Comparative Studies on Thermal, Mechanical, and Flame Retardant Properties of PBT Nanocomposites via Different Oxidation State Phosphorus-Containing Agents Modified Amino-CNTs
Abstract
:1. Introduction
2. Experimental Section
2.1. Raw Materials
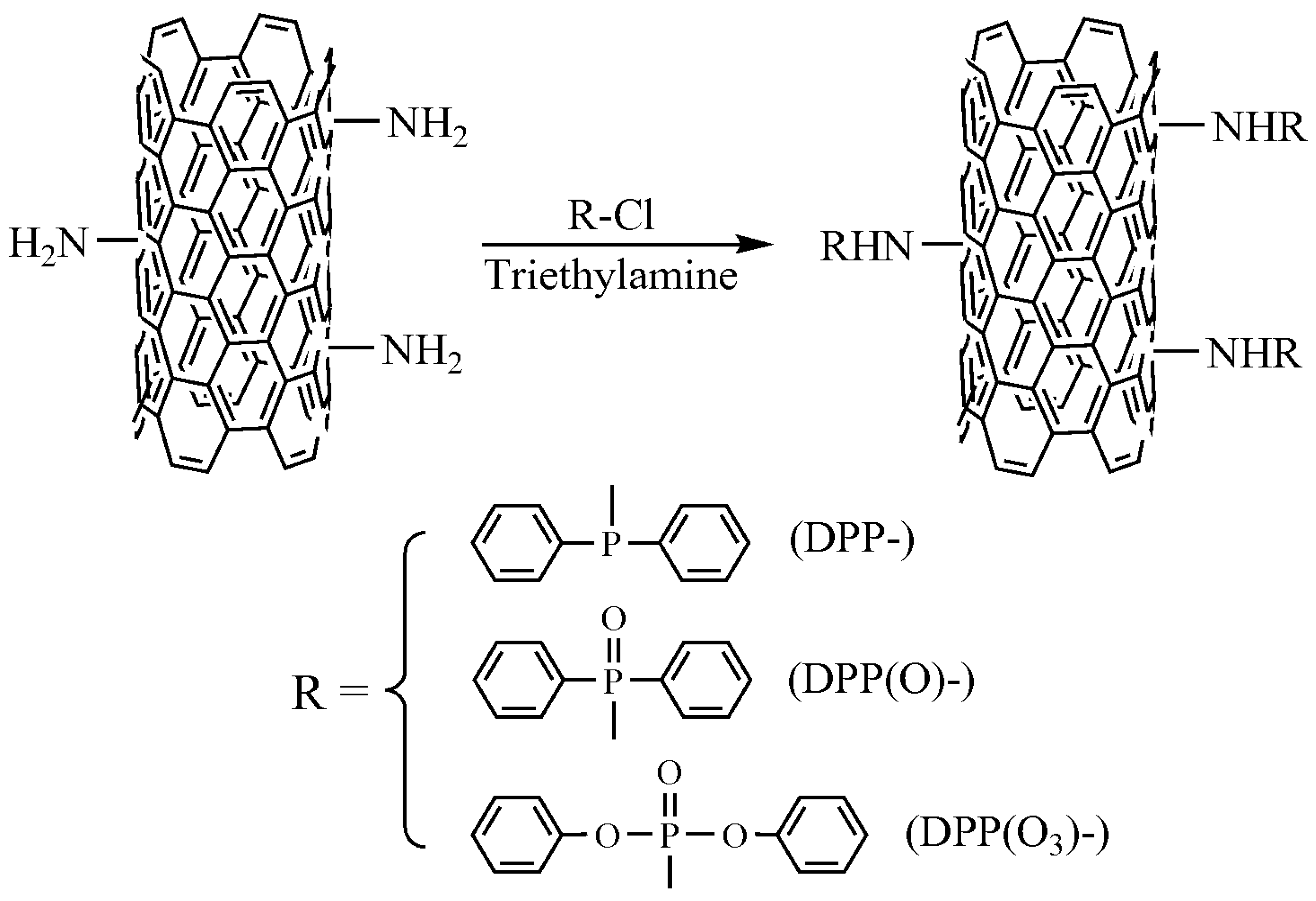
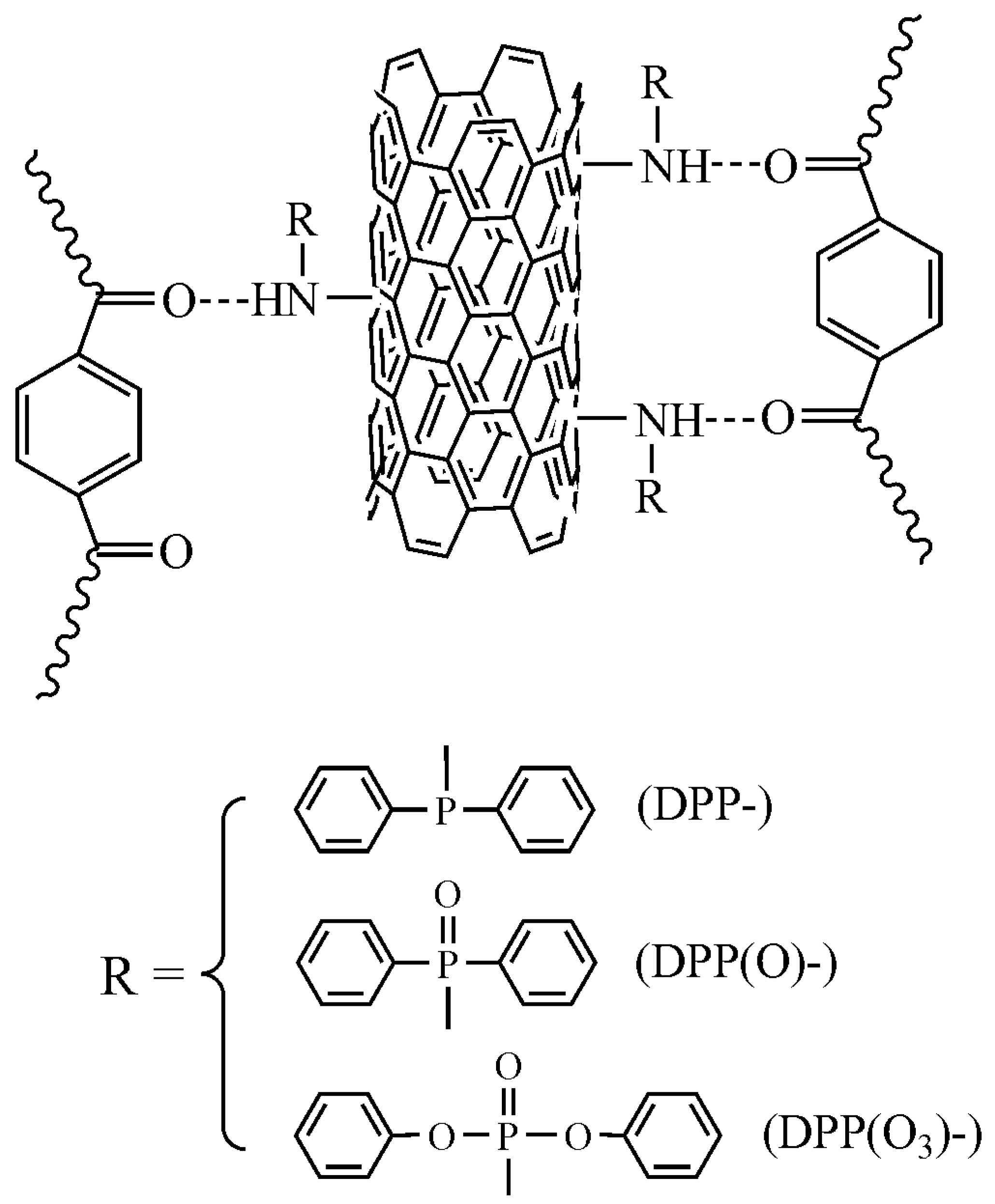
2.2. Preparation of Functionalized CNTs
2.3. Preparation of PBT/Functionalized CNTs Nanocomposites
2.4. Instruments and Measurements
3. Results and Discussion
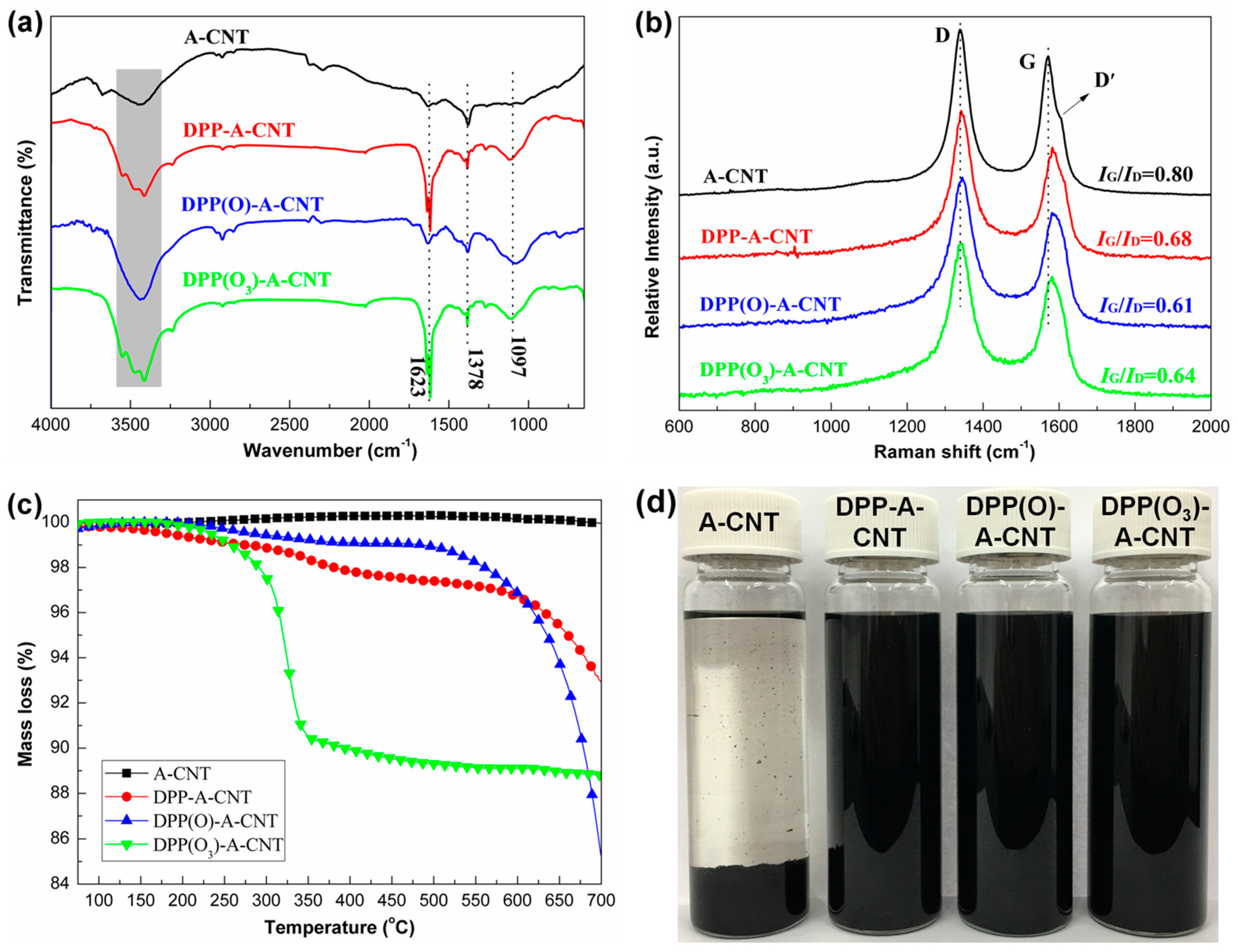
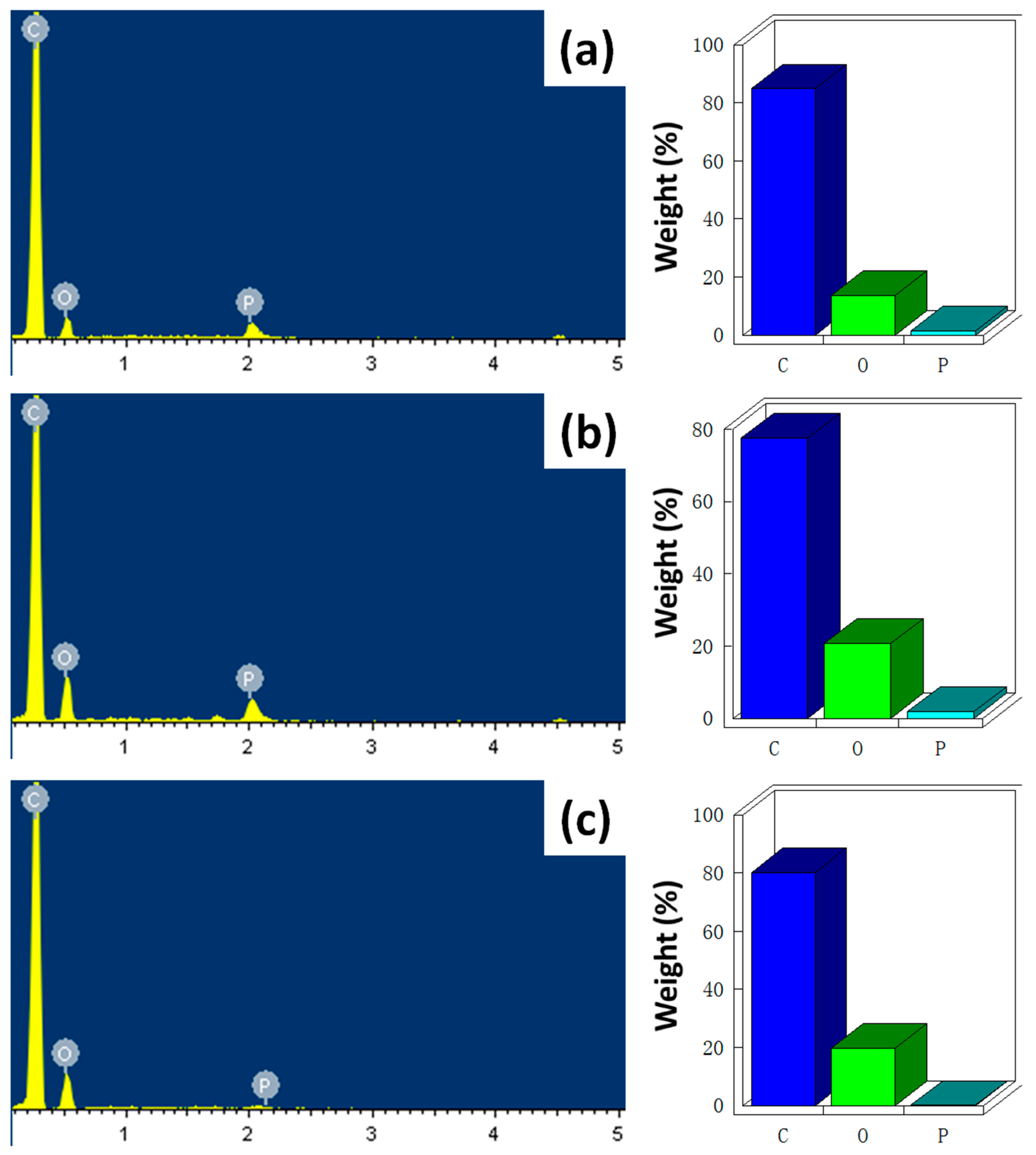
3.1. Characterization of Functionalized CNTs
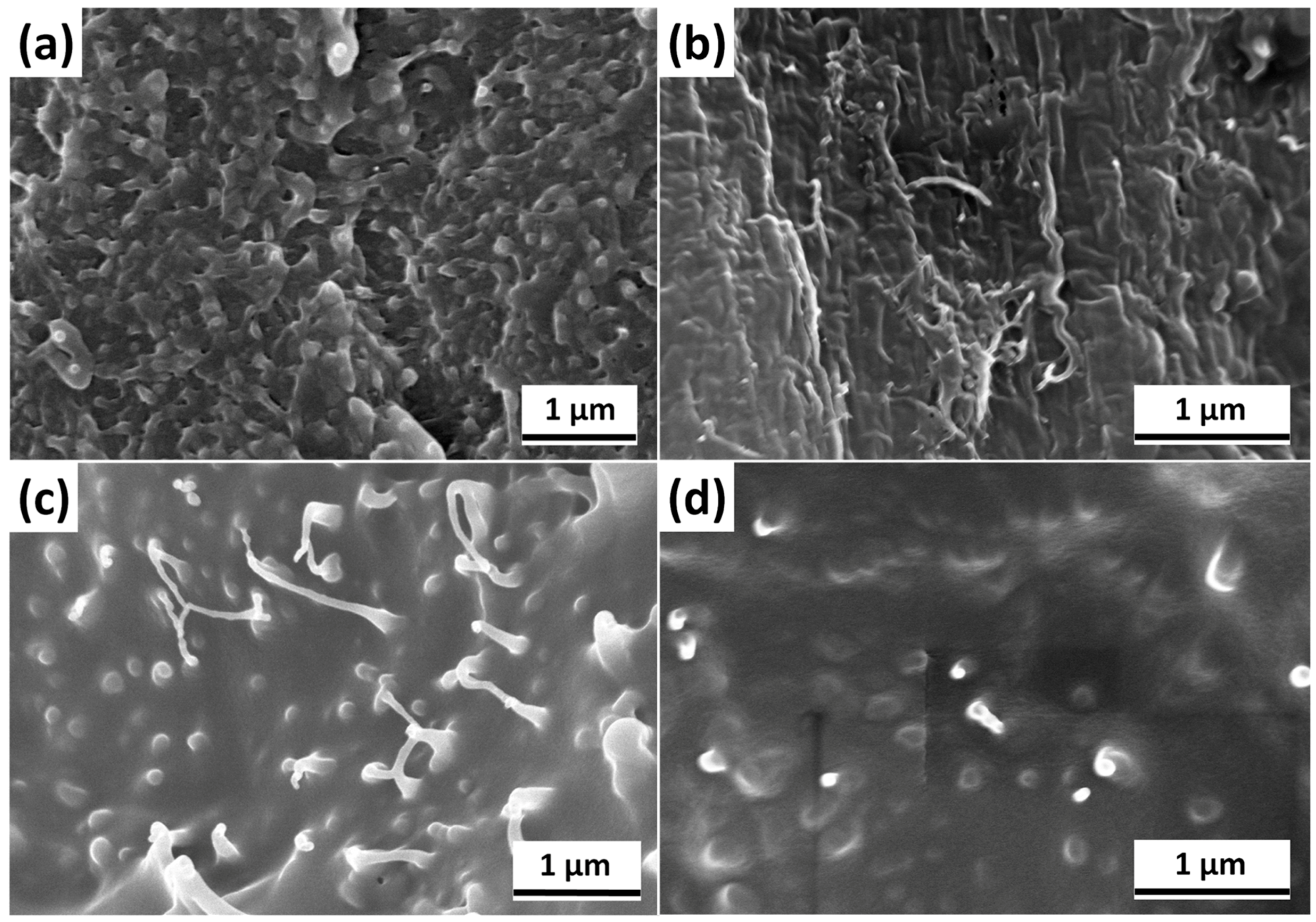
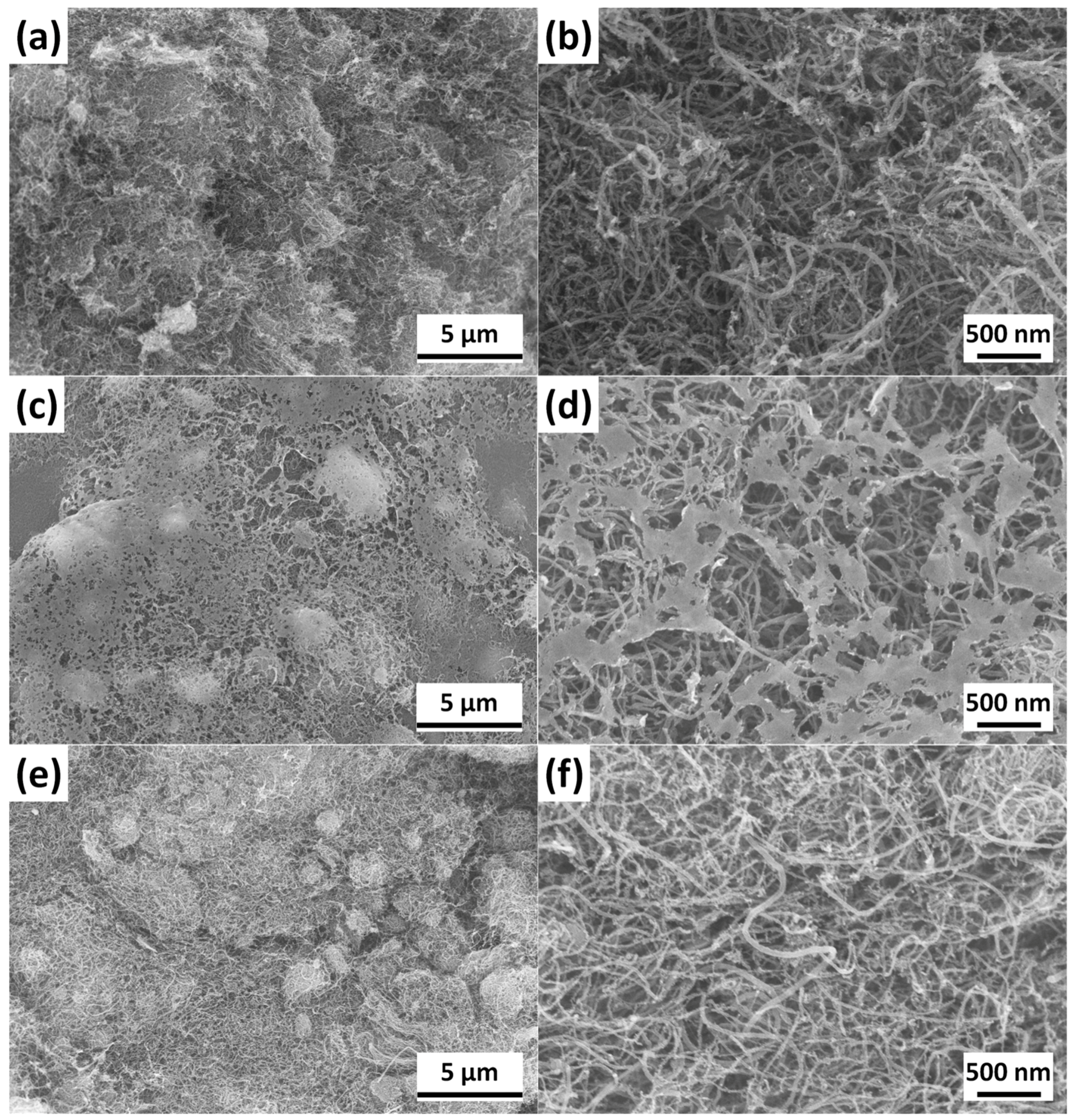
3.2. Morphological Analysis
3.3. Thermal Properties
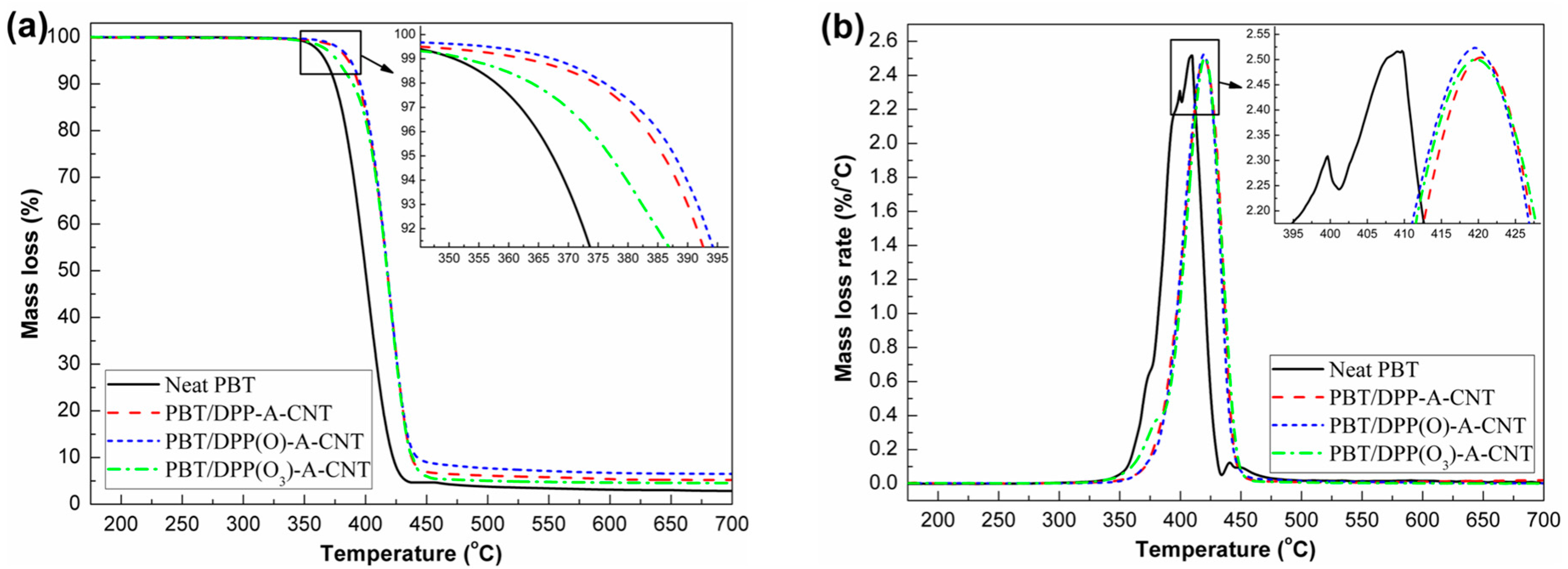
3.4. Thermal Decomposition Behaviors
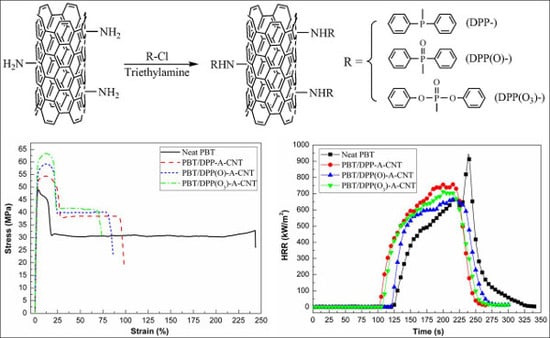
3.5. Tensile Properties
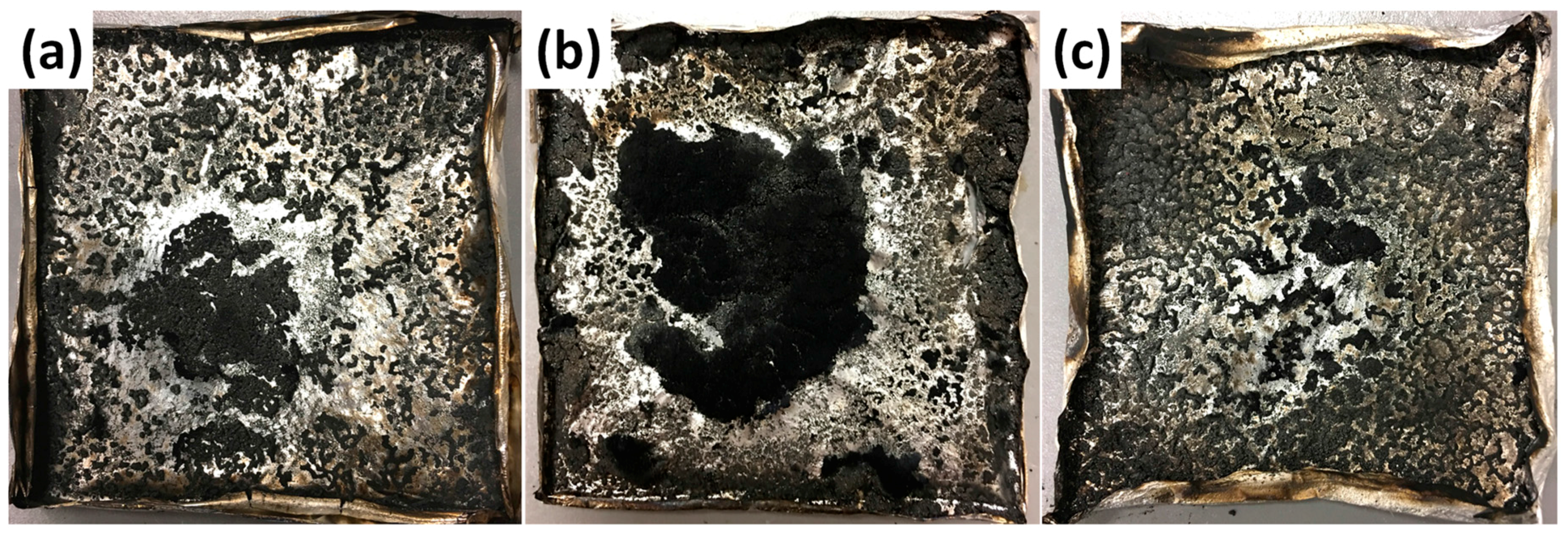
3.6. Flame Retardancy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Levchik, S.V.; Weil, E.D. Flame retardancy of thermoplastic polyesters—A review of the recent literature. Polym. Int. 2005, 54, 11–35. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. A review on thermal decomposition and combustion of thermoplastic polyesters. Polym. Adv. Technol. 2004, 15, 691–700. [Google Scholar] [CrossRef]
- Gianelli, W.; Camino, G.; Tabuani, D.; Bortolon, V.; Savadori, T.; Monticelli, O. Fire behaviour of polyester-clay nanocomposites. Fire Mater. 2006, 30, 333–341. [Google Scholar] [CrossRef]
- Yang, W.; Hu, Y.; Tai, Q.; Lu, H.; Song, L.; Yuen, R. Fire and mechanical performance of nanoclay reinforced glass-fiber/PBT composites containing aluminum hypophosphite particles. Compos. Part A Appl. Sci. Manuf. 2011, 42, 794–800. [Google Scholar] [CrossRef]
- Yang, W.; Kan, Y.; Song, L.; Hu, Y.; Lu, H.; Yuen, R. Effect of organo-modified montmorillonite on flame retardant poly(1,4-butylene terephthalate) composites. Polym. Adv. Technol. 2011, 22, 2564–2570. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Q.; Zhou, K.; Yang, W.; Hu, Y.; Gong, X. The influence of manganese-cobalt oxide/graphene on reducing fire hazards of poly(butylene terephthalate). J. Hazard. Mater. 2014, 278, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.E.; Wang, L.L.; Wang, M.Z.; Yuen, A.; Chen, T.; Yang, W.; Pan, T.Z.; Zhi, Y.R.; Lu, H.D. Simultaneous enhancements on the mechanical, thermal stability, and flame retardant properties of poly(1,4-butylene terephthalate) nanocomposites with a novel phosphorus-nitrogen-containing polyhedral oligomeric silsesquioxane. RSC Adv. 2017, 7, 54021–54030. [Google Scholar] [CrossRef]
- Braun, U.; Schartel, B. Flame retardancy mechanisms of aluminium phosphinate in combination with melamine cyanurate in glass-fibre-reinforced poly(1,4-butylene terephthalate). Macromol. Mater. Eng. 2008, 293, 206–217. [Google Scholar] [CrossRef]
- Yang, W.; Yang, B.; Lu, H.; Song, L.; Hu, Y. Effect of modified carbon nanotube on the thermal behavior, flame retardancy and mechanical properties of poly(1,4-butylene terephthalate)/aluminum phosphinate composites. Ind. Eng. Chem. Res. 2014, 53, 18489–18496. [Google Scholar] [CrossRef]
- Ma, H.Y.; Tong, L.F.; Xu, Z.B.; Fang, Z.P. Functionalizing carbon nanotubes by grafting on intumescent flame retardant: Nanocomposite synthesis, morphology, rheology, and flammability. Adv. Funct. Mater. 2008, 18, 414–421. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Winey, K.I. Polymer nanocomposites containing carbon nanotubes. Macromolecules 2006, 39, 5194–5205. [Google Scholar] [CrossRef]
- Fritzsche, J.; Lorenz, H.; Klueppel, M. CNT based elastomer-hybrid-nanocomposites with promising mechanical and electrical properties. Macromol. Mater. Eng. 2009, 294, 551–560. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Du, F.; Douglas, J.F.; Winey, K.I.; Harris, R.H.; Shields, J.R. Nanoparticle networks reduce the flammability of polymer nanocomposites. Nat. Mater. 2005, 4, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Star, A.; Stoddart, J.F.; Steuerman, D.; Diehl, M.; Boukai, A.; Wong, E.W.; Yang, X.; Chung, S.W.; Choi, H.; Heath, J.R. Preparation and properties of polymer-wrapped single-walled carbon nanotubes. Angew. Chem. Int. Ed. 2001, 40, 1721–1725. [Google Scholar] [CrossRef]
- Georgakilas, V.; Kordatos, K.; Prato, M.; Guldi, D.M.; Holzinger, M.; Hirsch, A. Organic functionalization of carbon nanotubes. J. Am. Chem. Soc. 2002, 124, 760–761. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xu, P.; Gui, H.; Wang, X.; Ding, Y. Effect of imidazolium phosphate and multiwalled carbon nanotubes on thermal stability and flame retardancy of polylactide. Compos. Part A Appl. Sci. Manuf. 2015, 77, 147–153. [Google Scholar] [CrossRef]
- Blanco, I.; Bottino, F.A.; Cicala, G.; Cozzo, G.; Latteri, A.; Recca, A. Synthesis and thermal characterization of new dumbbell shaped POSS/PS nanocomposites: Influence of the symmetrical structure of the nanoparticles on the dispersion/aggregation in the polymer matrix. Polym. Compos. 2015, 36, 1394–1400. [Google Scholar] [CrossRef]
- Blanco, I.; Bottino, F.A.; Bottino, P. Influence of symmetry/asymmetry of the nanoparticles structure on the thermal stability of polyhedral oligomeric silsesquioxane/polystyrene nanocomposites. Polym. Compos. 2012, 33, 1903–1910. [Google Scholar] [CrossRef]
- Xing, W.; Yang, W.; Yang, W.; Hu, Q.; Si, J.; Lu, H.; Yang, B.; Song, L.; Hu, Y.; Yuen, R. Functionalized carbon nanotubes with phosphorus-and nitrogen-containing agents: Effective reinforcer for thermal, mechanical, and flame-retardant properties of polystyrene nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 26266–26274. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Tong, L.; Xu, Z.; Fang, Z.; Jin, Y.; Lu, F. A novel intumescent flame retardant: Synthesis and application in ABS copolymer. Polym. Degrad. Stab. 2007, 92, 720–726. [Google Scholar] [CrossRef]
- Huang, Y.; Young, R. Microstructure and mechanical properties of pitch-based carbon fibres. J. Mater. Sci. 1994, 29, 4027–4036. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Ruan, S.; Gao, P.; Yang, X.G.; Yu, T. Toughening high performance ultrahigh molecular weight polyethylene using multiwalled carbon nanotubes. Polymer 2003, 44, 5643–5654. [Google Scholar] [CrossRef]
- Bahr, J.L.; Yang, J.; Kosynkin, D.V.; Bronikowski, M.J.; Smalley, R.E.; Tour, J.M. Functionalization of carbon nanotubes by electrochemical reduction of aryl diazonium salts: A bucky paper electrode. J. Am. Chem. Soc. 2001, 123, 6536–6542. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Shi, Z.; Fang, J.; Yin, J. One pot synthesis of multiwalled carbon nanotubes reinforced polybenzimidazole hybrids: Preparation, characterization and properties. Polymer 2009, 50, 5987–5995. [Google Scholar] [CrossRef]
- Koval’chuk, A.A.; Shevchenko, V.G.; Shchegolikhin, A.N.; Nedorezova, P.M.; Klyamkina, A.N.; Aladyshev, A.M. Effect of carbon nanotube functionalization on the structural and mechanical properties of polypropylene/MWCNT composites. Macromolecules 2008, 41, 7536–7542. [Google Scholar] [CrossRef]
- Kim, J.Y.; Han, S.I.; Hong, S. Effect of modified carbon nanotube on the properties of aromatic polyester nanocomposites. Polymer 2008, 49, 3335–3345. [Google Scholar] [CrossRef]
- London, L.; Bolton, L.; Samarakoon, D.; Sannigrahi, B.; Wang, X.; Khan, I. Effect of polymer stereoregularity on polystyrene/single-walled carbon nanotube interactions. RSC Adv. 2015, 5, 59186–59193. [Google Scholar] [CrossRef]
- Wu, C.S.; Liao, H.T. Study on the preparation and characterization of biodegradable polylactide/multi-walled carbon nanotubes nanocomposites. Polymer 2007, 48, 4449–4458. [Google Scholar] [CrossRef]
- Cheng, S.Z.; Pan, R.; Wunderlich, B. Thermal analysis of poly(butylene terephthalate) for heat capacity, rigid-amorphous content, and transition behavior. Macromol. Chem. Phys. 1988, 189, 2443–2458. [Google Scholar] [CrossRef]
- Nichols, M.E.; Robertson, R.E. The multiple melting endotherms from poly(butylene terephthalate). J. Polym. Sci. Polym. Phys. 1992, 30, 755–768. [Google Scholar] [CrossRef]
- Righetti, M.; Di Lorenzo, M. Melting process of poly(butylene terephthalate) analyzed by temperature-modulated differential scanning calorimetry. J. Polym. Sci. Polym. Phys. 2004, 42, 2191–2201. [Google Scholar] [CrossRef]
- Yasuniwa, M.; Tsubakihara, S.; Ohoshita, K.; Tokudome, S.I. X-ray studies on the double melting behavior of poly(butylene terephthalate). J. Polym. Sci. Polym. Phys. 2001, 39, 2005–2015. [Google Scholar] [CrossRef]
- Broza, G.; Kwiatkowska, M.; Rosłaniec, Z.; Schulte, K. Processing and assessment of poly(butylene terephthalate) nanocomposites reinforced with oxidized single wall carbon nanotubes. Polymer 2005, 46, 5860–5867. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, H.; Yang, B.; Lu, H.; Song, L.; Hu, Y. Facile preparation of modified carbon nanotube-reinforced PBT nanocomposites with enhanced thermal, flame retardancy, and mechanical properties. Polym. Compos. 2016, 37, 1812–1820. [Google Scholar] [CrossRef]
- Benedict, L.X.; Louie, S.G.; Cohen, M.L. Heat capacity of carbon nanotubes. Solid State Commun. 1996, 100, 177–180. [Google Scholar] [CrossRef]
- Berber, S.; Kwon, Y.-K.; Tománek, D. Unusually high thermal conductivity of carbon nanotubes. Phys. Rev. Lett. 2000, 84, 4613. [Google Scholar] [CrossRef] [PubMed]
- Bokobza, L. Multiwall carbon nanotube elastomeric composites: A review. Polymer 2007, 48, 4907–4920. [Google Scholar] [CrossRef]
- Meng, H.; Sui, G.; Fang, P.; Yang, R. Effects of acid-and diamine-modified MWNTs on the mechanical properties and crystallization behavior of polyamide 6. Polymer 2008, 49, 610–620. [Google Scholar] [CrossRef]
- Zhu, J.; Morgan, A.B.; Lamelas, F.J.; Wilkie, C.A. Fire properties of polystyrene-clay nanocomposites. Chem. Mater. 2001, 13, 3774–3780. [Google Scholar] [CrossRef]
- Zanetti, M.; Kashiwagi, T.; Falqui, L.; Camino, G. Cone calorimeter combustion and gasification studies of polymer layered silicate nanocomposites. Chem. Mater. 2002, 14, 881–887. [Google Scholar] [CrossRef]
- Zhou, K.; Gui, Z.; Hu, Y. The influence of graphene based smoke suppression agents on reduced fire hazards of polystyrene composites. Compos. Part A Appl. Sci. Manuf. 2016, 80, 217–227. [Google Scholar] [CrossRef]
- Dong, Y.; Gui, Z.; Hu, Y.; Wu, Y.; Jiang, S. The influence of titanate nanotube on the improved thermal properties and the smoke suppression in poly(methyl methacrylate). J. Hazard. Mater. 2012, 209, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Yu, Z.Z.; Cai, G.P.; Mai, Y.W. Recent developments in the fire retardancy of polymeric materials. Prog. Polym. Sci. 2013, 38, 1357–1387. [Google Scholar] [CrossRef]
- Purser, D.A. Toxic combustion product yields as a function of equivalence ratio and flame retardants in under-ventilated fires: Bench-large-scale comparisons. Polymers 2016, 8, 330. [Google Scholar] [CrossRef]
- Stec, A.A.; Rhodes, J. Smoke and hydrocarbon yields from fire retarded polymer nanocomposites. Polym. Degrad. Stab. 2011, 96, 295–300. [Google Scholar] [CrossRef]
- Hou, Y.B.; Hu, W.Z.; Gui, Z.; Hu, Y. A novel Co(II)-based metal-organic framework with phosphorus-containing structure: Build for enhancing fire safety of epoxy. Compos. Sci. Technol. 2017, 152, 231–242. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Grulke, E.; Hilding, J.; Groth, K.; Harris, R.; Butler, K.; Shields, J.; Kharchenko, S.; Douglas, J. Thermal and flammability properties of polypropylene/carbon nanotube nanocomposites. Polymer 2004, 45, 4227–4239. [Google Scholar] [CrossRef]
- Schartel, B.; Pötschke, P.; Knoll, U.; Abdel-Goad, M. Fire behaviour of polyamide 6/multiwall carbon nanotube nanocomposites. Eur. Polym. J. 2005, 41, 1061–1070. [Google Scholar] [CrossRef]
- Yu, B.; Shi, Y.Q.; Yuan, B.H.; Qiu, S.L.; Xing, W.Y.; Hu, W.Z.; Song, L.; Lo, S.M.; Hu, Y. Enhanced thermal and flame retardant properties of flame-retardant-wrapped graphene/epoxy resin nanocomposites. J. Mater. Chem. A 2015, 3, 8034–8044. [Google Scholar] [CrossRef]
- Shi, Y.; Fu, L.; Chen, X.; Guo, J.; Yang, F.; Wang, J.; Zheng, Y.; Hu, Y. Hypophosphite/Graphitic carbon nitride hybrids: Preparation and flame-retardant application in thermoplastic polyurethane. Nanomaterials 2017, 7, 259. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Xing, W.Y.; Guo, W.W.; Qiu, S.L.; Wang, X.; Lo, S.M.; Hu, Y. Thermal exfoliation of hexagonal boron nitride for effective enhancements on thermal stability, flame retardancy and smoke suppression of epoxy resin nanocomposites via sol-gel process. J. Mater. Chem. A 2016, 4, 7330–7340. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Y.R.; Yuen, A.; Chen, T.; Chan, M.C.; Peng, L.Z.; Yang, W.J.; Zhu, S.E.; Yang, B.H.; Hu, K.H.; et al. Synthesis of phosphorus-containing silane coupling agent for surface modification of glass fibers: Effective reinforcement and flame retardancy in poly(1,4-butylene terephthalate). Chem. Eng. J. 2017, 321, 257–267. [Google Scholar] [CrossRef]
- Yu, B.; Wang, X.; Qian, X.D.; Xing, W.Y.; Yang, H.Y.; Ma, L.Y.; Lin, Y.; Jiang, S.H.; Song, L.; Hu, Y.; et al. Functionalized graphene oxide/phosphoramide oligomer hybrids flame retardant prepared via in situ polymerization for improving the fire safety of polypropylene. RSC Adv. 2014, 4, 31782–31794. [Google Scholar] [CrossRef]
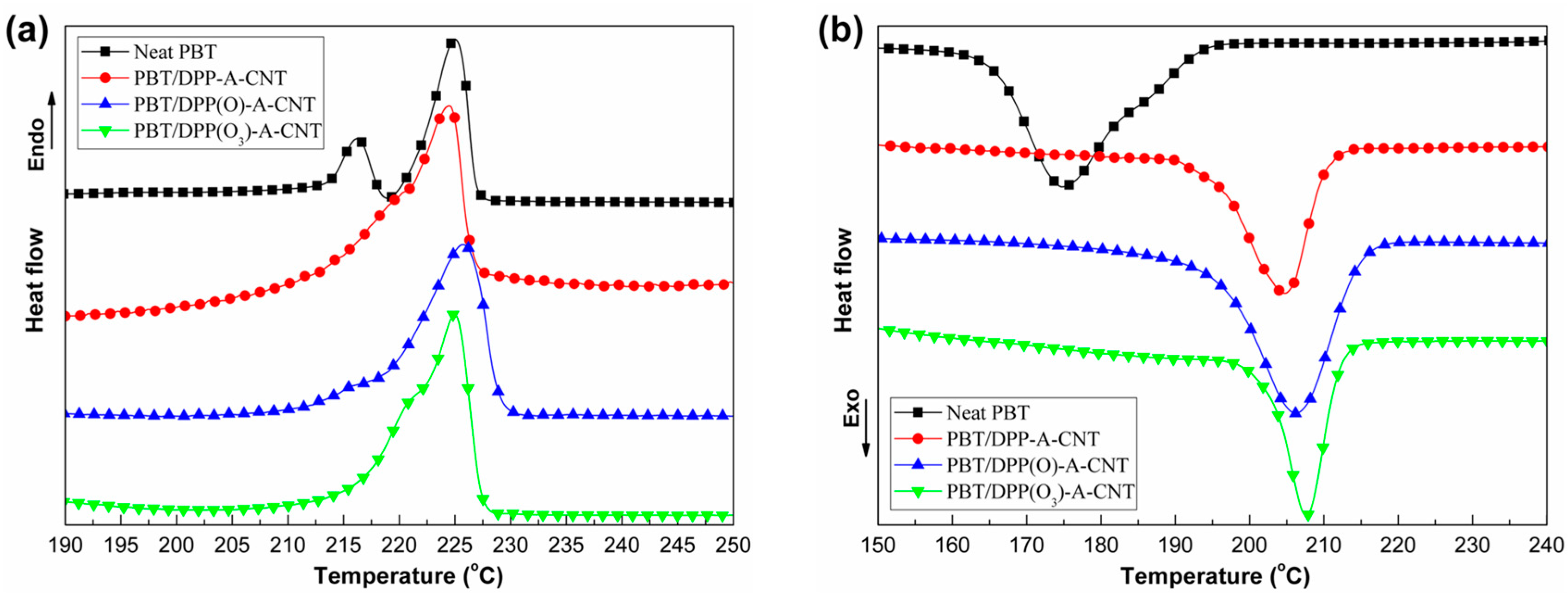


| Sample No. | Tm (°C) | Tc (°C) |
|---|---|---|
| Neat PBT | 225 | 174 |
| PBT/DPP-A-CNT | 224 | 205 |
| PBT/DPP(O)-A-CNT | 226 | 206 |
| PBT/DPP(O3)-A-CNT | 225 | 208 |
| Sample No. | T−1% (°C) | T−5% (°C) | Tmax (°C) | Residue at 700 °C (wt %) |
|---|---|---|---|---|
| Neat PBT | 351 | 365 | 410 | 2.9 |
| PBT/DPP-A-CNT | 362 | 386 | 420 | 5.2 |
| PBT/DPP(O)-A-CNT | 366 | 388 | 419 | 6.5 |
| PBT/DPP(O3)-A-CNT | 352 | 377 | 419 | 4.5 |
| Sample No. | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| Neat PBT | 49.5 ± 2.1 | 244 ± 10 |
| PBT/DPP-A-CNT | 55.1 ± 1.9 | 99 ± 15 |
| PBT/DPP(O)-A-CNT | 58.5 ± 1.8 | 86 ± 12 |
| PBT/DPP(O3)-A-CNT | 62.1 ± 2.7 | 74 ± 11 |
| Sample No. | TTI (s) | PHRR (kW/m2) | THR (MJ/m2) | PSPR (m2/s) | TSP (m2/kg) | PCO2P (g/s) | PCOP (g/s) | Residue (wt %) |
|---|---|---|---|---|---|---|---|---|
| Error | ±2 | ±15 | ±0.5 | ±0.01 | ±20 | ±0.02 | ±0.005 | ±0.2 |
| Neat PBT | 120 | 944 | 74.7 | 0.207 | 573 | 0.987 | 0.0300 | 2.9 |
| PBT/DPP-A-CNT | 101 | 759 | 78.4 | 0.213 | 583 | 0.866 | 0.0293 | 5.6 |
| PBT/DPP(O)-A-CNT | 117 | 668 | 70.9 | 0.189 | 546 | 0.760 | 0.0228 | 7.4 |
| PBT/DPP(O3)-A-CNT | 104 | 710 | 75.5 | 0.183 | 544 | 0.838 | 0.0273 | 4.8 |
| Sample No. | Element Content (wt %) | ||
|---|---|---|---|
| C | O | P | |
| PBT/DPP-A-CNT | 84.82 | 13.58 | 1.60 |
| PBT/DPP(O)-A-CNT | 77.51 | 20.63 | 1.86 |
| PBT/DPP(O3)-A-CNT | 80.17 | 19.70 | 0.13 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.-E.; Wang, L.-L.; Chen, H.; Yang, W.; Yuen, A.C.-Y.; Chen, T.B.-Y.; Luo, C.; Bi, W.-M.; Hu, E.-Z.; Zhang, J.; et al. Comparative Studies on Thermal, Mechanical, and Flame Retardant Properties of PBT Nanocomposites via Different Oxidation State Phosphorus-Containing Agents Modified Amino-CNTs. Nanomaterials 2018, 8, 70. https://doi.org/10.3390/nano8020070
Zhu S-E, Wang L-L, Chen H, Yang W, Yuen AC-Y, Chen TB-Y, Luo C, Bi W-M, Hu E-Z, Zhang J, et al. Comparative Studies on Thermal, Mechanical, and Flame Retardant Properties of PBT Nanocomposites via Different Oxidation State Phosphorus-Containing Agents Modified Amino-CNTs. Nanomaterials. 2018; 8(2):70. https://doi.org/10.3390/nano8020070
Chicago/Turabian StyleZhu, San-E, Li-Li Wang, Hao Chen, Wei Yang, Anthony Chun-Yin Yuen, Timothy Bo-Yuan Chen, Cheng Luo, Wen-Mei Bi, En-Zhu Hu, Jian Zhang, and et al. 2018. "Comparative Studies on Thermal, Mechanical, and Flame Retardant Properties of PBT Nanocomposites via Different Oxidation State Phosphorus-Containing Agents Modified Amino-CNTs" Nanomaterials 8, no. 2: 70. https://doi.org/10.3390/nano8020070