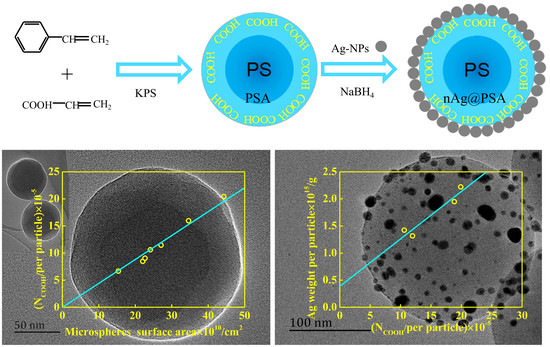
Preparation and Structural Analysis of Nano-Silver Loaded Poly(styrene-co-acrylic acid) Core-Shell Nanospheres with Defined Shape and Composition
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Poly(styrene-co-acrylic acid) (PSA) Composite Nanospheres
2.3. Preparation of nAg@PSA Composite Nanospheres
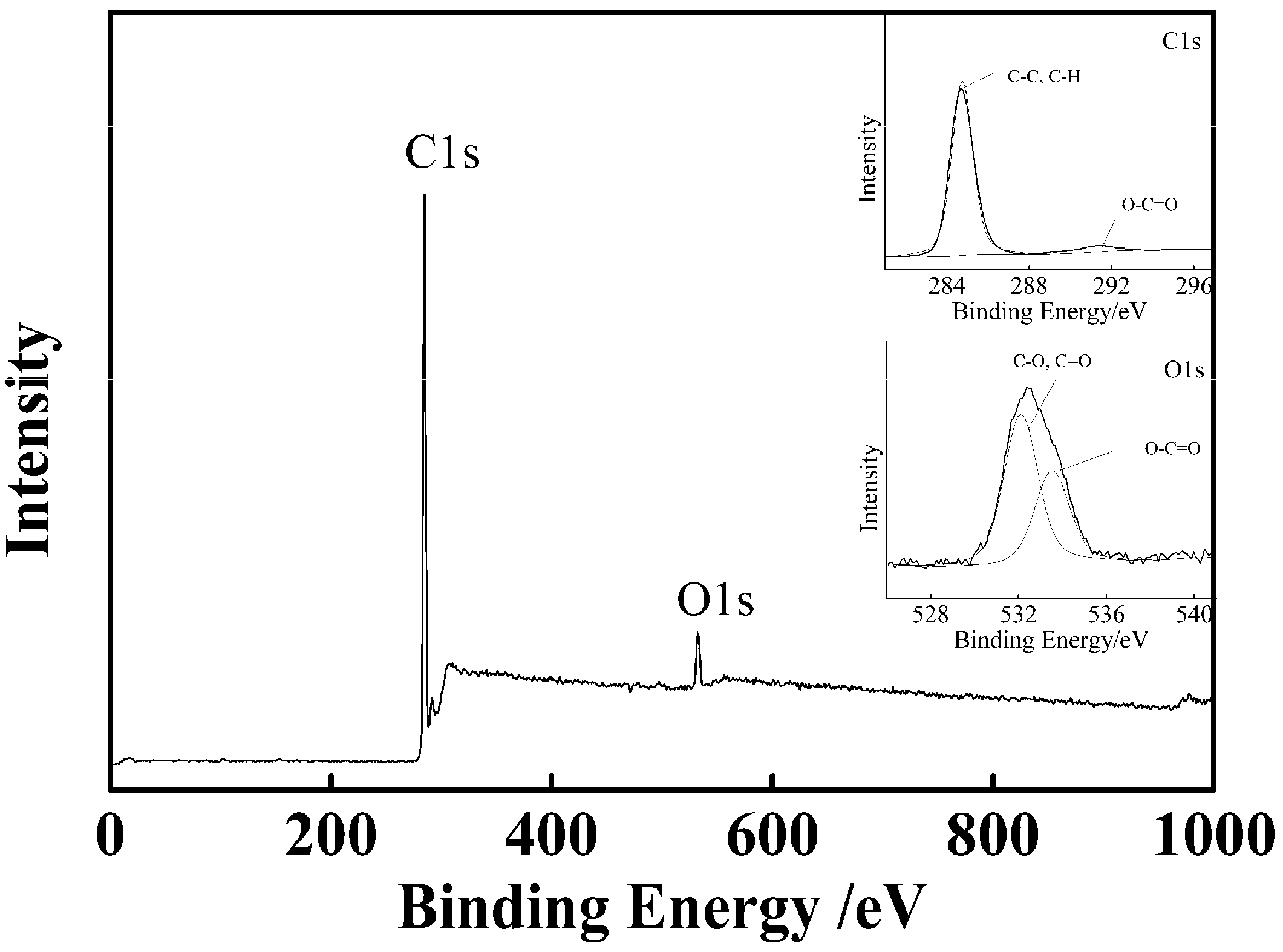
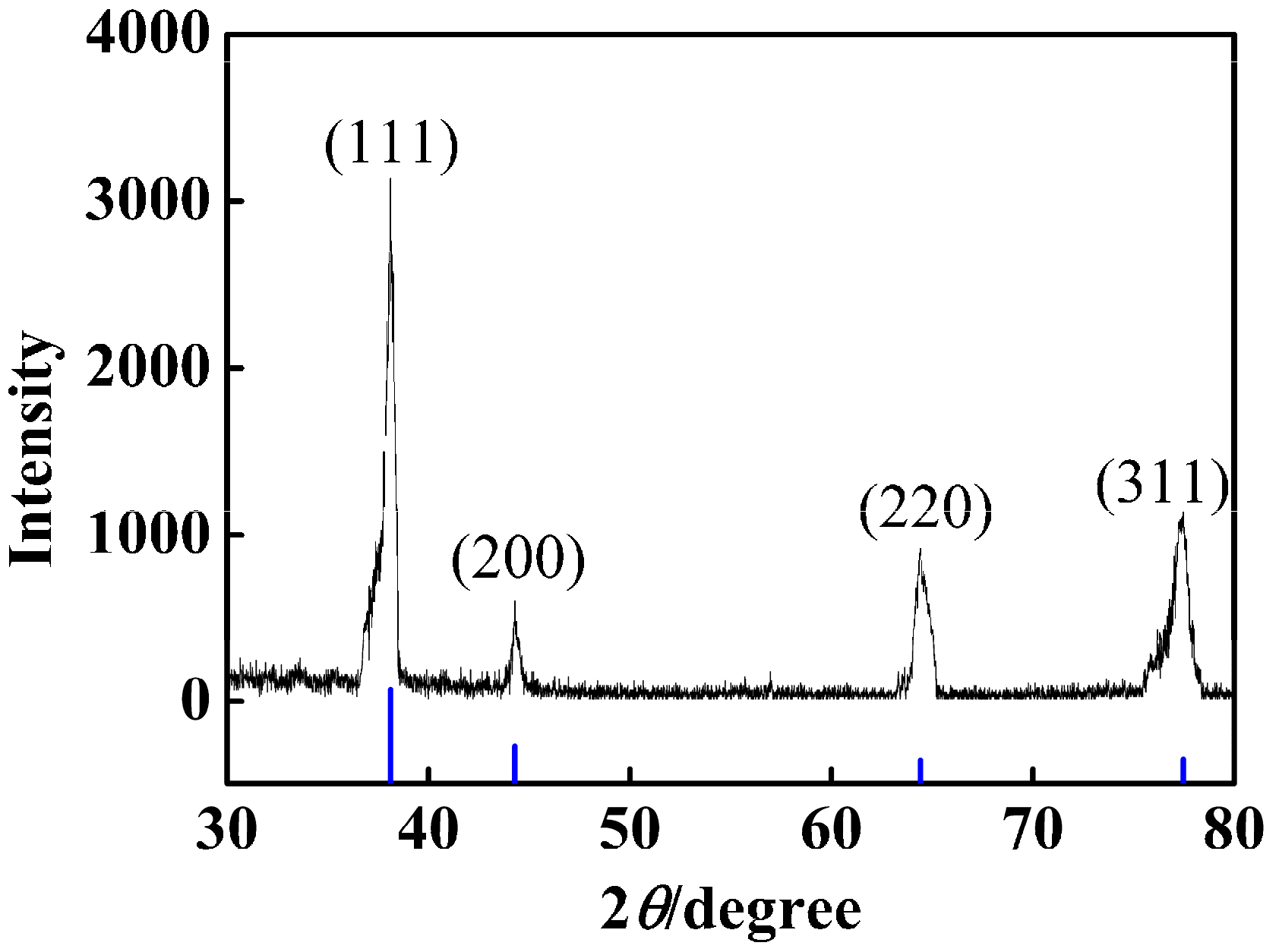
2.4. Characterization of PSA Nanospheres and nAg@PSA Nanocomposites
3. Results and Discussion
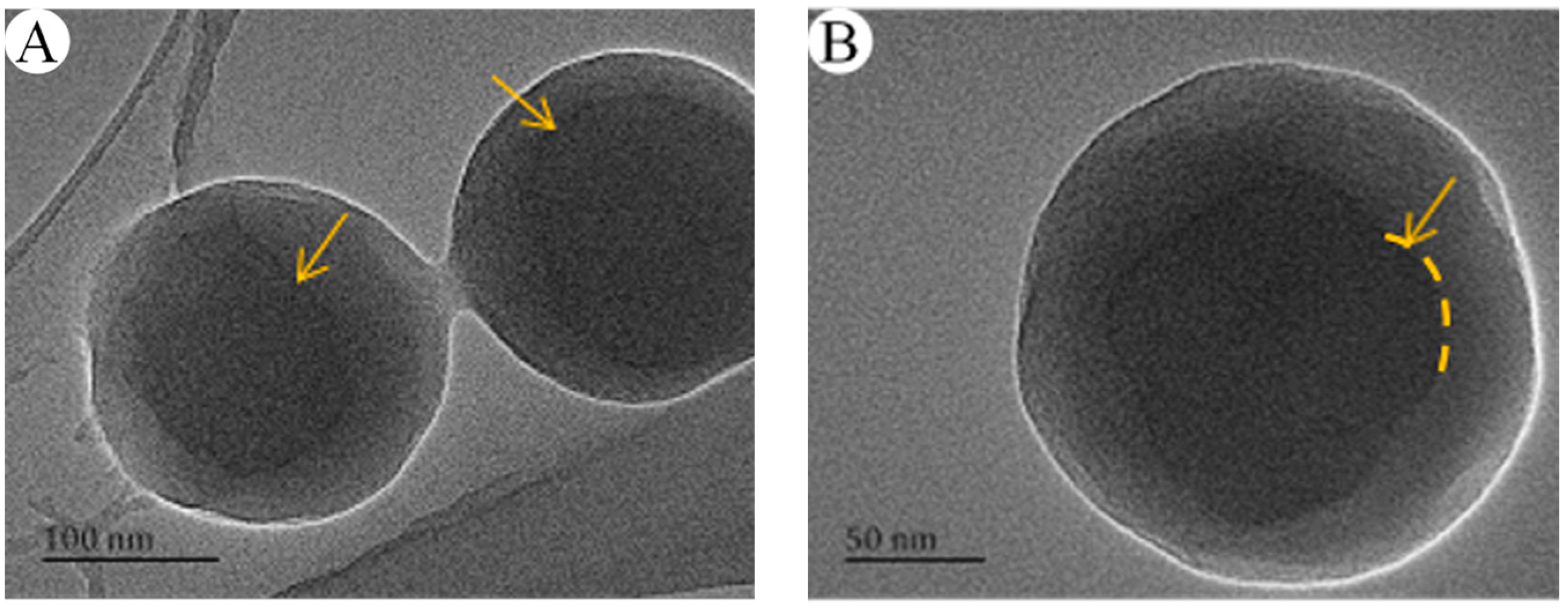
3.1. Effect of Different Factors on the Particle Size and Morphology of PSA Nanospheres
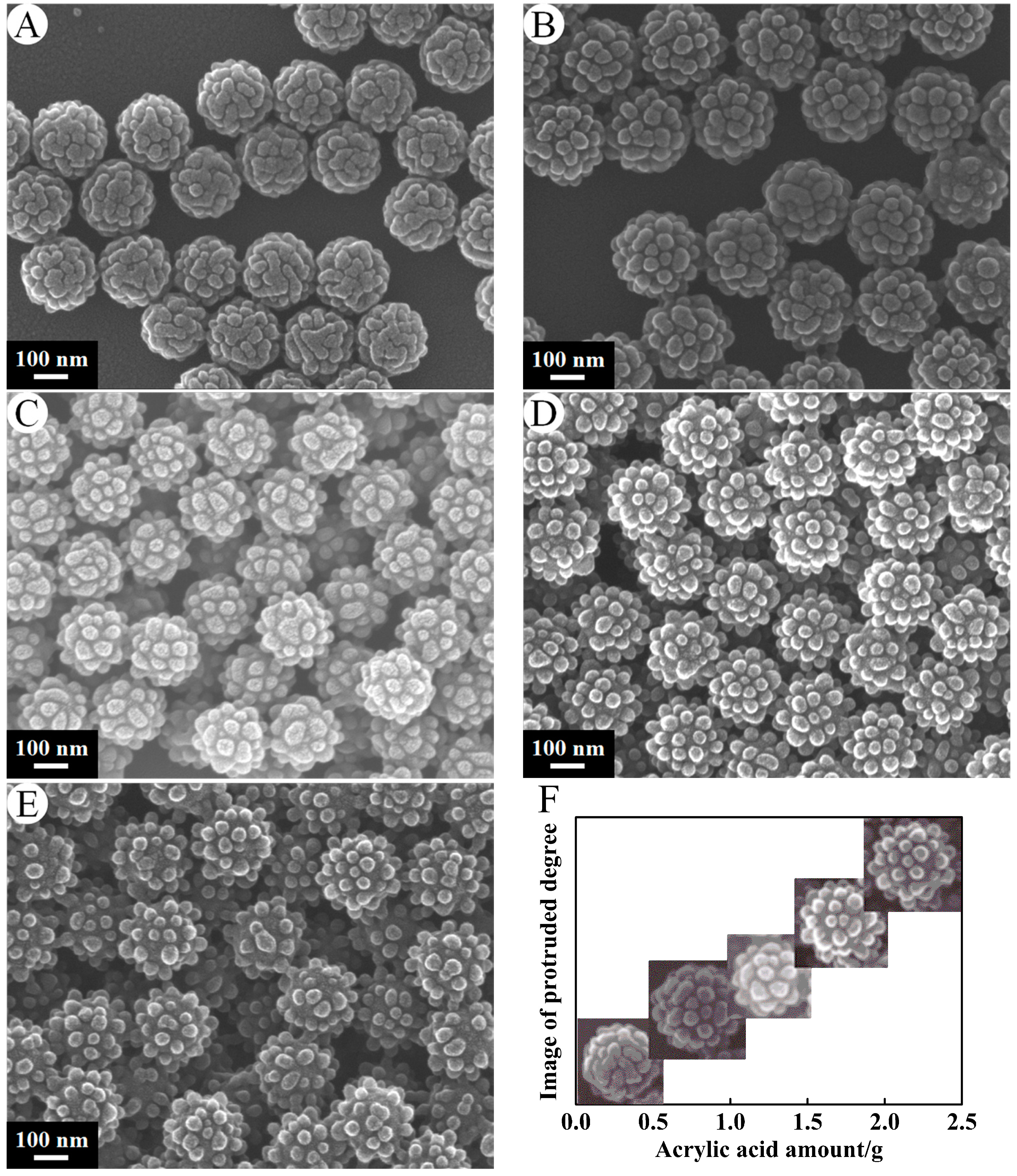
3.1.1. Amount of Acrylic Acid
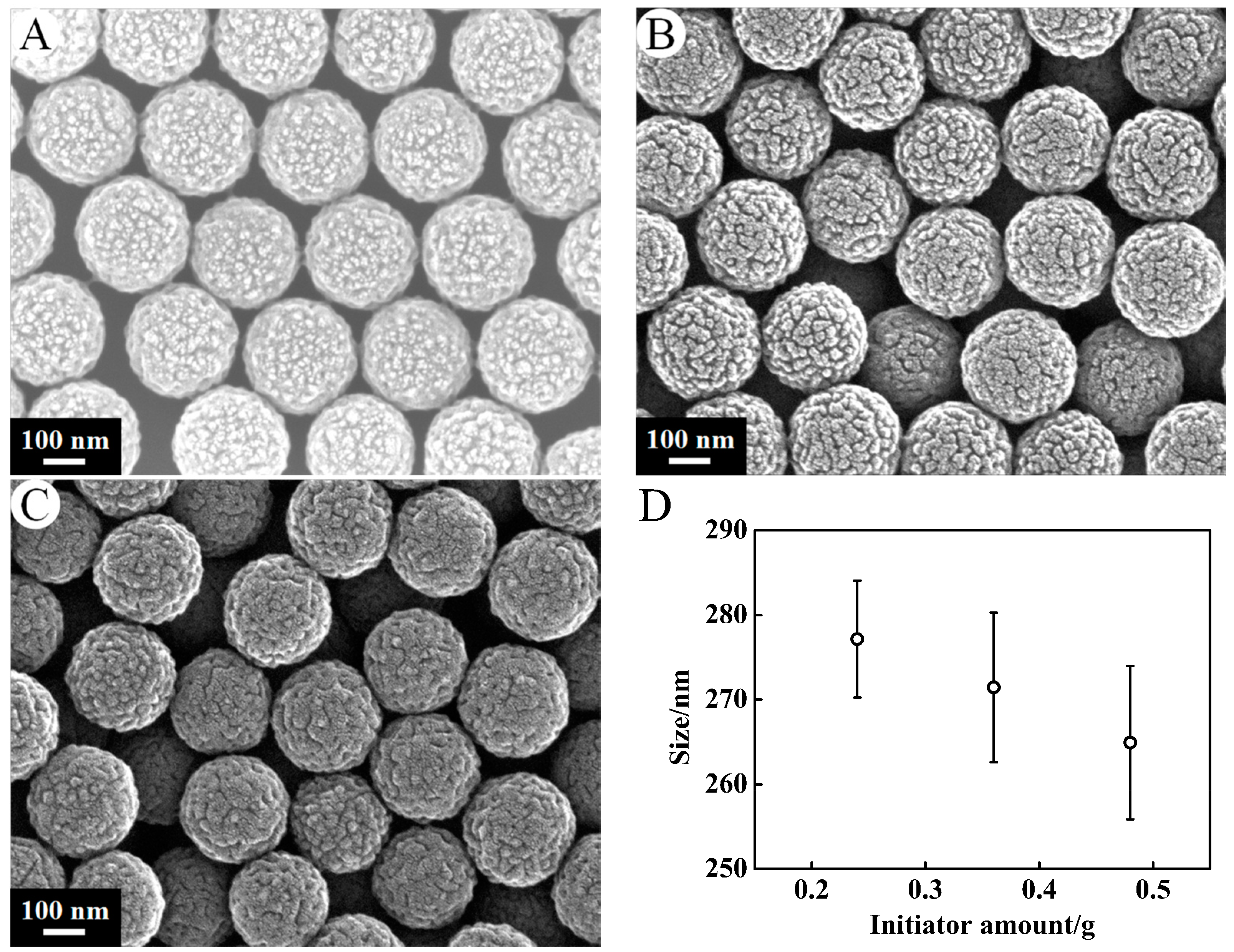
3.1.2. Amount of Initiator
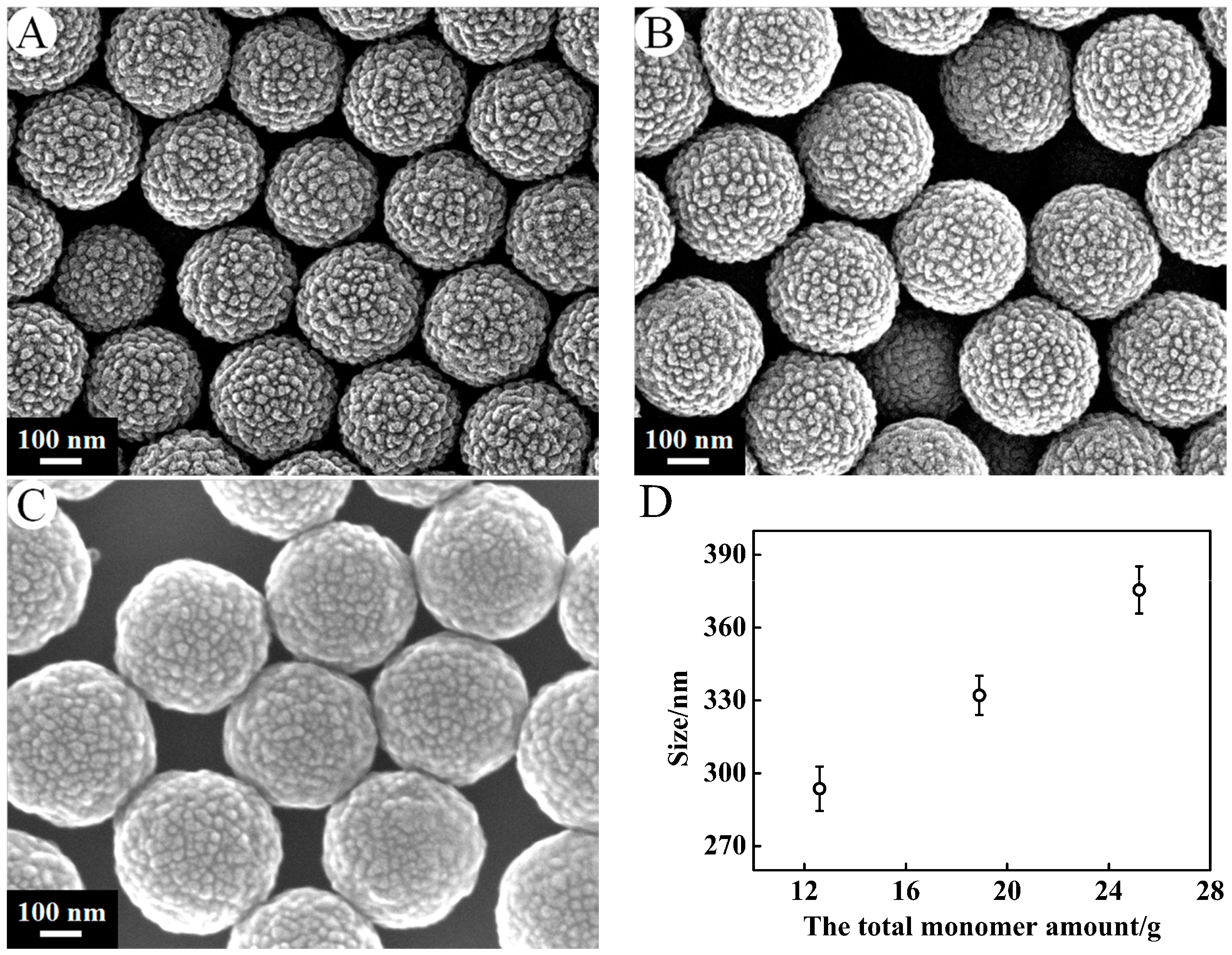
3.1.3. Monomer Concentration
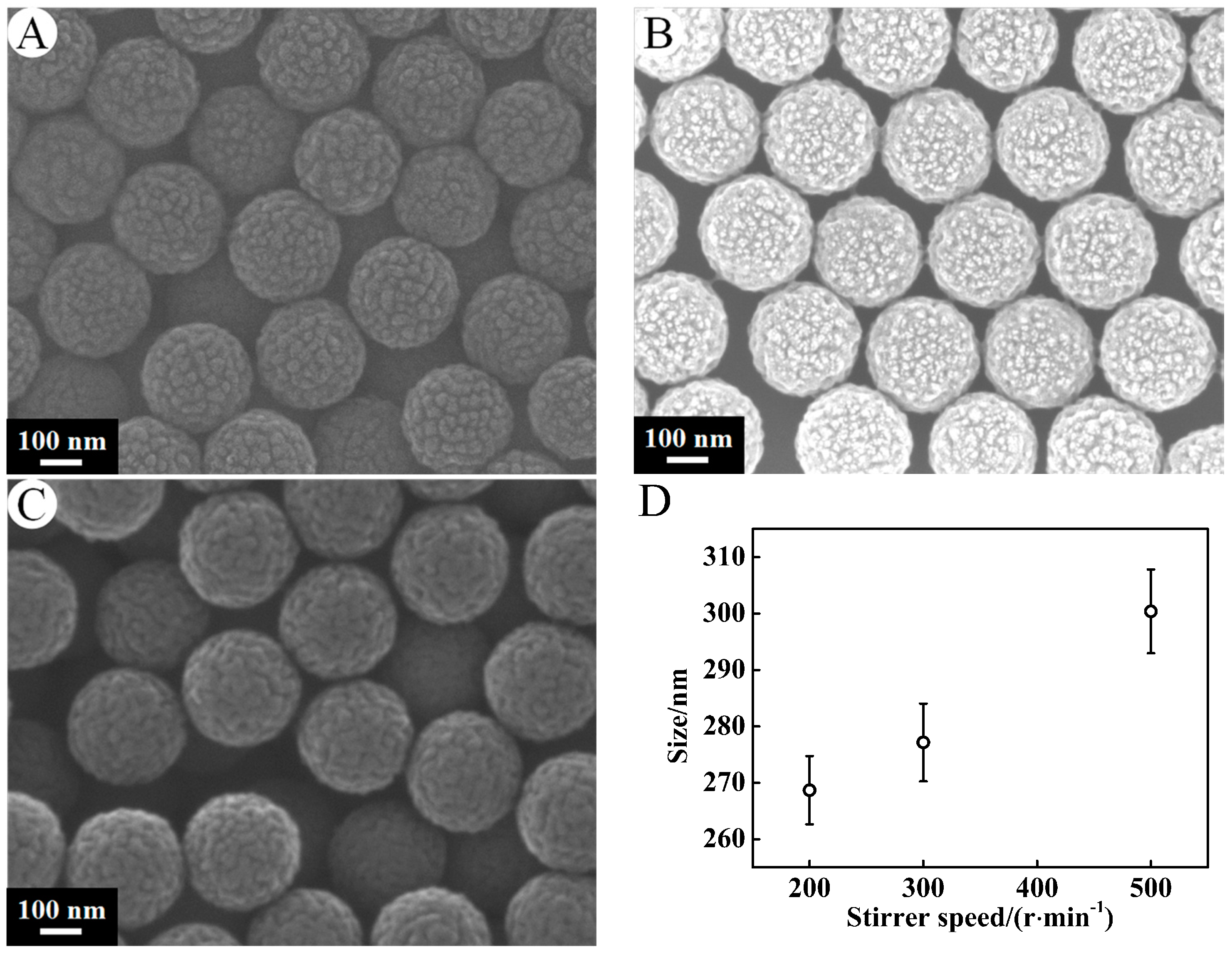
3.1.4. Stirrer Speed
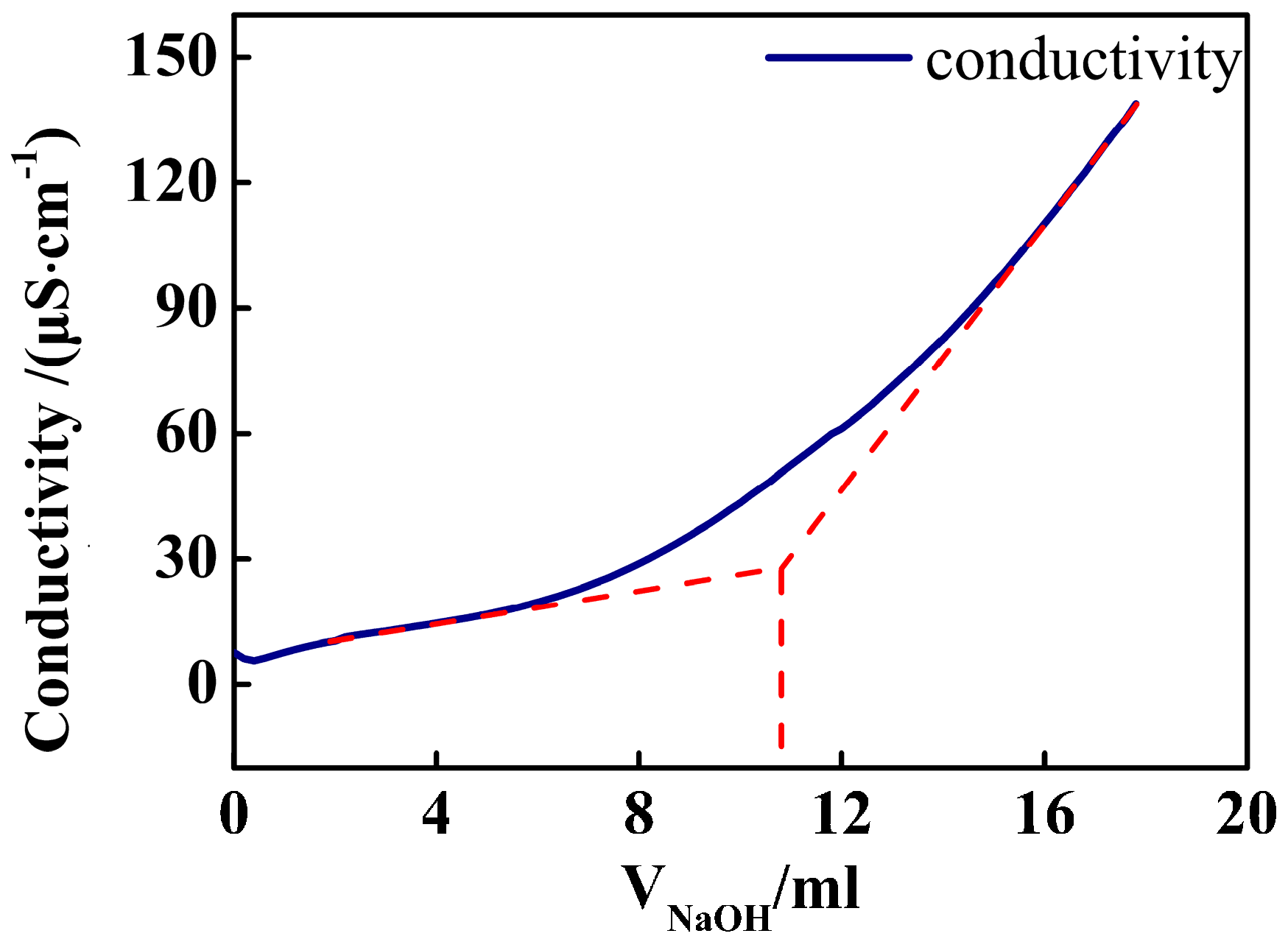
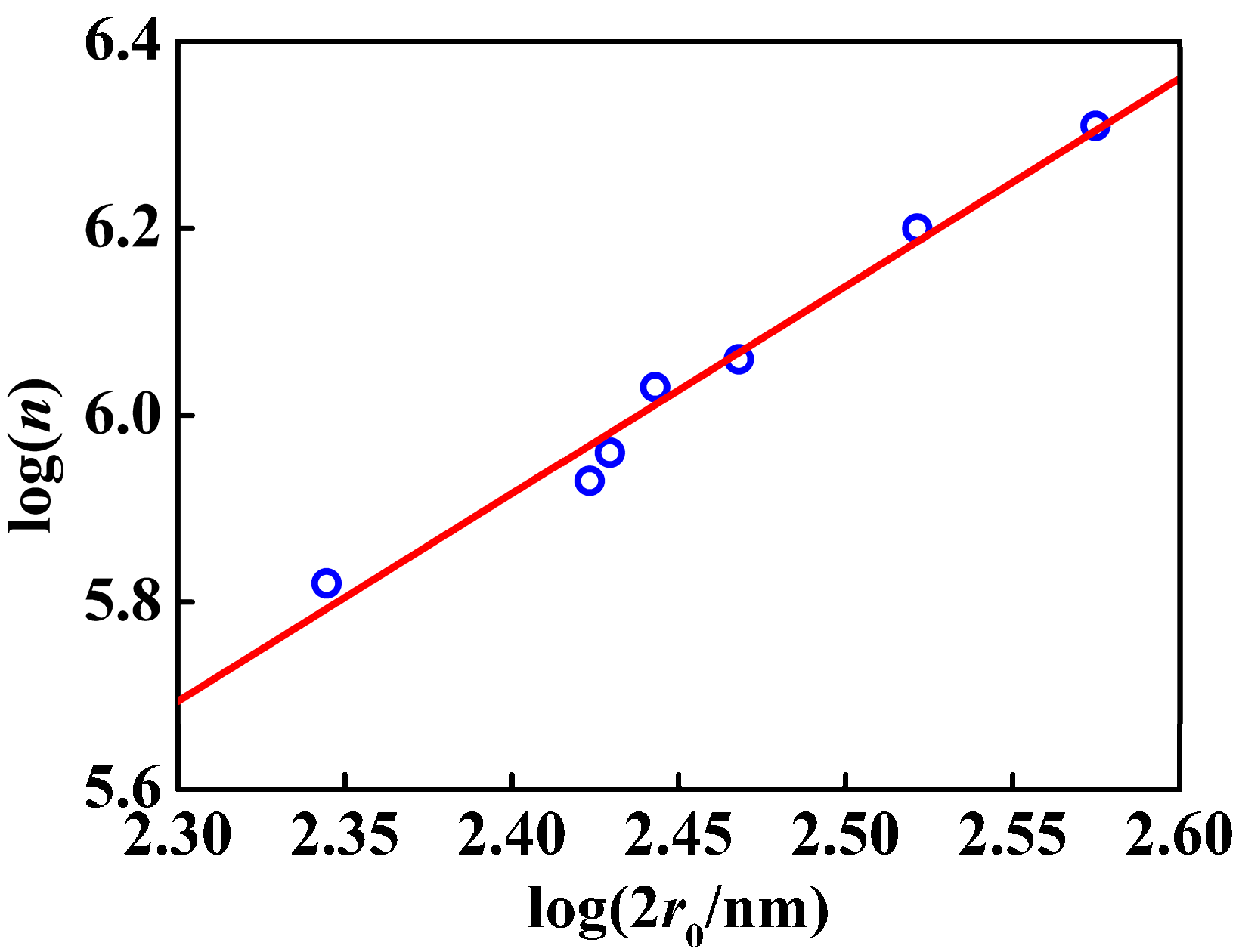
3.2. Amount of Carboxyl in PSA Nanospheres
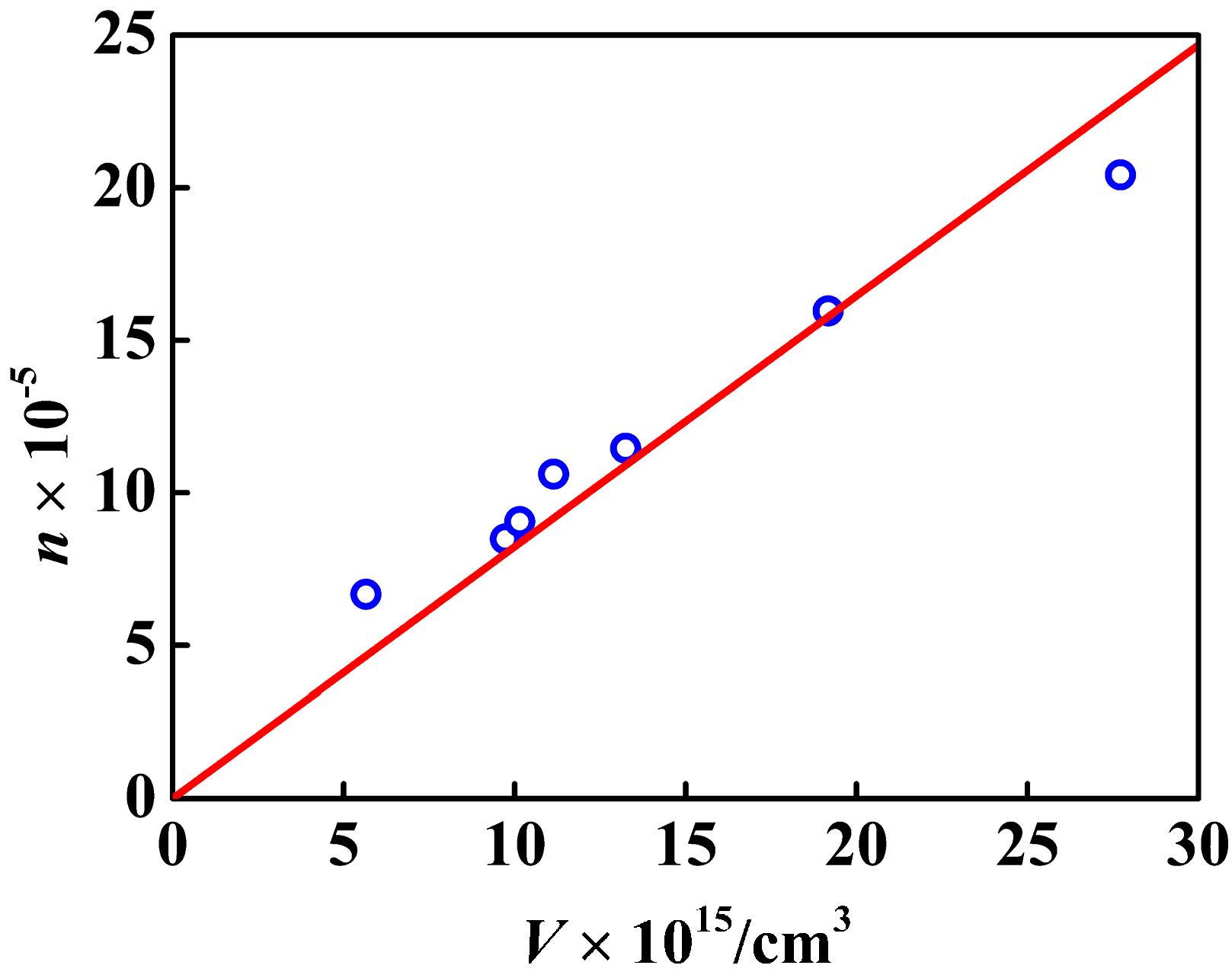
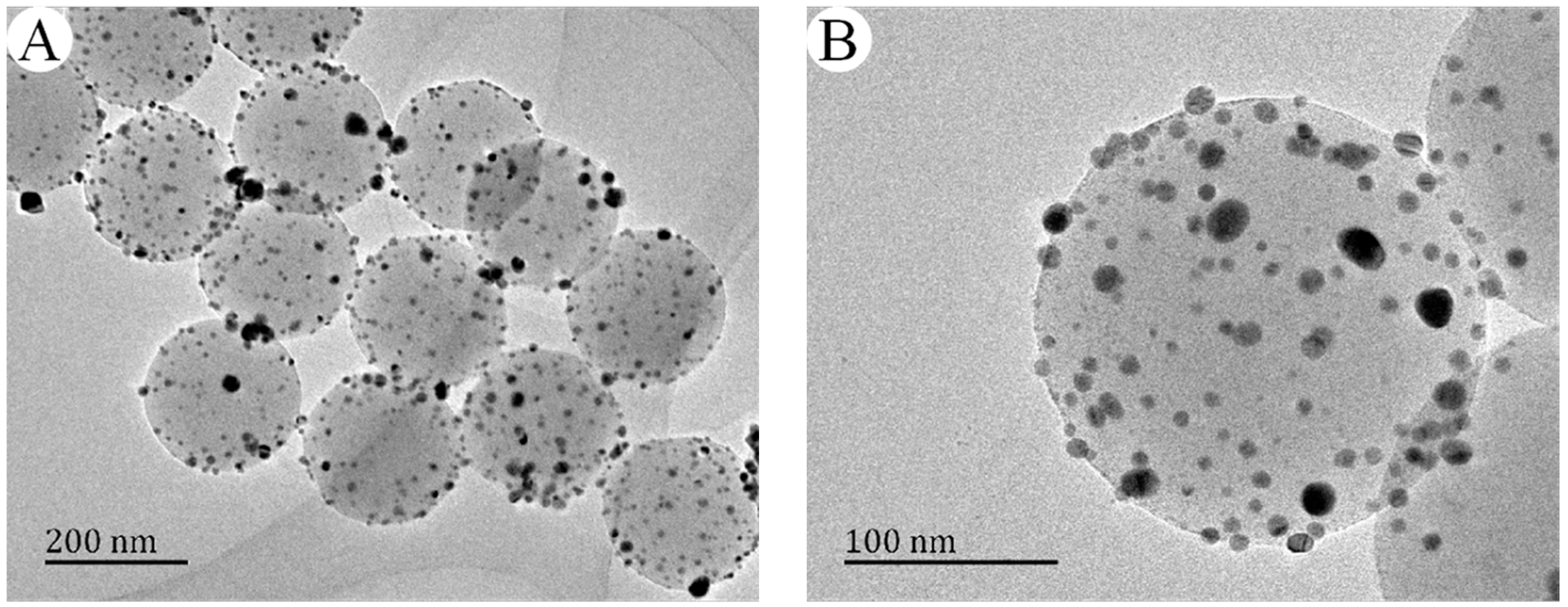
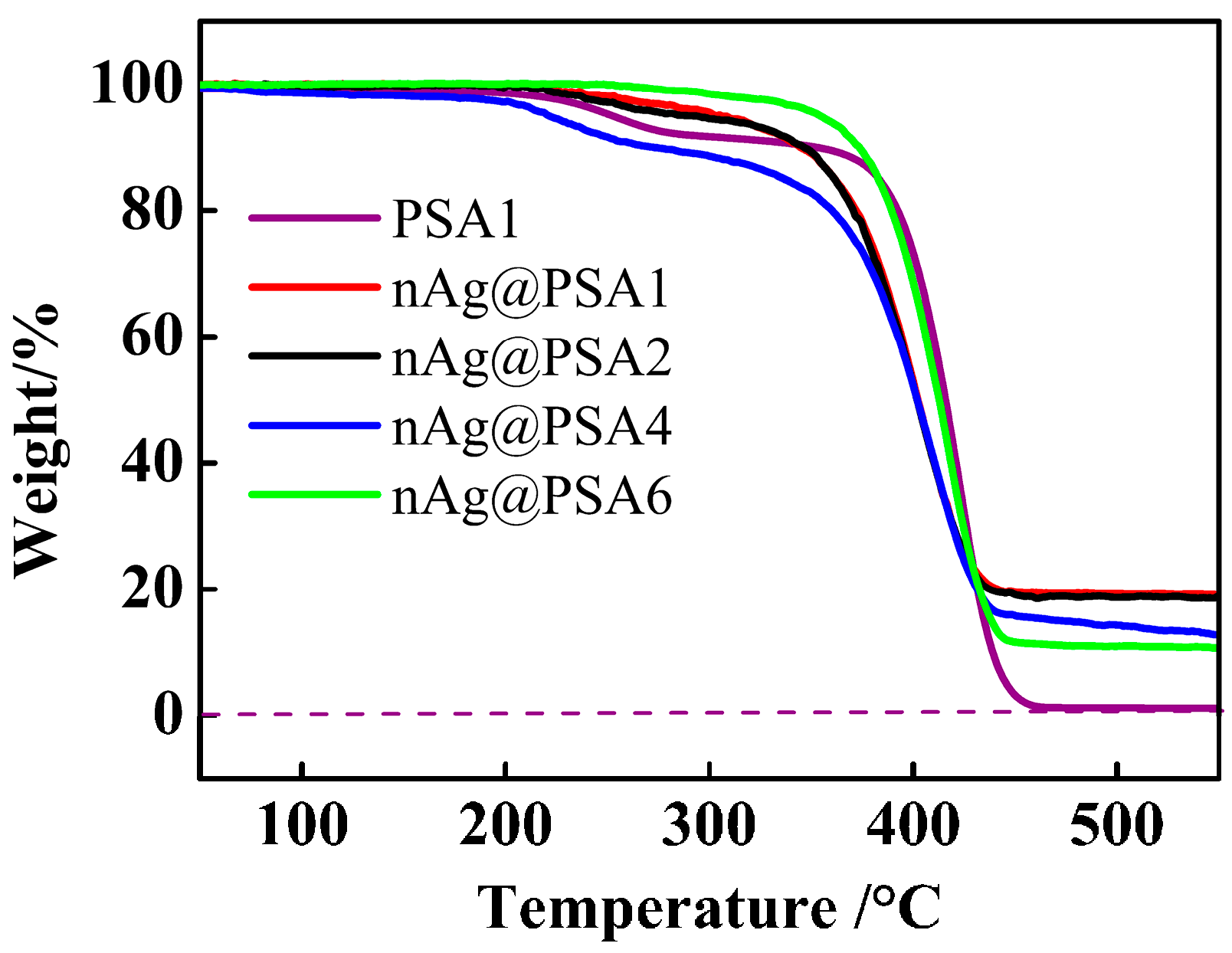
3.3. Effect of the Amount of Carboxyl on the Deposition of Ag Nanoparticles
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wen, F.; Zhang, W.; Wei, G.; Wang, Y.; Zhang, J.; Zhang, M.; Shi, L. Synthesis of noble metal nanoparticles embedded in the shell layer of core-shell poly (styrene-co-4-vinylpyridine) micospheres and their application in catalysis. Chem. Mater. 2008, 20, 2144–2150. [Google Scholar] [CrossRef]
- Kim, J.-H.; Bryan, W.W.; Randall Lee, T. Preparation, characterization, and optical properties of gold, silver, and gold-silver alloy nanoshells having silica cores. Langmuir 2008, 24, 11147–11152. [Google Scholar] [CrossRef] [PubMed]
- Baer, R.; Neuhauser, D.; Weiss, S. Enhanced absorption induced by a metallic nanoshell. Nano Lett. 2004, 4, 85–88. [Google Scholar] [CrossRef]
- Zeng, J.; Yang, J.; Lee, J.Y.; Zhou, W. Preparation of carbon-supported core-shell au-pt nanoparticles for methanol oxidation reaction: The promotional effect of the au core. J. Phys. Chem. B 2006, 110, 24606–24611. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.; van Blaaderen, A. Metallodielectric colloidal core-shell particles for photonic applications. Langmuir 2002, 18, 524–534. [Google Scholar] [CrossRef]
- Hu, J.-W.; Li, J.-F.; Ren, B.; Wu, D.-Y.; Sun, S.-G.; Tian, Z.-Q. Palladium-coated gold nanoparticles with a controlled shell thickness used as surface-enhanced raman scattering substrate. J. Phys. Chem. C 2007, 111, 1105–1112. [Google Scholar] [CrossRef]
- Jiang, Z.-J.; Liu, C.-Y.; Sun, L.-W. Catalytic properties of silver nanoparticles supported on silica spheres. J. Phys. Chem. B 2005, 109, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, S.; Averitt, R.; Westcott, S.; Halas, N. Nanoengineering of optical resonances. Chem. Phys. Lett. 1998, 288, 243–247. [Google Scholar] [CrossRef]
- Jackson, J.; Halas, N. Silver nanoshells: Variations in morphologies and optical properties. J. Phys. Chem. B 2001, 105, 2743–2746. [Google Scholar] [CrossRef]
- Oldenburg, S.J.; Westcott, S.L.; Averitt, R.D.; Halas, N.J. Surface enhanced raman scattering in the near infrared using metal nanoshell substrates. J. Phys. Chem. 1999, 111, 4729–4735. [Google Scholar] [CrossRef]
- Zhang, W.; Lei, X.; Wang, Z.; Zheng, D.; Tam, W.Y.; Chan, C.T.; Sheng, P. Robust photonic band gap from tunable scatterers. Phys. Rev. Lett. 2000, 84, 2853. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chan, C.; Zhang, W.; Ming, N.; Sheng, P. Three-dimensional self-assembly of metal nanoparticles: Possible photonic crystal with a complete gap below the plasma frequency. Phys. Rev. B 2001, 64, 113108. [Google Scholar] [CrossRef]
- Liang, Z.; Susha, A.S.; Caruso, F. Metallodielectric opals of layer-by-layer processed coated colloids. Adv. Mater. 2002, 14, 1160–1164. [Google Scholar] [CrossRef]
- Chen, C.-W.; Serizawa, T.; Akashi, M. Preparation of platinum colloids on polystyrene nanospheres and their catalytic properties in hydrogenation. Chem. Mater. 1999, 11, 1381–1389. [Google Scholar] [CrossRef]
- Siiman, O.; Burshteyn, A. Preparation, microscopy, and flow cytometry with excitation into surface plasmon resonance bands of gold or silver nanoparticles on aminodextran-coated polystyrene beads. J. Phys. Chem. B 2000, 104, 9795–9810. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Wang, S.; Zhan, P.; Wang, Z.; Ming, N. Facile methods to coat polystyrene and silica colloids with metal. Adv. Funct. Mater. 2004, 14, 1089–1096. [Google Scholar] [CrossRef]
- Wang, W.; Asher, S.A. Photochemical incorporation of silver quantum dots in monodisperse silica colloids for photonic crystal applications. J. Am. Chem. Soc. 2001, 123, 12528–12535. [Google Scholar] [CrossRef] [PubMed]
- Piao, L.; Park, S.; Lee, H.B.; Kim, K.; Kim, J.; Chung, T.D. Single gold microshell tailored to sensitive surface enhanced raman scattering probe. Anal. Chem. 2009, 82, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wang, Z.; Pan, L.; Zhao, S.; Zhu, S. Ag-coated Fe3O4@SiO2 three-ply composite microspheres: Synthesis, characterization, and application in detecting melamine with their surface-enhanced raman scattering. J. Phys. Chem. C 2010, 114, 7738–7742. [Google Scholar] [CrossRef]
- Cassagneau, T.; Caruso, F. Contiguous silver nanoparticle coatings on dielectric spheres. Adv. Mater. 2002, 14, 732–736. [Google Scholar] [CrossRef]
- Mayer, A.; Grebner, W.; Wannemacher, R. Preparation of silver-latex composites. J. Phys. Chem. B 2000, 104, 7278–7285. [Google Scholar] [CrossRef]
- Fang, K.; Ren, B. A facile method for preparing colored nanospheres of poly(styrene-co-acrylic acid). Dyes Pigm. 2014, 100, 50–56. [Google Scholar] [CrossRef]
- Song, C.; Chang, Y.; Cheng, L.; Xu, Y.; Chen, X.; Zhang, L.; Zhong, L.; Dai, L. Preparation, characterization, and antibacterial activity studies of silver-loaded poly(styrene-co-acrylic acid) nanocomposites. Mater. Sci. Eng. C 2014, 36, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Zhang, Y.; Wang, X.; Xu, J.; Wang, D.; Zhang, W. Synthesis of porous poly(styrene-co-acrylic acid) microspheres through one-step soap-free emulsion polymerization: Whys and wherefores. J. Colloid Interface Sci. 2012, 368, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Aoki, K.J.; Chen, J.; Nishiumi, T. Which controls conductivity of sulfonic latex suspension, hydrogen ion or latex core? Int. J. Electrochem. Sci. 2014, 9, 2649–2661. [Google Scholar]





| Sample Code | AA/g | St/g | KPS/g | Stirrer Speed/r·min−1 |
|---|---|---|---|---|
| PSA0 | 0.1 | 2 | 0.04 | 400 |
| PSA1 | 0.6 | 6 | 0.12 | 300 |
| PSA2 | 1.2 | 6 | 0.12 | 300 |
| PSA3 | 1.5 | 6 | 0.12 | 300 |
| PSA4 | 1.8 | 6 | 0.12 | 300 |
| PSA5 | 2.1 | 6 | 0.12 | 300 |
| PSA6 | 0.3 | 6 | 0.24 | 300 |
| PSA7 | 0.3 | 6 | 0.36 | 300 |
| PSA8 | 0.3 | 6 | 0.48 | 300 |
| PSA9 | 0.6 | 12 | 0.24 | 300 |
| PSA10 | 0.9 | 18 | 0.24 | 300 |
| PSA11 | 1.2 | 24 | 0.24 | 300 |
| PSA12 | 0.3 | 6 | 0.24 | 200 |
| PSA13 | 0.3 | 6 | 0.24 | 500 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhao, X.; Wang, Y.; Zhu, L.; Yang, L.; Li, G.; Sha, Z. Preparation and Structural Analysis of Nano-Silver Loaded Poly(styrene-co-acrylic acid) Core-Shell Nanospheres with Defined Shape and Composition. Nanomaterials 2017, 7, 234. https://doi.org/10.3390/nano7090234
Zhang J, Zhao X, Wang Y, Zhu L, Yang L, Li G, Sha Z. Preparation and Structural Analysis of Nano-Silver Loaded Poly(styrene-co-acrylic acid) Core-Shell Nanospheres with Defined Shape and Composition. Nanomaterials. 2017; 7(9):234. https://doi.org/10.3390/nano7090234
Chicago/Turabian StyleZhang, Jin, Xiaoyu Zhao, Yanfei Wang, Liang Zhu, Libin Yang, Gang Li, and Zuoliang Sha. 2017. "Preparation and Structural Analysis of Nano-Silver Loaded Poly(styrene-co-acrylic acid) Core-Shell Nanospheres with Defined Shape and Composition" Nanomaterials 7, no. 9: 234. https://doi.org/10.3390/nano7090234