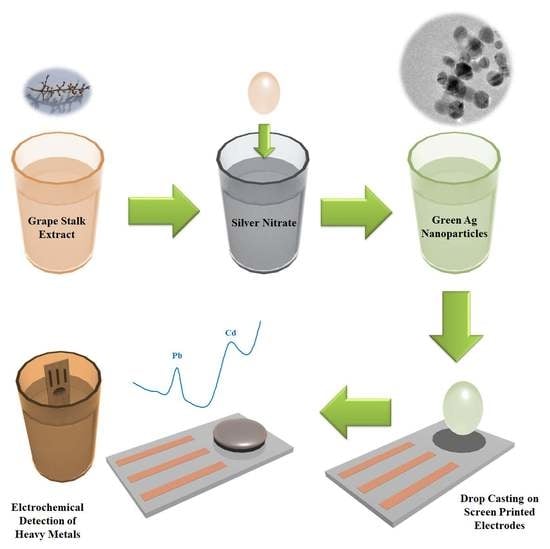
Green Synthesis of Ag Nanoparticles Using Grape Stalk Waste Extract for the Modification of Screen-Printed Electrodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of Grape Stalk Extract
Determination of Total Phenolic and Reducing Sugars
2.3. Synthesis of Silver Nanoparticles Using Grape Stalk as Reducing Agent
2.4. Characterization of Ag Nanoparticles
2.4.1. UV/Vis Spectroscopy
2.4.2. Electron Microscopy Characterization
2.4.3. Fourier Transformed Infrared (FTIR) Spectroscopy
2.4.4. Electrochemical Characterization of Modified SPCNFE
3. Results and Discussion
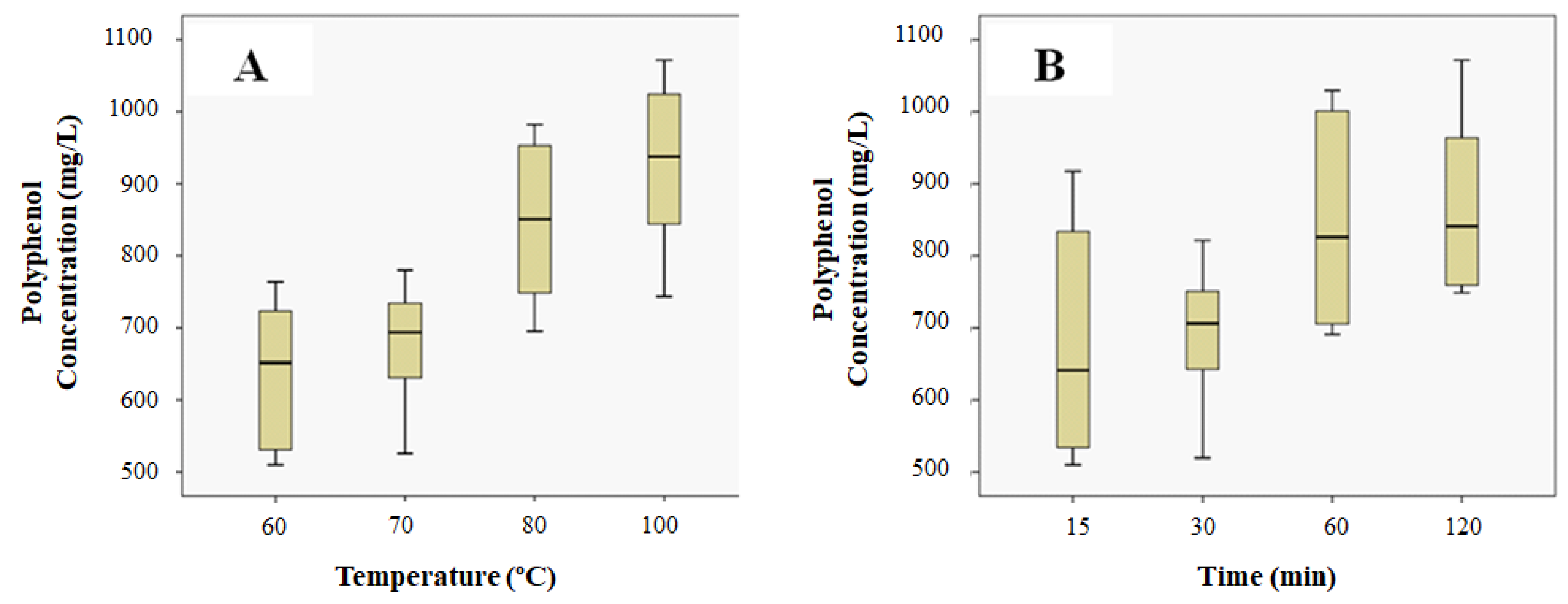
3.1. Grape Stalk Extracts
3.2. Silver Nanoparticles Synthesis
3.2.1. FTIR Characterization of Grape Stalk
3.2.2. Study of Factors Affecting Ag-NPs Synthesis
Effect of pH
Effect of Contact Time
Effect of Extract/Silver Solution (v/v) Ratio
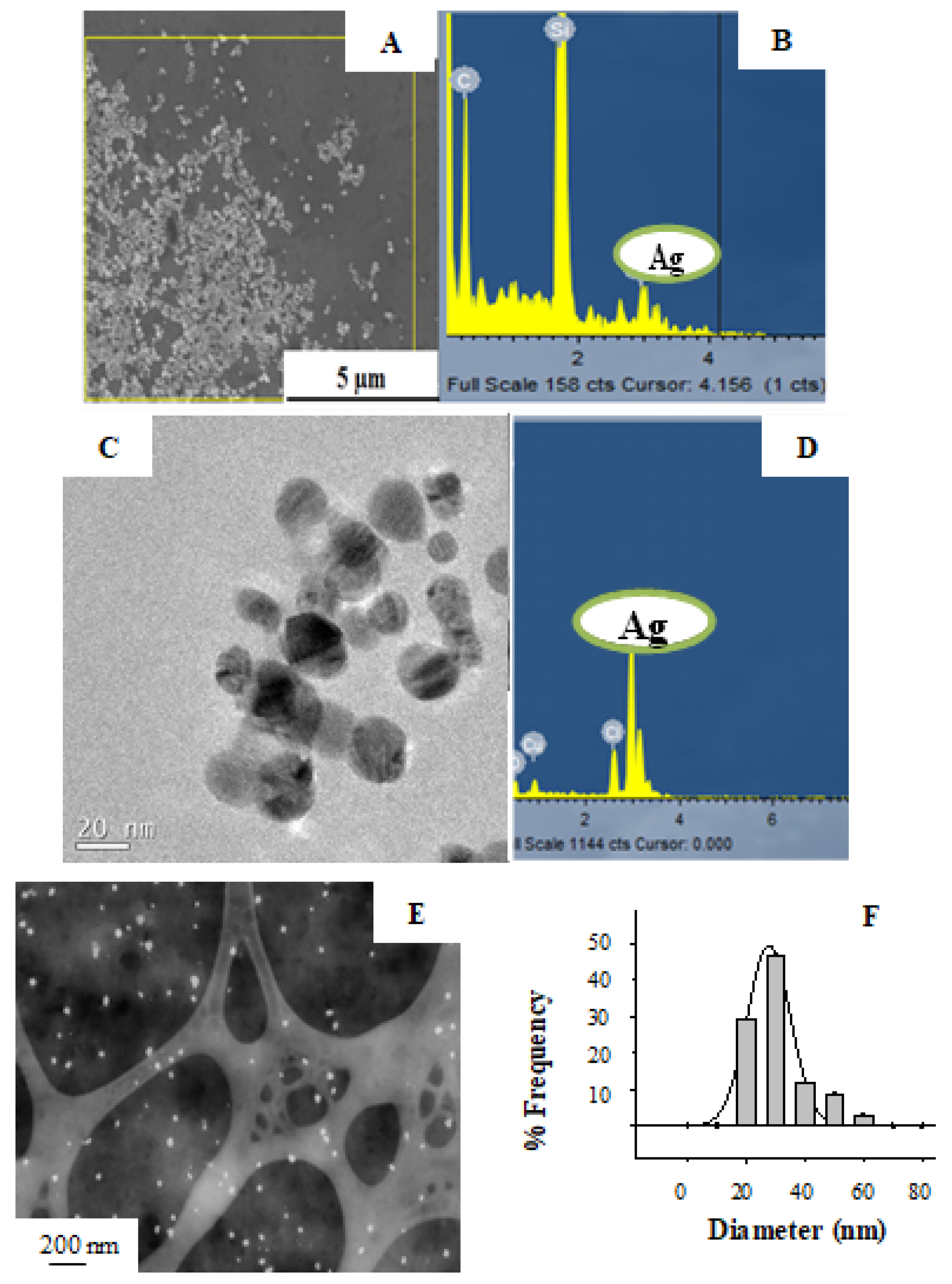
3.2.3. Electron Microscopy Characterization
3.2.4. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Synthesis of nanoparticles by microorganisms and their application in enhancing microbiological reaction rates. Chemosphere 2011, 82, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 2015. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—An updated report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Srikar, S.K.; Giri, D.D.; Pal, D.B.; Mishra, P.K.; Upadhyay, S.N. Green synthesis of silver nanoparticles: A review. Green Sustain. Chem. 2016, 6, 34–56. [Google Scholar] [CrossRef]
- He, Y.; Du, Z.; Lv, H.; Jia, Q.; Tang, Z.; Zheng, X.; Zhang, K.; Zhao, F. Green synthesis of silver nanoparticles by Chrysanthemum morifolium Ramat. extract and their application in clinical ultrasound gel. Int. J. Nanomed. 2013, 8, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, V.; Vasanthi, S.; Shalini, S.; Ali Shah, S.A.; Harish, R. Green synthesis of silver nanoparticles using Atrocarpus altilis leaf extract and the study of their antimicrobial and antioxidant activity. Mater. Lett. 2016, 180, 264–267. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, P.; Ichwan, S.J.A.; Parine, N.R.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Intracellular biosynthesis of Au and Ag nanoparticles using ethanolic extract of Brassica oleracea L. and studies on their physicochemical and biological properties. J. Environ. Sci. (China) 2015, 29, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Yang, D.; Chen, Z.; Megharaj, M.; Naidu, R. Characterization of bimetallic Fe/Pd nanoparticles by grape leaf aqueous extract and identification of active biomolecules involved in the synthesis. Sci. Total Environ. 2016, 562, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Venkateswarlu, S.; Natesh Kumar, B.; Prathima, B.; Anitha, K.; Jyothi, N.V. V A novel green synthesis of Fe3O4-Ag core shell recyclable nanoparticles using Vitis vinifera stem extract and its enhanced antibacterial performance. Phys. B Condens. Matter 2015, 457, 30–35. [Google Scholar] [CrossRef]
- Dziwoń, K.; Pulit-Prociak, J.; Banach, M. Green technologies in obtaining nanomaterials–using white grapes (Vitis vinifera) in the processes for the preparation of silver nanoparticles. Chemik 2015, 69, 33–38. [Google Scholar]
- Krishnaswamy, K.; Vali, H.; Orsat, V. Value-adding to grape waste: Green synthesis of gold nanoparticles. J. Food Eng. 2014, 142, 210–220. [Google Scholar] [CrossRef]
- Pujol, D.; Liu, C.; Fiol, N.; Olivella, M.À.; Gominho, J.; Villaescusa, I.; Pereira, H. Chemical characterization of different granulometric fractions of grape stalks waste. Ind. Crops Prod. 2013, 50, 494–500. [Google Scholar] [CrossRef]
- Muñoz, J.; Bastos-Arrieta, J.; Muñoz, M.; Muraviev, D.N.; Céspedes, F.; Baeza, M. Simple green routes for the customized preparation of sensitive carbon nanotubes/epoxy nanocomposite electrodes with functional metal nanoparticles. RSC Adv. 2014, 4, 44517–44524. [Google Scholar] [CrossRef]
- Pérez-Ràfols, C.; Bastos-Arrieta, J.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; de Pablo, J.; Esteban, M. Ag nanoparticles drop-casting modification of screen-printed electrodes for the simultaneous voltammetric determination of Cu(II) and Pb(II). Sensors 2017, 17, 1458. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liang, X.; Niyungeko, C.; Zhou, J.; Xu, J.; Tian, G. A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 2018, 178, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Economou, A. Screen-printed electrodes modified with “green” metals for electrochemical stripping analysis of toxic elements. Sensors 2018, 18, 1032. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ràfols, C.; Serrano, N.; Díaz-cruz, J.M.; Ariño, C.; Esteban, M. New approaches to antimony film screen-printed electrodes using carbon-based nanomaterials substrates. Anal. Chim. Acta 2016, 916, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.I. Textbook of Quantitative Chemical Analysis, 5th ed.; Pearson Education Limited: Harlow, UK, 1989. [Google Scholar]
- Ramteke, C.; Chakrabarti, T.; Sarangi, B.K.; Pandey, R. Synthesis of silver nanoparticles from the aqueous extract of leaves of ocimum sanctum for enhanced antibacterial activity. Hindawi Publ. Corp. J. Chem. 2013, 2013, 278925. [Google Scholar] [CrossRef]
- Xu, H.; Wang, L.; Su, H.; Gu, L.; Han, T.; Meng, F.; Liu, C. Making good use of food wastes: green synthesis of highly stabilized silver nanoparticles from grape seed extract and their antimicrobial activity. Food Biophys. 2015, 10, 12–18. [Google Scholar] [CrossRef]
- Tchaikovskaya, O.N.; Basyl’, O.K.; Sultimova, N.B. Proton-acceptor and proton-donor properties of phenol and its substitutes. Russ. Phys. J. 2005, 48, 1245–1250. [Google Scholar] [CrossRef]
- Lerma-garcía, M.; Ávila, M.; Simó-alfonso, E.F.; Ríos, Á.; Zougagh, M. Synthesis of gold nanoparticles using phenolic acids and its application in catalysis. J. Mater. Environ. Sci. 2014, 5, 1919–1926. [Google Scholar]
- Mehanna, N.S.; Hassan, Z.M.R.; El-Din, H.M.F.; Ali, A.A.-E.; Amarowicz, R.; El-Messery, T.M. Effect of interaction phenolic compounds with milk proteins on cell line. Food Nutr. Sci. 2014, 5, 2130–2146. [Google Scholar] [CrossRef]
- François, E.M.; Marcelle, L.S.; Cecile, O.E.; Agnes, A.N.; Djiopang, Y.S.; Fanny, A.E.M.; Lidwine, N.; Harouna, M.; Emmanuel, M.M. Unexplored vegetal green synthesis of silver nanoparticles: A preliminary study with Corchorus olitorus Linn and Ipomea batatas (L.) Lam. African J. Biotechnol. 2016, 15, 341–349. [Google Scholar] [CrossRef]
- Demirbas, A.; Welt, B.A.; Ocsoy, I. Biosynthesis of red cabbage extract directed Ag NPs and their effect on the loss of antioxidant activity. Mater. Lett. 2016, 179, 20–23. [Google Scholar] [CrossRef]
- Velgosová, O.; Mražíková, A.; Marcinčáková, R. Influence of pH on green synthesis of Ag nanoparticles. Mater. Lett. 2016, 180, 336–339. [Google Scholar] [CrossRef]
- Ajitha, B.; Kumar Reddy, Y.A.; Reddy, P.S.; Jeon, H.-J.; Ahn, C.W. Role of capping agents in controlling silver nanoparticles size, antibacterial activity and potential application as optical hydrogen peroxide sensor. RSC Adv. 2016, 6, 36171–36179. [Google Scholar] [CrossRef]
- Khalil, M.M.H.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab. J. Chem. 2014, 7, 1131–1139. [Google Scholar] [CrossRef]
- Carrillo-López, L.M.; Morgado-González, A.; Morgado-González, A. Biosynthesized silver nanoparticles used in preservative solutions for Chrysanthemum cv. Puma. J. Nanomater. 2016, 2016. [Google Scholar] [CrossRef]
- Prakash, S.; Shahi, V.K. Inproved sensitive detection of Pb2+ and Cd2+ in water samples at electrodeposited silver nanonuts on a glassy carbon electrode. Anal. Methods 2011, 3, 2134–2139. [Google Scholar] [CrossRef]
- Serrano, N.; Alberich, A.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. Coating methods, modifiers and applications of bismuth screen-printed electrodes. Trends Anal. Chem. 2013, 46, 15–29. [Google Scholar] [CrossRef]
- Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. Antimony- based electrodes for analytical determinations. Trends Anal. Chem. 2016, 77, 203–213. [Google Scholar] [CrossRef]
- Pérez-Ràfols, C.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. Glutathione modified screen-printed carbon nanofiber electrode for the voltammetric determination of metal ions in natural samples. Talanta 2016, 155, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Serrano, N.; González-Calabuig, A.; del Valle, M. Crown ether-modified electrodes for the simultaneous stripping voltammetric determination of Cd(II), Pb(II) and Cu(II). Talanta 2015, 138, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Serrano, N.; Prieto-Simón, B.; Cetó, X.; del Valle, M. Array of peptide-modified electrodes for the simultaneous determination of Pb(II), Cd(II) and Zn(II). Talanta 2014, 125, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ràfols, C.; Serrano, N.; Manuel Díaz-Cruz, J.; Ariño, C.; Esteban, M. Penicillamine-modified sensor for the voltammetric determination of Cd(II) and Pb(II) ions in natural samples. Talanta 2015, 144, 569–573. [Google Scholar] [CrossRef] [PubMed]





| Sample Preparation pH | Diameter (nm) |
|---|---|
| 2 | 55.4 ± 0.3 |
| 4 | 27.7 ± 0.6 |
| 6 | 54.3 ± 0.1 |
| 8 | 9.0 ± 0.2 |
| Sample | Analytical Parameter | DPASV Calibration | |
|---|---|---|---|
| Pb(II) | Cd(II) | ||
| SPCNFE—pH4.W3 | Sensitivity (nA µg−1 L) | 62 (1) | 46 (2) |
| R2 | 0.998 | 0.993 | |
| Linear range a (µg L−1) | 8.9–100.4 | 9.5–39.7 | |
| LOD (µg L−1) | 2.7 | 2.8 | |
| SPCNFE—pH4.W4 | Sensitivity (nA µg−1 L) | 26.0 (0.7) | 8.5 (0.5) |
| R2 | 0.996 | 0.993 | |
| Linear range a (µg L−1) | 13.3–100.4 | 16.0–39.7 | |
| LOD (µg L−1) | 4.0 | 4.8 | |
| SPCNFE—pH6.W3 | Sensitivity (nA µg−1 L) | 21.2 (0.5) | 5.1 (0.5) |
| R2 | 0.996 | 0.980 | |
| Linear range a (µg L−1) | 12.3–100.4 | 26.9–39.7 | |
| LOD (µg L−1) | 3.7 | 8.1 | |
| SPCNFE—pH6.W4 | Sensitivity (nA µg−1 L) | 43 (1) | 13.8 (0.8) |
| R2 | 0.993 | 0.989 | |
| Linear range a (µg L−1) | 16.2–100.4 | 10.0–27.5 | |
| LOD (µg L−1) | 4.9 | 3.0 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastos-Arrieta, J.; Florido, A.; Pérez-Ràfols, C.; Serrano, N.; Fiol, N.; Poch, J.; Villaescusa, I. Green Synthesis of Ag Nanoparticles Using Grape Stalk Waste Extract for the Modification of Screen-Printed Electrodes. Nanomaterials 2018, 8, 946. https://doi.org/10.3390/nano8110946
Bastos-Arrieta J, Florido A, Pérez-Ràfols C, Serrano N, Fiol N, Poch J, Villaescusa I. Green Synthesis of Ag Nanoparticles Using Grape Stalk Waste Extract for the Modification of Screen-Printed Electrodes. Nanomaterials. 2018; 8(11):946. https://doi.org/10.3390/nano8110946
Chicago/Turabian StyleBastos-Arrieta, Julio, Antonio Florido, Clara Pérez-Ràfols, Núria Serrano, Núria Fiol, Jordi Poch, and Isabel Villaescusa. 2018. "Green Synthesis of Ag Nanoparticles Using Grape Stalk Waste Extract for the Modification of Screen-Printed Electrodes" Nanomaterials 8, no. 11: 946. https://doi.org/10.3390/nano8110946
APA StyleBastos-Arrieta, J., Florido, A., Pérez-Ràfols, C., Serrano, N., Fiol, N., Poch, J., & Villaescusa, I. (2018). Green Synthesis of Ag Nanoparticles Using Grape Stalk Waste Extract for the Modification of Screen-Printed Electrodes. Nanomaterials, 8(11), 946. https://doi.org/10.3390/nano8110946