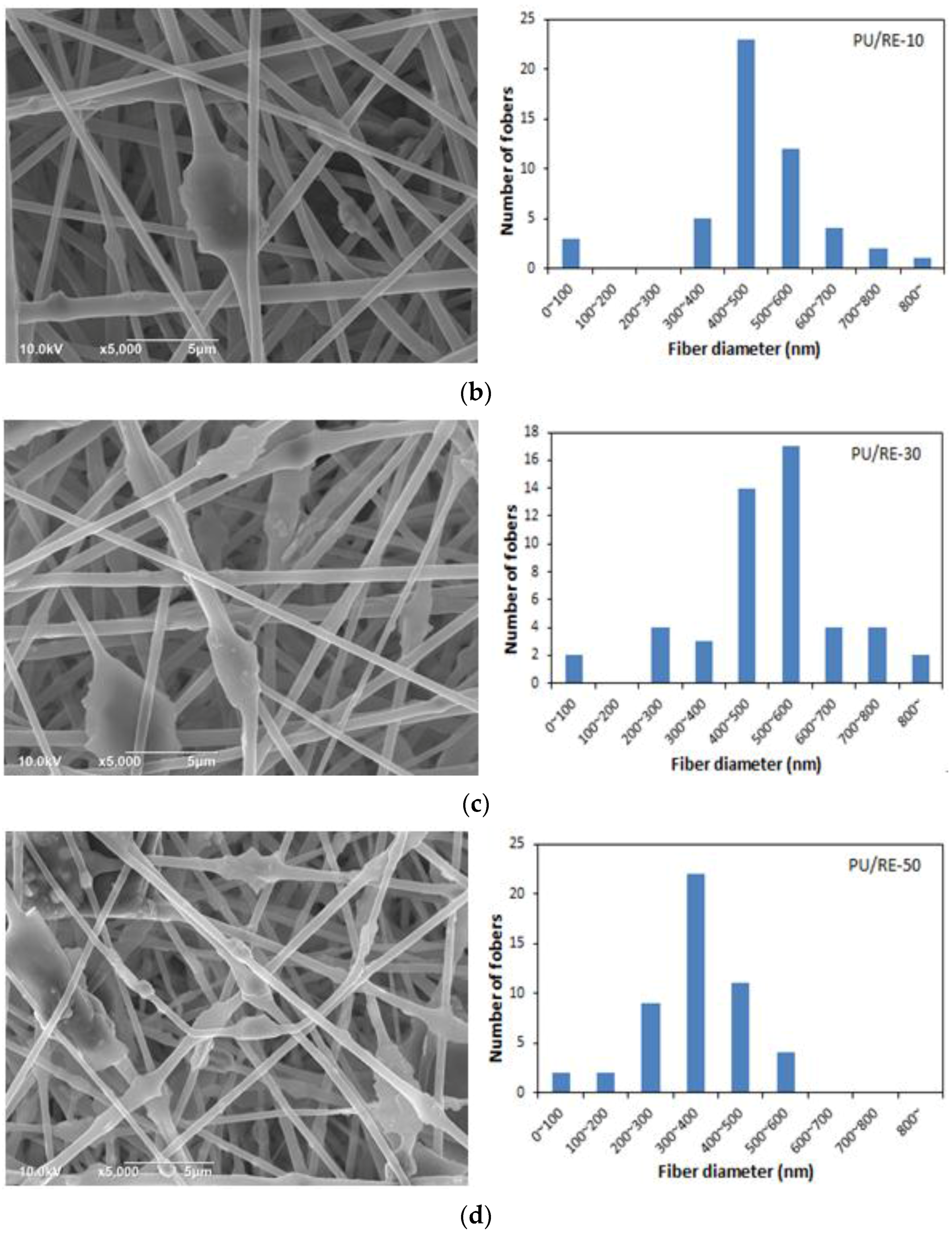
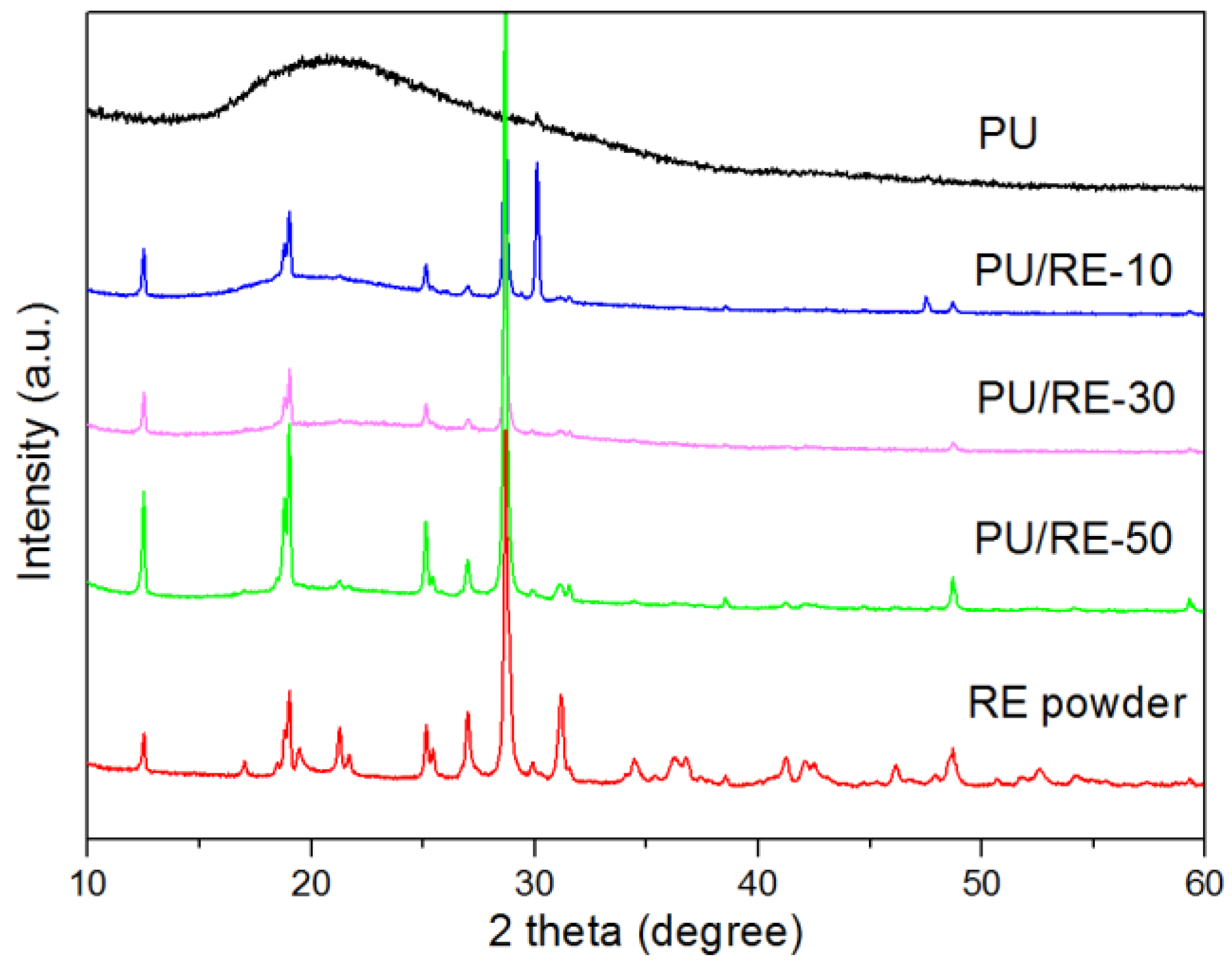
3.2. Morphological Characteristics of the PU/RE Nanofibrous Membranes
3.3. Morphological Characteristics of the PU/RE Nanofibrous Membranes
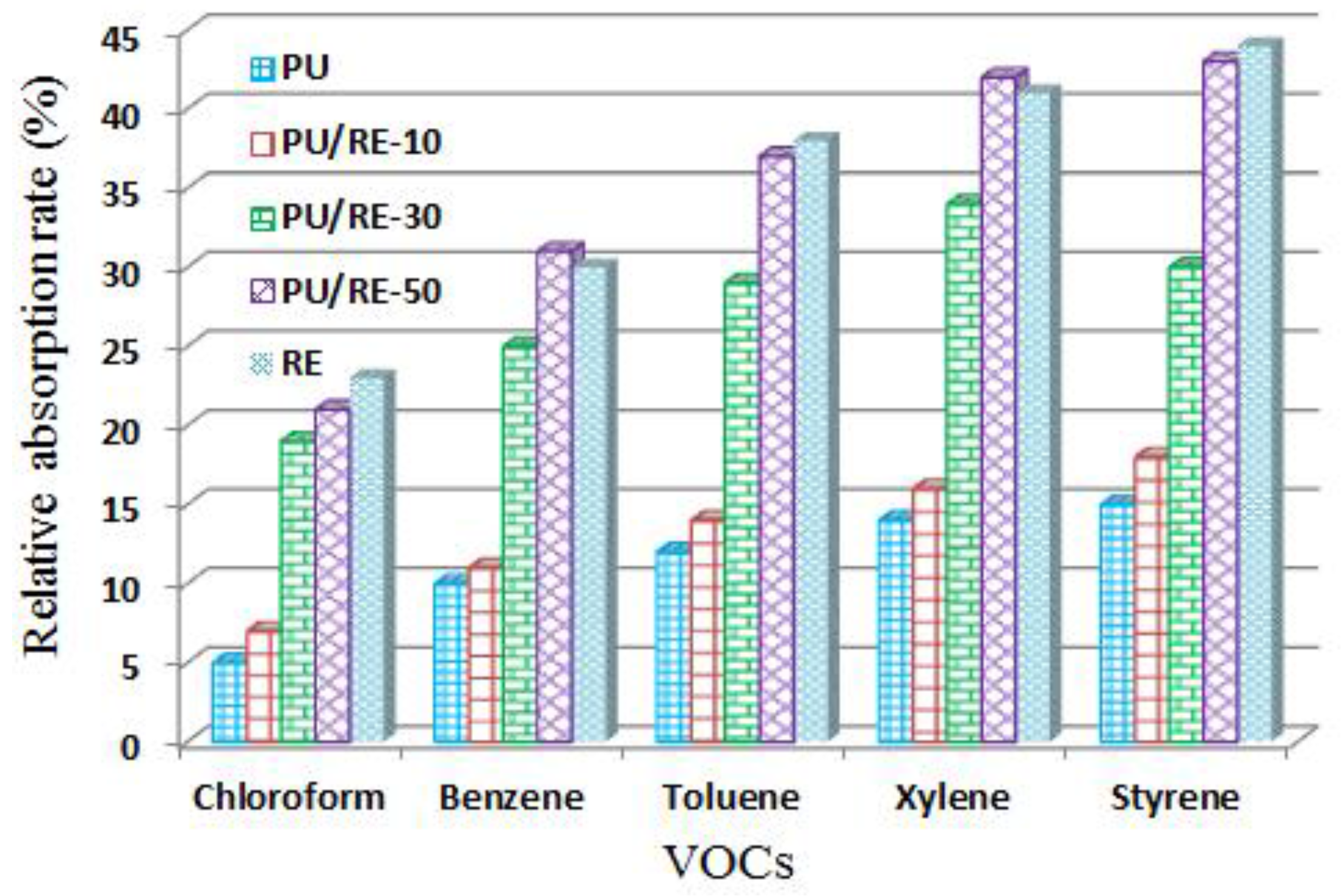
3.4. Absorption Characteristics of the Composite PU/RE Nanofibrous Membranes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kawamura, K.; Vestergaard, M.; Ishiyama, M.; Nagatani, N.; Hashiba, T.; Tamiya, E. Development of a novel hand-held toluene gas sensor: Possible use in the prevention and control of sick building syndrome. Measurement 2006, 39, 490–496. [Google Scholar] [CrossRef]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Bartzis, J.; Wolkoff, P.; Stranger, M.; Efthimiou, G.; Tolis, E.I.; Maes, F.; Nørgaard, A.W.; Ventura, G.; Kalimeri, K.K.; Goelen, E.; et al. On organic emissions testing from indoor consumer products’ use. J. Hazard. Mater. 2015, 285, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Mendell, M.J. Indoor residential chemical emissions as risk factors for respiratory and allergic effects in children: A review. Indoor Air 2007, 17, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Temel, F.; Tabakci, M. Calix[4]arene coated QCM sensors for detection of VOC emissions: Methylene chloride sensing studies. Talanta 2016, 15, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Takahara, N.; Korposh, S.; Yang, D.H.; Toko, K.; Kunitake, T. Nanoassembled thin film gas sensors. III. Sensitive detection of amine odors using TiO2/poly (acrylic acid) ultrathin film quartz crystal microbalance sensors. Anal. Chem. 2010, 82, 2228–2236. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Du, B. Selective detection of trace p-xylene by polymer-coated QCM sensors. Sens. Actuators B 2012, 166, 753–760. [Google Scholar] [CrossRef]
- Yoo, H.Y.; Bruckenstein, S. A novel quartz crystal microbalance gas sensor based on porous film coatings. A high sensitivity porous poly(methylmethacrylate) water vapor sensor. Anal. Chim. Acta 2013, 785, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, T.; Hashimoto, T.; Ueda, T.; Nakagoe, O.; Kamada, K.; Sasahara, T.; Tanabe, S.; Shimizu, Y. Adsorption/combustion-type VOC sensors employing mesoporous γ-alumina co-loaded with noble-metal and oxide. Sens. Actuators B 2015, 220, 1091–1104. [Google Scholar] [CrossRef]
- Sundarrajan, S.; Tan, K.L.; Lim, S.H.; Ramakrishna, S. Electrospun Nanofibers for Air Filtration Applications. Procedia Eng. 2014, 75, 159–163. [Google Scholar] [CrossRef]
- Fletcher, A.J.; Yüzak, Y.; Thomas, K.M. Adsorption and desorption kinetics for hydrophilic and hydrophobic vapors on activated carbon. Carbon 2006, 44, 989–1004. [Google Scholar] [CrossRef]
- Ramos, M.E.; Bonelli, P.R.; Cukierman, A.L.; Ribeiro Carrott, M.M.L.; Carrott, P.J.M. Adsorption of volatile organic compounds onto activated carbon cloths derived from a novel regenerated cellulosic precursor. J. Hazard. Mater. 2010, 177, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.Y.; Ju, Y.W.; Kim, M.Y.; Jung, H.R.; Kim, H.J.; Lee, W.J. Adsorption of toluene on carbon nanofibers prepared by electrospinning. Sci. Total Environ. 2008, 393, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, T.; Fan, X.; Na, H. Adsorption and desorption of small molecule volatile organic compounds over carbide-derived carbon. Carbon 2014, 67, 712–720. [Google Scholar] [CrossRef]
- Bai, Y.; Huang, Z.H.; Wang, M.X.; Kang, F. Adsorption of benzene and ethanol on activated carbon nanofibers prepared by electrospinning. Adsorption 2013, 19, 1035–1043. [Google Scholar] [CrossRef]
- Scholten, E.; Bromberg, L.; Rutledge, G.C.; Hatton, T.A. Electrospun Polyurethane Fibers for Absorption of Volatile Organic Compounds from Air. ACS Appl. Mater. Interfaces 2011, 3, 3902–3909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, P.Q.; Zhou, H.G.; Li, C.; Wu, S.P.; Xiao, Y. Characteristics of using layered double hydroxides to reduce the VOCs from bituminous materials. Constr. Build. Mater. 2016, 123, 69–77. [Google Scholar] [CrossRef]
- Gil, R.R.; Ruiz, B.; Lozano, M.S.; Martín, M.J.; Fuent, E. VOCs removal by adsorption onto activated carbons from biocollagenic wastes of vegetable tanning. Chem. Eng. J. 2014, 245, 80–88. [Google Scholar] [CrossRef]
- Kim, H.J.; Pant, H.R.; Choi, N.J.; Kim, C.S. Composite electrospun fly ash/polyurethane fibers for absorption of volatile organic compounds from air. Chem. Eng. J. 2013, 230, 244–250. [Google Scholar] [CrossRef]
- Ge, J.C.; Kim, J.H.; Choi, N.J. Electrospun polyurethane/loess powder hybrids and their absorption of volatile organic compounds. Adv. Mater. Sci. Eng. 2016, 2016, 8521259. [Google Scholar] [CrossRef]
- Kim, H.J.; Pant, H.R.; Choi, N.J.; Kim, C.S. Fly Ash/Polyurethane Thin Film for the Adsorption of Volatile Organic Compounds (VOCs) from Air. Fibers Polym. 2014, 15, 1393–1398. [Google Scholar] [CrossRef]
- Purwar, R.; Goutham, K.S.; Srivastava, C.M. Electrospun Sericin/PVA/Clay nanofibrous mats for antimicrobial air filtration mask. Fibers Polym. 2016, 17, 1206–1216. [Google Scholar] [CrossRef]
- Sun, G.; Sun, L.; Xie, H.; Liu, J. Electrospinning of nanofibers for energy applications. Nanomaterials 2016, 6, 129. [Google Scholar] [CrossRef]
- Ercolano, G.; Farina, F.; Cavaliere, S.; Jones, D.J.; Rozière, J. Nickel based electrospun materials with tuned morphology and composition. Nanomaterials 2016, 6, 236. [Google Scholar] [CrossRef]
- Liu, Y.; Park, M.; Ding, B.; Kim, J.; El-Newehy, M.; Al-Deyab, S.S.; Kim, H.Y. Facile electrospun polyacrylonitrile/poly(acrylic acid) nanofibrous membranes for high efficiency particulate air filtration. Fibers Polym. 2015, 16, 629–633. [Google Scholar] [CrossRef]
- Rohatgi, C.V.; Dutta, N.K.; Choudhury, N.R. Separator membrane from crosslinked poly(Vinyl Alcohol) and poly(methyl vinyl ether-alt-maleic anhydride). Nanomaterials 2015, 5, 398–414. [Google Scholar] [CrossRef]
- Kim, H.J.; Pant, H.R.; Park, C.H.; Tijing, L.D.; Choi, N.J.; Kim, C.S. Hydrothermal growth of mop-brush-shaped ZnO rods on the surface of electrospun nylon-6 nanofibers. Ceram. Int. 2013, 39, 3095–3102. [Google Scholar] [CrossRef]
- Kumbar, S.G.; Nukavarapu, S.P.; James, R.; Nair, L.S.; Laurencin, C.T. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials 2008, 29, 4100–4107. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kang, Y.J.; Choi, N.J. Optical-fiber electronic speckle pattern interferometry for quantitative measurement of defects on aluminum liners in composite pressure vessels. J. Opt. Soc. Korea 2013, 17, 50–56. [Google Scholar] [CrossRef]
- Naumov, A.V. Review of the world market of rare-earth metals. Russ. J. Non-Ferr. Met. 2008, 49, 14–22. [Google Scholar]
- Charalampides, G.; Vatalis, K.I.; Apostoplos, B.; Ploutarch-Nikolas, B. Rare earth elements: Industrial applications and economic dependency of europe. Procedia Econ. Financ. 2015, 24, 126–135. [Google Scholar] [CrossRef]
- Guo, H.; Xue, B.; Chen, M. Catalytic oxidation of VOCs over the structured bimetallic catalyst 0.1% Pt-0.75% CeO2/SSWM. Sustain. Environ. Res. 2015, 25, 167–170. [Google Scholar]
- Carabineiro, S.; Konsolakis, M.; Marnellos, G.; Asad, M.; Soares, O.; Tavares, P.; Pereira, M.; Melo Órfão, J.; Figueiredo, J. Ethyl acetate abatement on copper catalysts supported on ceria doped with rare earth oxides. Molecules 2016, 21, 644. [Google Scholar] [CrossRef] [PubMed]
- Konsolakis, M.; Carabineiro, S.A.C.; Marnellos, G.E.; Asad, M.F.; Soares, O.S.G.P.; Pereira, M.F.R.; Órfão, J.J.M.; Figueiredo, J.L. Volatile organic compounds abatement over copper-based catalysts: Effect of support. Inorg. Chim. Acta 2017, 455, 473–482. [Google Scholar] [CrossRef]
- Yang, L.; Tao, D.; Yang, X.; Li, Y.; Guo, Y. Synthesis, characterization, and antibacterial activities of some rare Earth metal complexes of pipemidic acid. Chem. Pharm. Bull. 2003, 51, 494. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, S.; Wang, X.; Yu, J.; Ding, B. Efficient and reusable polyamide-56 nanofiber/nets membrane with bimodal structures for air filtration. J. Colloid Interface Sci. 2015, 457, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Gopal, R.; Kaur, S.; Ma, Z.; Chan, C.; Ramakrishna, S.; Matsuura, T. Electrospun nanofibrous filtration membrane. J. Membr. Sci. 2006, 281, 581–586. [Google Scholar] [CrossRef]
- Wang, Z.; Lou, S.; Li, P. Enhanced orange-red emission of Sr3La(PO4)3:Ce3+, Mn2+ via energy transfer. J. Lumin. 2014, 156, 87–90. [Google Scholar] [CrossRef]
- Li, Q.; Pang, J.; Wang, B.; Tao, D.; Xu, X.; Sun, L.; Zhai, J. Preparation, characterization and microwave absorption properties of barium-ferrite-coated fly-ash cenospheres. Adv. Powder Technol. 2013, 24, 288–294. [Google Scholar] [CrossRef]
- Iwasiki, M.; Shinjoh, H. Analysis of the adsorption state and desorption kinetics of NO2 over Fe–zeolite catalyst by FT-IR and temperature-programmed desorption. Phys. Chem. Chem. Phys. 2010, 12, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
| Spinning Solutions (PU/RE) | Viscosity (cps) | Conductivity (ms/m) | Fiber Diameter (nm) | BET Surface Area (m2/g) | |
|---|---|---|---|---|---|
| Distribution | Mean | ||||
| PU | 379.0 | 0.172 | 227–877 | 489 | 6.853 |
| PU/RE-10 | 439.0 | 0.183 | 357–966 | 513 | 7.147 |
| PU/RE-30 | 464.2 | 0.191 | 262–871 | 524 | 7.729 |
| PU/RE-50 | 504.6 | 0.315 | 179–591 | 356 | 11.207 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, J.C.; Choi, N.J. Fabrication of Functional Polyurethane/Rare Earth Nanocomposite Membranes by Electrospinning and Its VOCs Absorption Capacity from Air. Nanomaterials 2017, 7, 60. https://doi.org/10.3390/nano7030060
Ge JC, Choi NJ. Fabrication of Functional Polyurethane/Rare Earth Nanocomposite Membranes by Electrospinning and Its VOCs Absorption Capacity from Air. Nanomaterials. 2017; 7(3):60. https://doi.org/10.3390/nano7030060
Chicago/Turabian StyleGe, Jun Cong, and Nag Jung Choi. 2017. "Fabrication of Functional Polyurethane/Rare Earth Nanocomposite Membranes by Electrospinning and Its VOCs Absorption Capacity from Air" Nanomaterials 7, no. 3: 60. https://doi.org/10.3390/nano7030060






