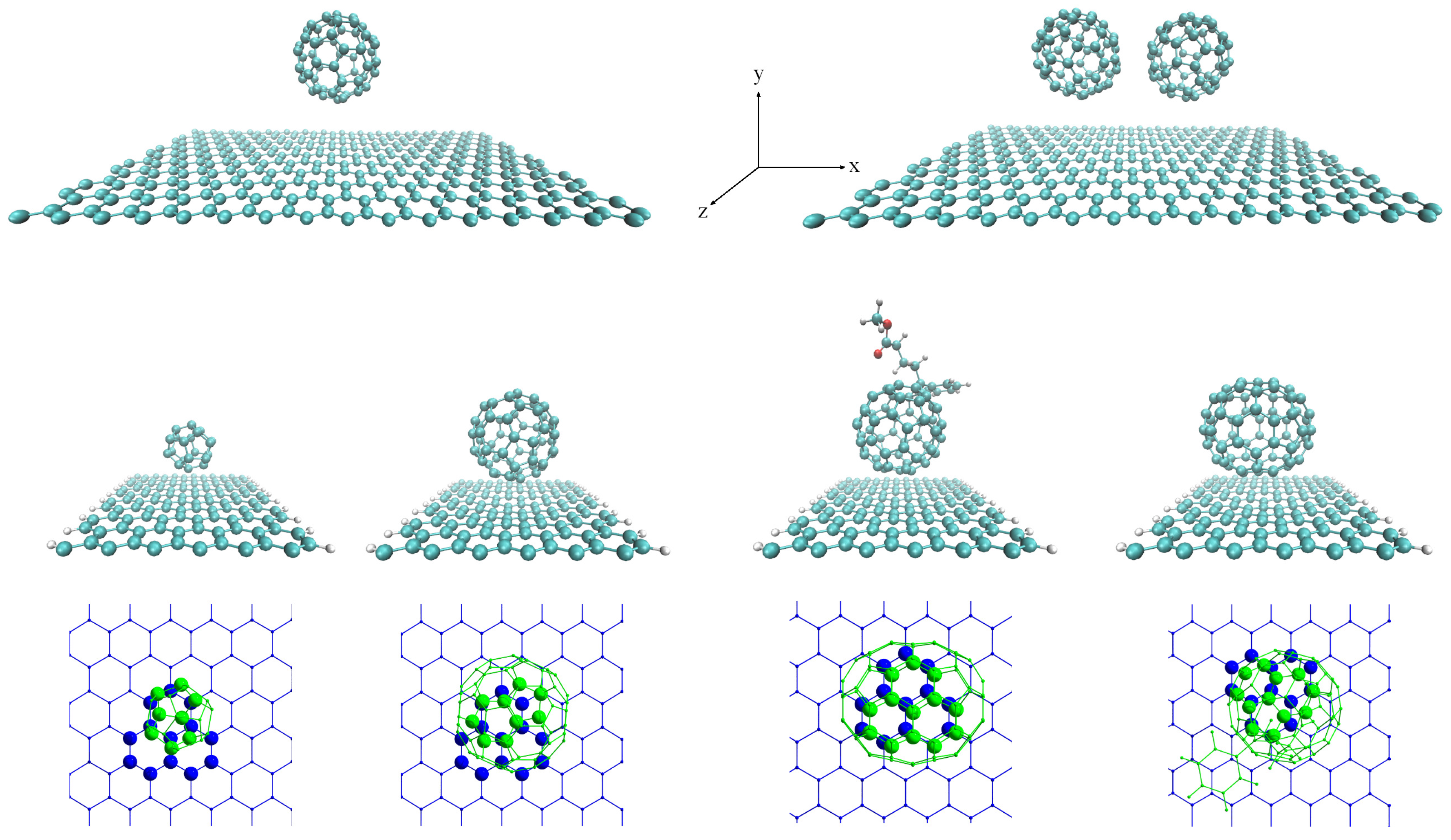
In
Table 1, we show some geometrical and electronic structure parameters for each complex studied. Three different widths of GRs are considered:
Å (8GR),
Å (10GR) and
Å (12GR). When the fullerenes are physisorbed on SLG or nGRs (n = 8, 10 or 12), the equilibrium distance of the fullerenes varies between
Å and
Å. These distances depend on several factors such as the contact area, the widths of the nGRs, the size, shape and orientation of the fullerenes, and the vdW exchange-correlation functional employed. Our results of the binding energy of fullerenes on SLG and nGR are in the range of
to
eV, where the largest value corresponds to the 10GR–C
system, and the smallest value to the 8GR–C
system. The difference between the smallest and the largest value of the binding energy is due to the decrease in the contact area. This is confirmed by the results displayed in
Table 1, where we observe that the largest binding energy, for each nGR–fullerene system, corresponds to the C
fullerene, which is the biggest fullerene considered in this work.
The adsorption energy is also influenced by the finite width of the nGRs, and in
Table 1 it is possible to compare the values of the
corresponding to the different systems. In all cases, we observe that the binding energy for nGR–C
is lower than that corresponding to the SLG–C
system, which suggests that the edges of the nGRs induce a reduction in the binding of the fullerenes. Our result for the binding energy of SLG–C
is
eV, and this value is in agreement with previous theoretical calculations [
14], that inform on a value of
eV using a vdW-DF1 level of theory. This last result is consistent with the reported experimental value for C
physisorbed on graphite [
15]. In contrast, the adsorption energy of all the fullerenes increases with the width of the GRs because the edges effect becomes less important.
2.1. Fullerenes Adsorbed on a Graphene Monolayer
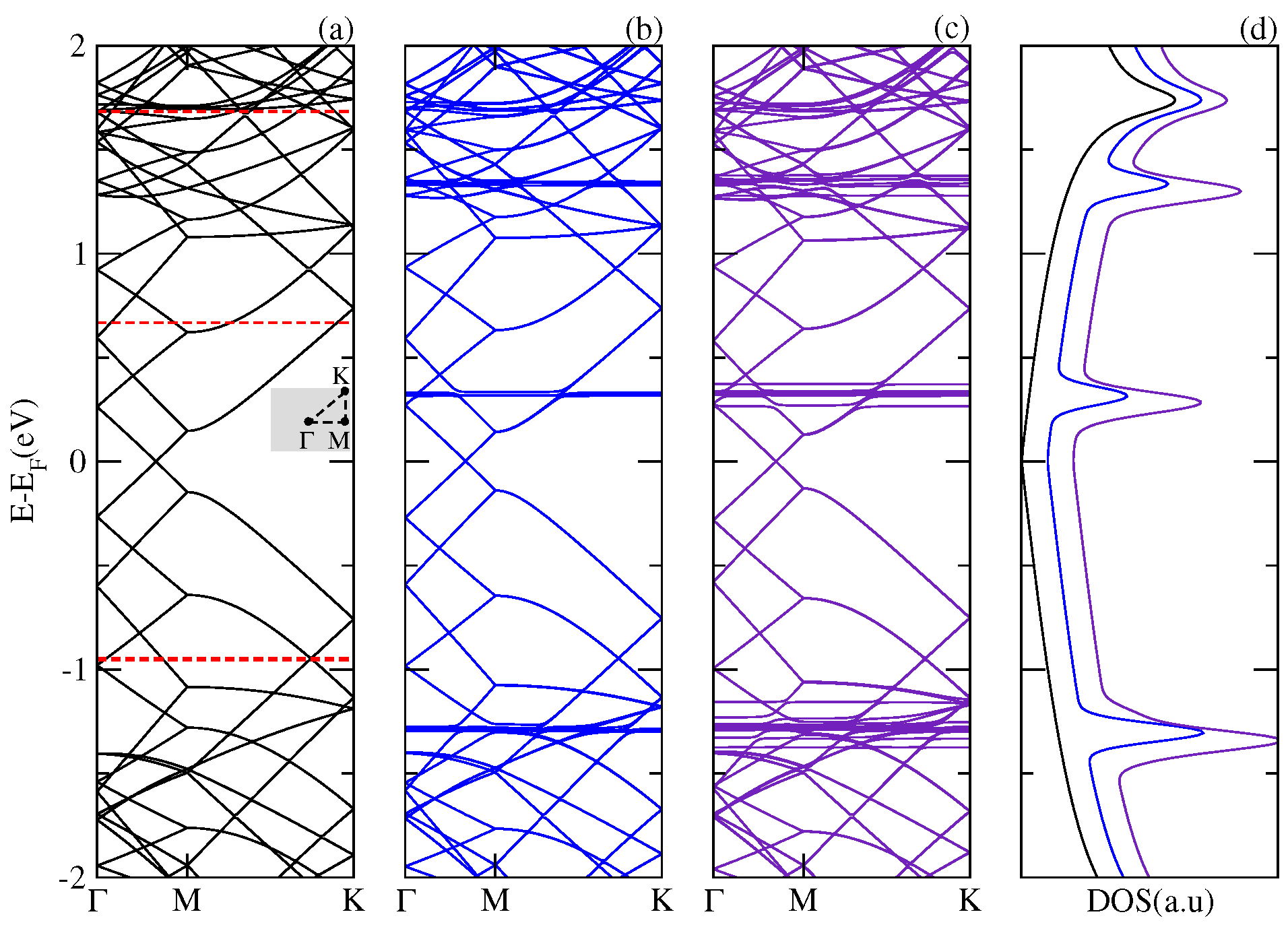
Figure 1 shows the electronic band structure and the total DOS of the single layer graphene and of the complexes composed of a C
physisorbed on a graphene layer (SLG–C
) and two fullerenes adsorbed on a graphene layer (SLG–2C
). It is apparent from the low energies results that the energy spectrum of the hybrid structure SLG–C
is basically a superposition of the energy spectrum corresponding to each component of the composite. The energy levels of the C
molecule appear as dispersionless bands around the Fermi energy (E
) of the system. The presence of these states is reflected in the DOS as resonances inserted between the characteristic Van Hove peaks of the graphene. The same overall features are observed for the SLG–2C
complex; however, in this case, the interaction between the two fullerenes splits the levels forming stripes of dispersionless bands around the original levels of the fullerene. The resonances in the DOS are enhanced and slightly displaced with respect to those corresponding to the SLG–C
system. We found that the adsorption of the C
fullerenes on the SLG does not modify the low energy states of the pristine graphene maintaining its metallic character.
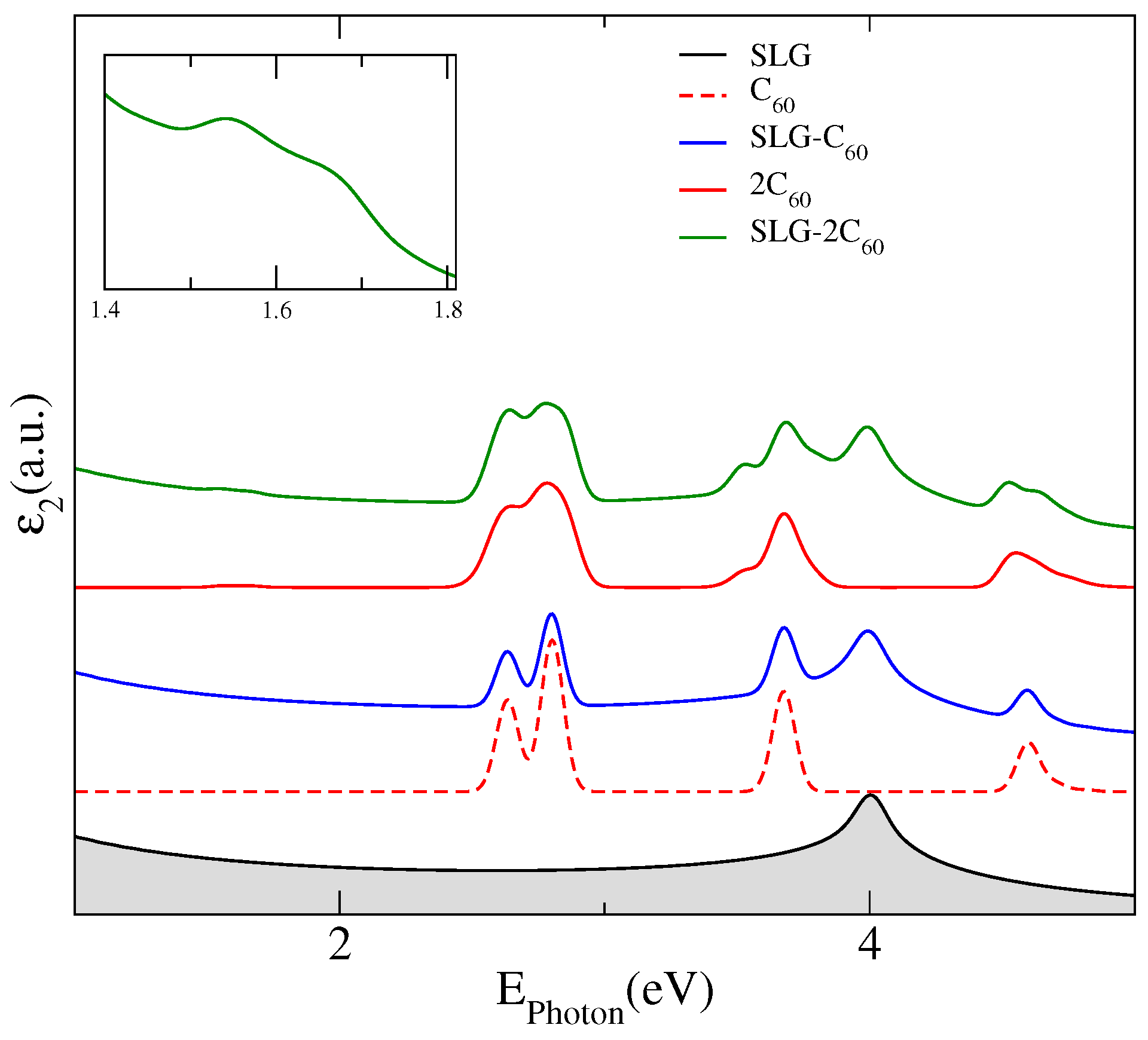
The main effects of the adsorption of fullerenes on graphene are manifested in its optical response, which is displayed in
Figure 2 where we have plotted the imaginary part of the dielectric constant for a pristine SLG (black line), an isolated C
(dashed red line) and 2C
fullerenes (red line) and, for the complexes SLG–C
(blue line) and SLG–2C
(green line). The absorption spectra of the hybrid systems present noticeable differences with the SLG spectrum in the visible energy region where two prominent peaks appear from interband transitions between the new states stemming from the adsorbed fullerenes. The extra interband transitions, at the high energy region, also modify the distinctive absorption peak in graphene coming from band-to-band transitions near the saddle-point singularity at the M-point. Unlike the case of one C
adsorbed on a SLG, for which the optical spectrum of the hybrid system is mainly a superposition of the spectrum of each component, in the complex SLG–2C
the interaction between both fullerenes produces a symmetry breaking leading to a splitting of the peaks associated to transitions between fullerene levels, which can be observed at energies of about 3.5 eV and at 4.5 eV in the corresponding spectrum. However, the most remarkable result for the optical spectrum of the hybrid SLG–2C
is the presence of a low-energy absorption peak located at 1.54 eV, which is not attributable to any transition between graphene energy levels or between fullerene levels but seems to correspond to transitions between graphene–fullerene strongly hybridized states to levels of the first flat miniband associated to C
levels that are forbidden for isolated fullerenes on graphene. We have included an inset in
Figure 2 to highlight this peak, which should be more intense for a greater number of interacting molecules.
2.2. Fullerenes Adsorbed on Armchair Nanoribbons
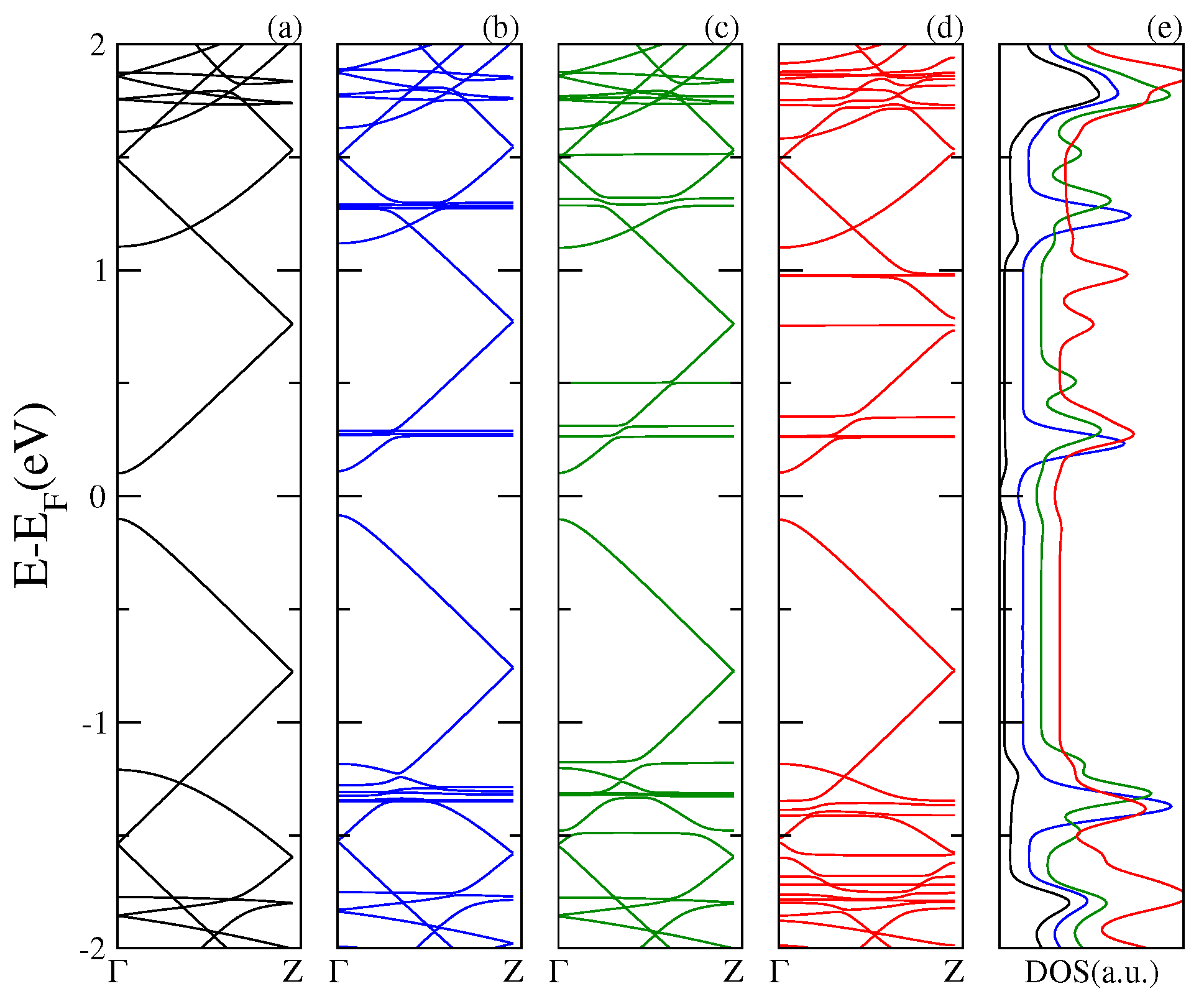
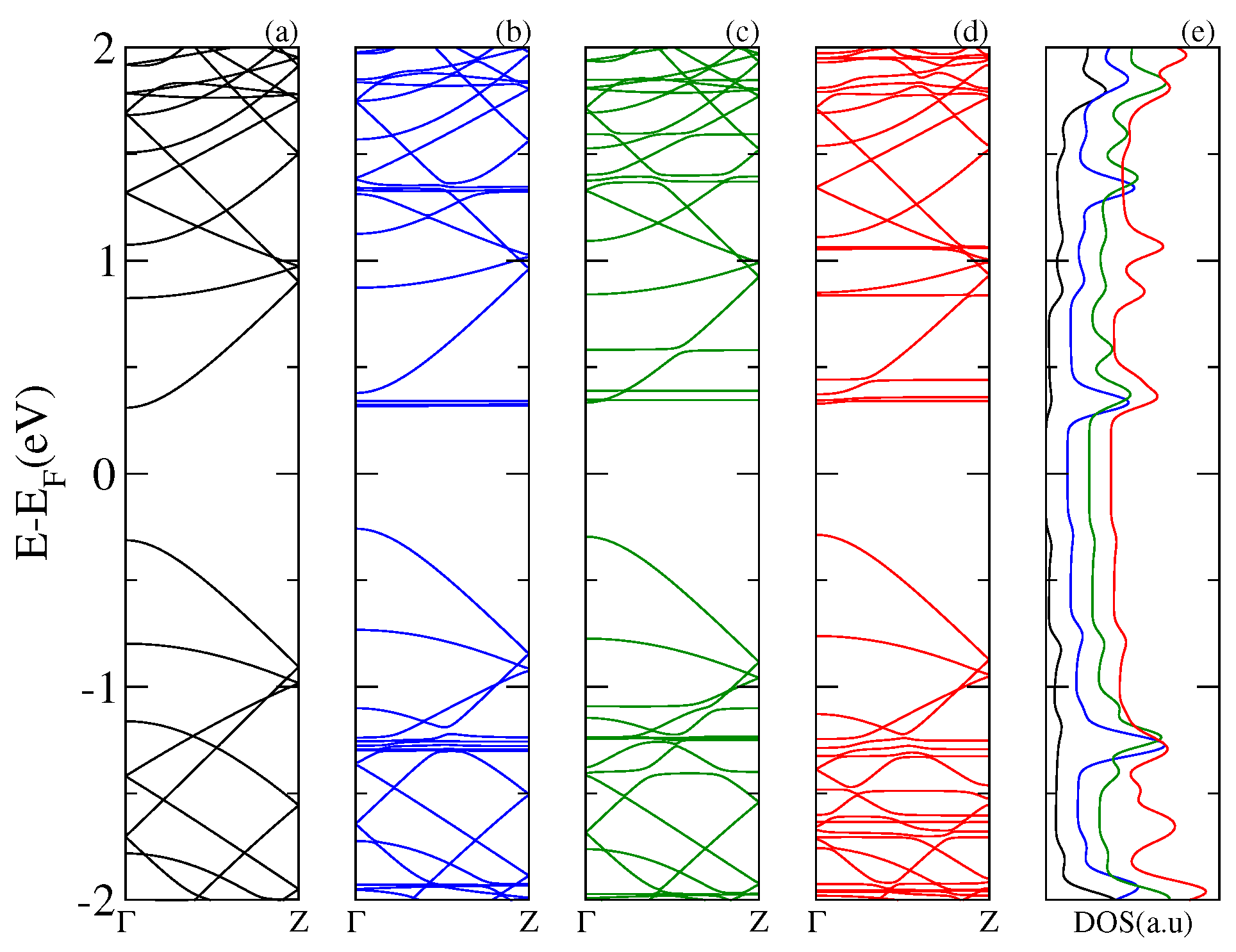
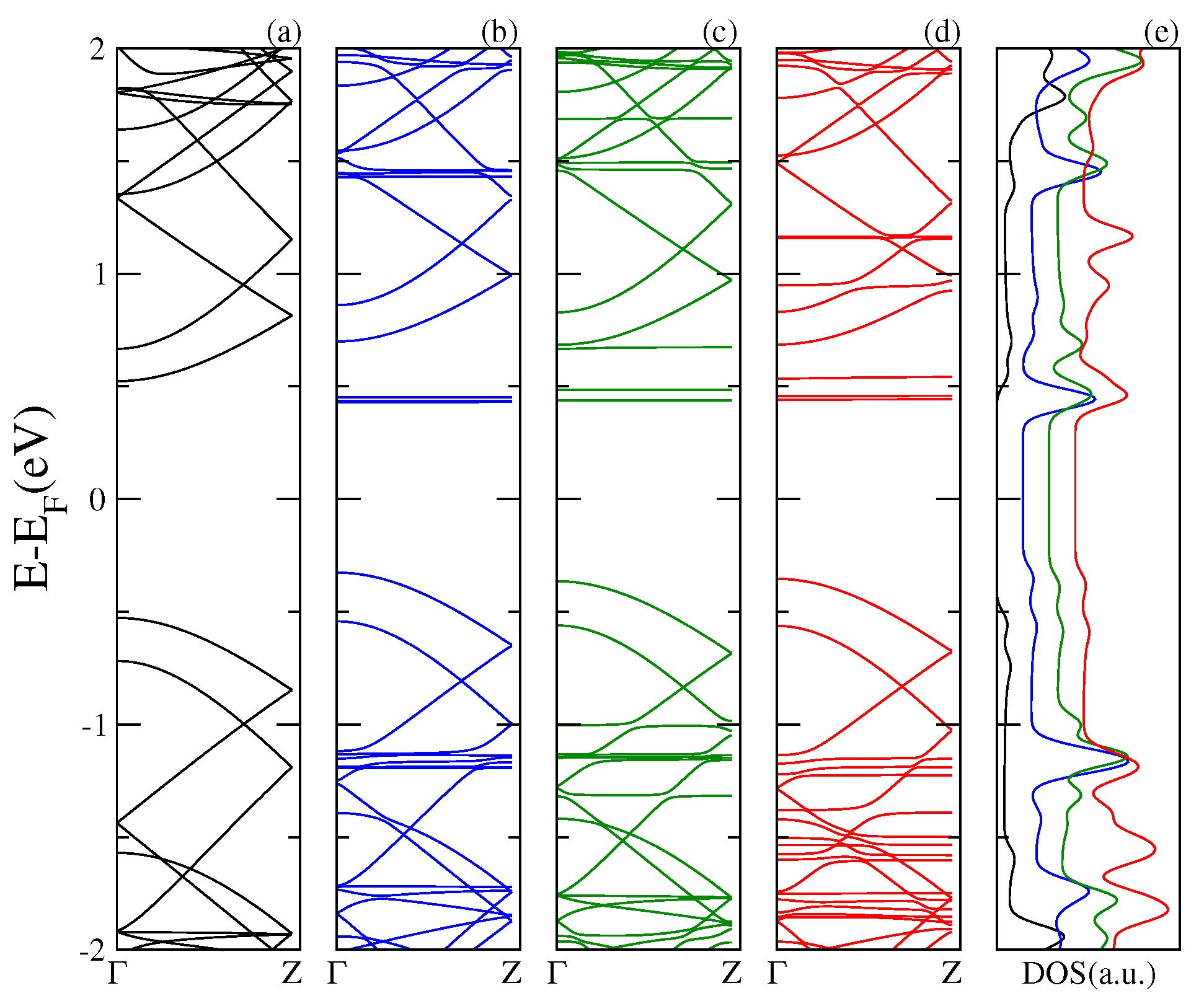
Next, in
Figure 3,
Figure 4 and
Figure 5, we display the electronic band structure and corresponding DOS for armchair nanoribbons of different widths: nGR, n = 8, 12, 10. The band spectrum is plotted as a function of the wave vector along the
z direction. Each figure shows results for the (a) pristine nGR, and the hybrids: (b) nGR–C
; (c) nGR–PCMB; and (d) nGR–C
. In (e) the corresponding DOS for the nanostructures is depicted.
Similarly, as in the case of the SLG–fullerenes systems, the energy spectrum of nGRs with adsorbed fullerenes displays a superposition of π bands coming from the pristine nanoribbon and localized states generated by the adsorption of the fullerene molecules. As a general observation, we can infer that the modification of the electronic structure of each nGR due to the fullerene adsorption is strongly dependent on the size of the fullerene molecule. The DOS for the studied complexes shows the emergence of many new resonances apart from those proper of the geometrical characteristics of the nanoribbons.
Our results show that the main alterations in the electronic band structure of the pristine nanoribbons occur for the GRs of smaller band gap, 8GR (
Figure 3) and 12GR (
Figure 4). For these cases, the
π bands of the graphene nanoribbons are clearly modified as a consequence of the re-hybridization with the molecule orbitals. In particular, this is more evident in
Figure 4 for the complexes with molecules adsorbed on the narrowest nanoribbon in which the states near the Fermi energy are strongly affected. This is manifested in the corresponding DOS as an increase of the resonance strengths.
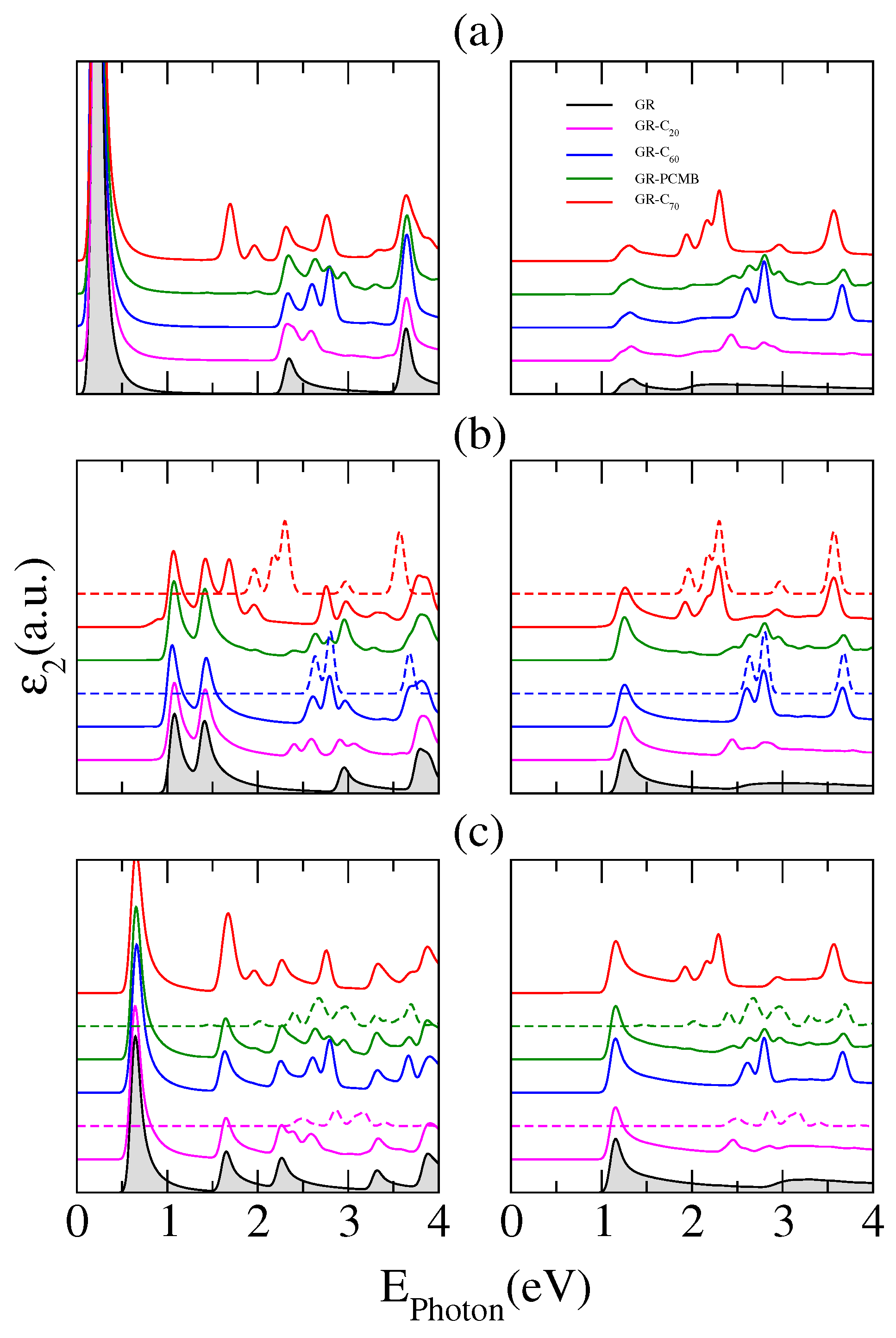
The optical response of the hybrid structures shows many rich features, as noted in
Figure 6 where the corresponding absorption spectra are plotted. Distinct panels present calculations of the imaginary part of the dielectric constant as a function of the photon energy for fullerene molecules adsorbed on (a) 8GR; (b) 10GR and (c) 12GR nanoribbons. The curves displayed in each panel correspond to the optical spectrum of the different complexes, vertically shifted for clarity. We have also included results for the molecule C
, the smallest known fullerene. For comparison, each panel incorporates—shadowed in gray—the corresponding optical response of the pristine nanoribbon forming the complex. The left panels exhibit the optical response for parallel light polarization (along the length of the ribbon-
z) and the right panels for perpendicular light polarization (across the ribbon-
x). It can be observed that the principal signature of the adsorption of molecules on the nanoribbons is the strong light absorption in the range between 2 and 3 eV. This is true for all nanoribbons considered, independent of their width and of the light polarization considered.
In the range of lower energies (E
eV) and for longitudinally polarized photons, the optical absorption of the different systems shows a strong dependence on the nanoribbon width, exhibiting a greater number of absorption peaks for wider nanoribbons. This is an expected behavior for pristine GRs [
16]. In this range of energy, the optical response is dominated by the nanoribbon electronic properties, except for the nGR–C
systems due to the stronger interaction between the parts of the complex. For these systems, we can realize the appearance of new transitions between states hybridized of the nanoribbon–fullerene complex.
For higher energy regions, the interpretation of the origin of the different absorption peaks is more complicated due to the superposition, or hybridization of the nanoribbon and fullerene states. For comparison, we have included in dashed lines the optical response of isolated C
and C
fullerenes in
Figure 6b, and in
Figure 6c those of isolated PCMB and C
. This effectively permits us to realize the unveiling of new optical resonances derived from transitions between hybridized states of the nanoribbons and fullerenes. The strong peak at about 1.69 eV for the complex 10GR–C
is conspicuous, and it does not correspond to any transition between states associated to the C
molecule or to transitions between states associated to the nanoribbon, but clearly it corresponds to a transition between hybridized states. This can be observed in the columns (a) and (b) of
Figure 7 where the electronic contribution to the band structure of the complex, coming from the carbon atoms of the nanoribbon and those from the atoms of the C
, has been calculated separately (fat-band representation).
It has been shown before that GRs exhibit a strongly anisotropic optical response due to the symmetry selection rules [
16], and this can be observed in
Figure 6 for the absorption spectra of pristine nanoribbons where only one absorption peak, located at low photon energies, is noticeable for light polarized perpendicular to the nanoribbons (shadow curves). This peak remains without distortion for all considered hybrid structures and clearly corresponds to transitions between nanoribbon states. Unlike the case of polarization along the nanoribbon edges, at the high energy range the optical spectra for transversely polarized light show only resonant transitions between states of isolated fullerenes. This is clear from the comparison between the spectra of the hybrid structures and the bare fullerenes (dashed curves). Thereby, the absorption spectra for transversely polarized photons of the nGR–fullerene systems enable us to distinguish those peaks resulting from transitions between fullerene states, between nanoribbon states, or from transitions between hybridized states of the complex.
The optical transitions of the nanoribbon–fullerene hybrid systems can be tuned by the application of an external static electric field across the nanoribbon edges. We have calculated the electro-absorption spectrum of two representative hybrids which have shown a rich absorption spectra in the visible range of light.
Figure 8 displays calculations of the imaginary part of the dielectric function for a 10GR–C
(upper panel) and a 10GR–C
(lower panel) system, for incident light linearly polarized (a) parallel to the ribbon and (b) perpendicular to the ribbon. The curves displayed in both panels correspond to different electric field intensities, scanned from 0.0 to 1.0 V/Å, and vertically shifted for clarity. The position of the optical absorption peaks of the pristine 10GR has been highlighted with a red line for comparison. It is clear from the results that due to the breaking of symmetry caused by the electric field, some original absorption peaks associated with the pristine GR are suppressed, while new extra peaks associated with forbidden transitions for zero electric fields, appear in the optical spectra. The common feature observed in the optical spectra of both structures is the characteristic redshift of the absorption edge induced by the electric field. This is an expected result because the structure of the infrared absorption mainly originates from transitions between states of the 10GR [
13,
17]. For photon energies higher than 1.5 eV, it is possible to differentiate two types of resonances in terms of its dependence with the electric field intensity. The magnitude of the absorption peaks associated with transitions between nanoribbon states increases with the field strength, and the corresponding positions move to lower energies. In contrast, the position of the resonances associated with transitions between fullerene states is not strongly affected by the electric field except for the intensities considered.
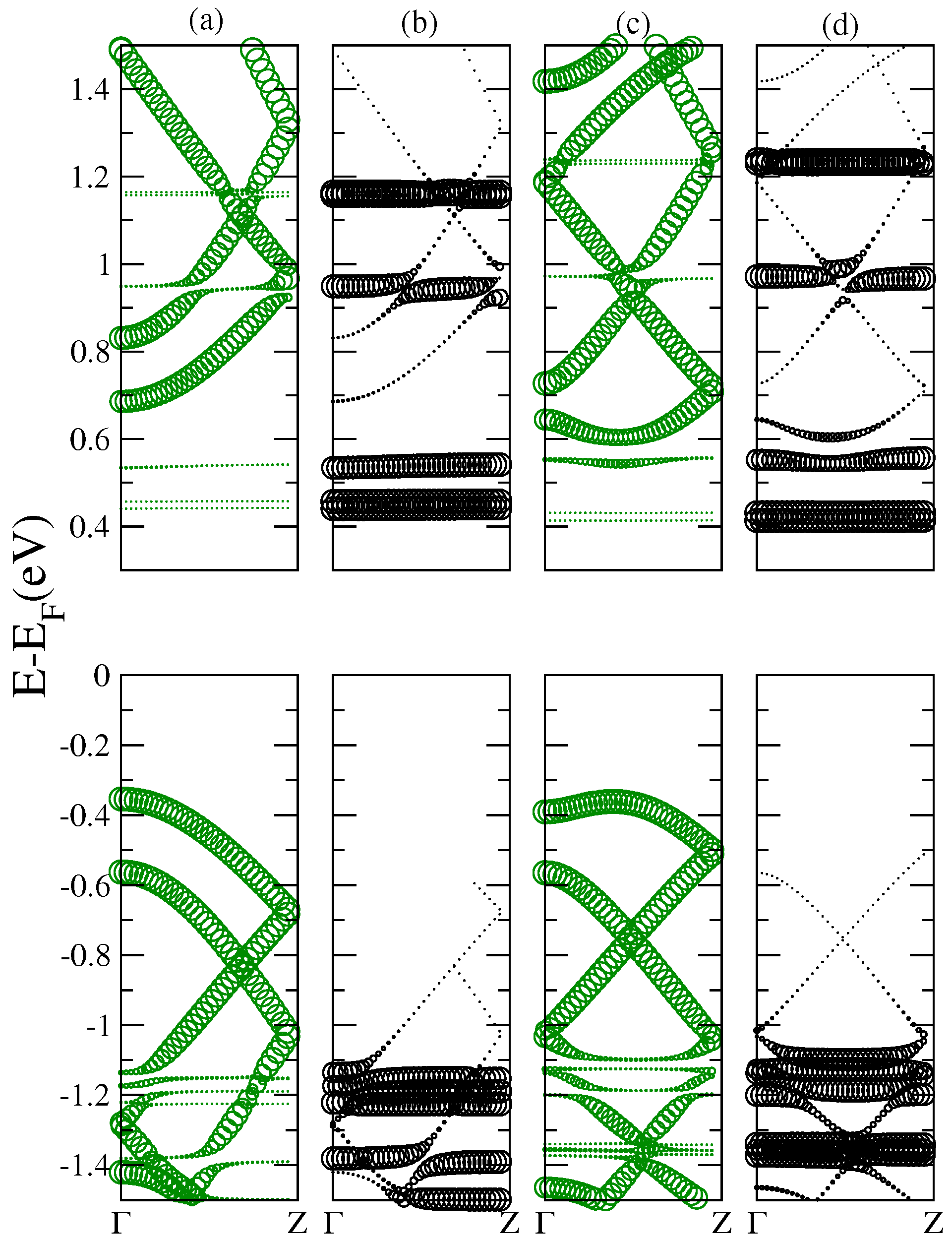
An important result provided by the electro-absorption spectra is the feasibility to analyze and detect those optical resonances derived from transitions between states of an energy-band of the nanoribbon to energy levels of the fullerene. Let us look for instance at the absorption peak located at 1.69 eV for the complex 10GR–C
in the lower panel (a). For increasing fields, the fullerene levels are split and new optical transitions are allowed leading to two resonances in the spectrum. It can be noticed that for light longitudinally polarized, the spectra are enriched with many new transitions coming from nanoribbon–fullerene hybridizing states. This is clear for the structure 10GR–C
where for photon energies larger than 2 eV the breaking of the degeneracy of the fullerene levels by the field admits new transitions between hybridizing states. In
Figure 8, we show the band structure of the complex 10GR–C
in the fat-band representation, which allows a better visualization of the electronic contribution of the carbon atoms from the nanoribbon and those from the atoms of the C
. Columns (a) and (b) show the case of zero electric field and columns (c) and (d) show the case of an applied electric field of
V/Å. The contribution to the band structure of the 10GR carbon atoms is displayed in (a) and (c) and the contribution of the C
carbon atoms is displayed in (b) and (d). The biggest circle means a major contribution of the corresponding atoms to a specific point in the band structure.
2.3. Graphene-Based Hybrid Structures as Components of Solar Cells
Theoretical studies on the possible use of GNRs as low band gap donor materials for solar cells had been reported by S. Osella et al. [
18]. In their paper, they proposed finite GNRs of different shapes and widths which could meet the required electronic and optical properties to be a good candidate for C
-based solar cells. The former results on the optoelectronic properties of graphene-based hybrid structures give support to the idea of using them as possible donor–acceptor systems for organic photovoltaic cells. To perform a qualitative study, we have included an analysis on the type of energy band alignment between the nGRs (donor) and the fullerenes (acceptor) studied.
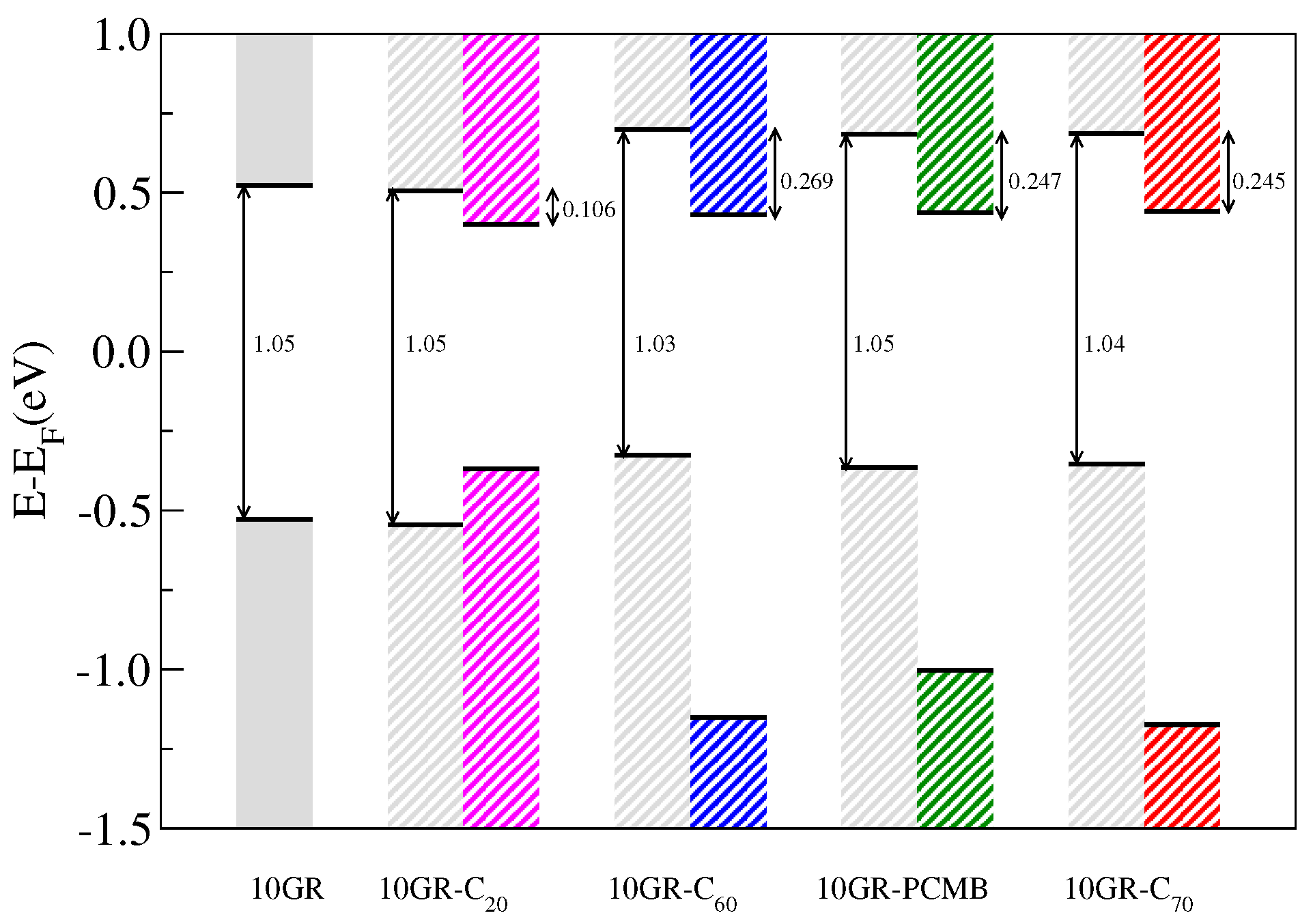
Figure 9 shows a schematic representation of the alignment of the highest valence band energy (HVBE) and lowest conduction band energy (LCBE) in the Γ point associated with the 10GR and the HOMO and LUMO molecular levels of the different fullerenes considered in the complex 10GR–fullerene. In the scheme, we observe changes in the position of the HVBE and LCBE with respect to the Fermi level (
in this case) when the fullerenes are physisorbed; in particular, for fullerenes C
, PCMB and C
the HVBE and LCBE are shifted about
eV with respect to the Fermi level. However, in the case of the smallest fullerene C
, the position of the LCBE and HVBE energies remains almost invariant, and this is because these shifts are caused by the redistribution of charge originated by the van der Waals interaction between the components of the complex, which grows with the size of the fullerene. On the other hand, it can be observed in
Table 1 that the difference between the LCBE and the HVBE energies virtually does not change with the presence of the fullerenes and that the difference in energy between the HOMO and LUMO states associated with each fullerene only shows minor changes of at most
eV. For the systems 10GR–C
/–PCMB/–C
, we observe that the bands display a type-II alignment, which is an ideal characteristic for photovoltaic device operations [
2]. Another important aspect that emerges from the scheme shown in
Figure 10 is the values of the band offset of the conduction bands. These are fundamental parameters in the development of heterostructured devices [
19], where few meV values are needed to favor the electron transfer from the photoexcited donor to the ground-state acceptor [
18]. Finally, the band offset and the donor band gap could be employed to determine the maximum quantum conversion efficiency of an excitonic solar cell [
20]. For our systems, we get a maximum conduction band offset (
) of
eV corresponding to the 10GR–C
system and a minimum
of
eV corresponding to the 10GR–C
system, which is not a system with type-II band alignment. Despite the fact that our results do not include many body corrections such as the GW or the Bethe–Salpeter equation (BSE), which are necessary to calculate the correct position of the conduction bands and a best value to the optical band gap, they are a first qualitative approximation for the analysis of possible candidates to be used as excitonic solar cells. This level of theoretical calculation was employed previously to study other heterojunctions for photovoltaic devices [
20].