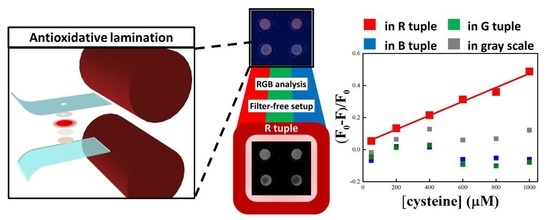
Laminated Copper Nanocluster Incorporated Antioxidative Paper Device with RGB System-Assisted Signal Improvement
Abstract
:1. Introduction
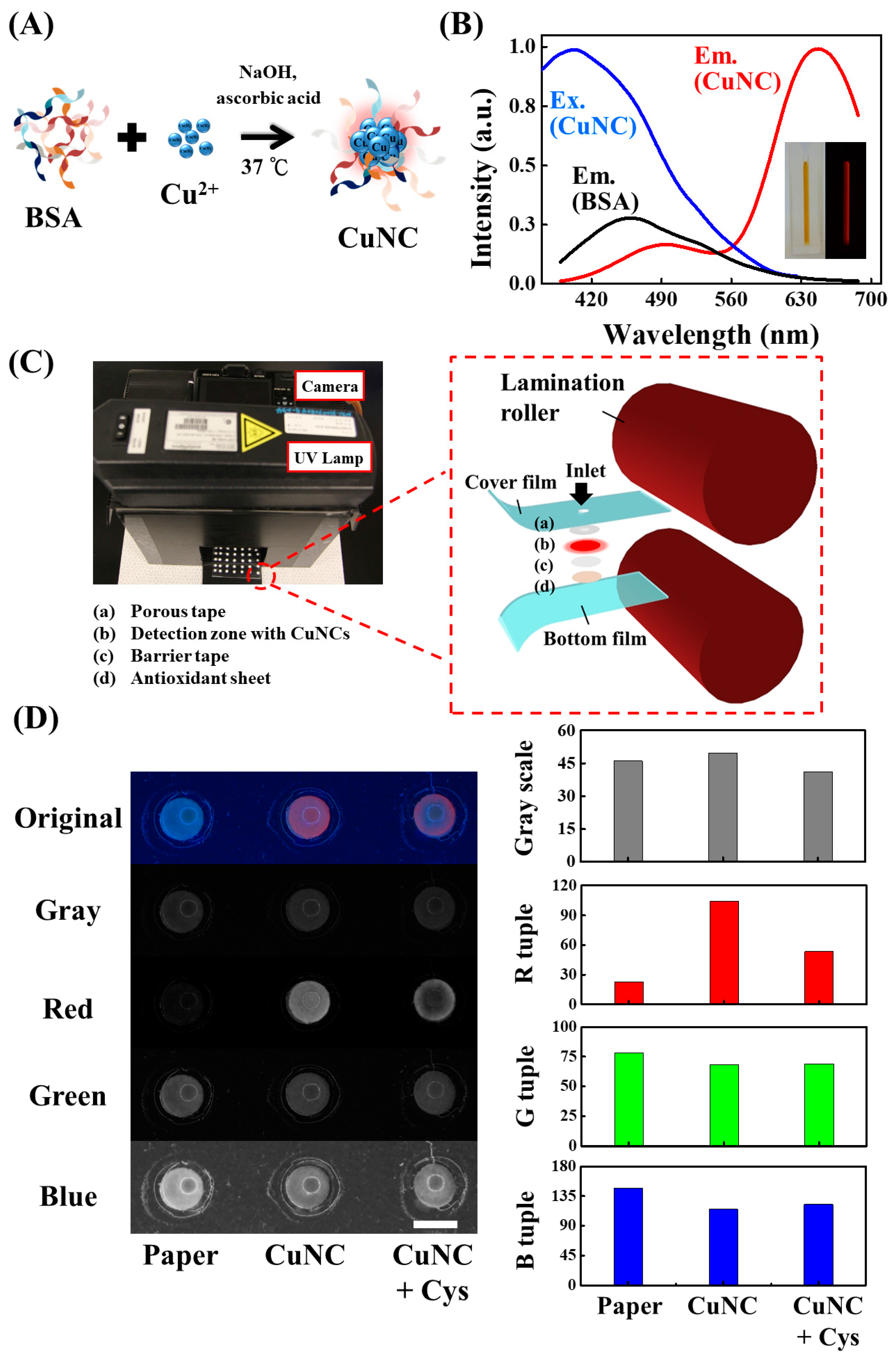
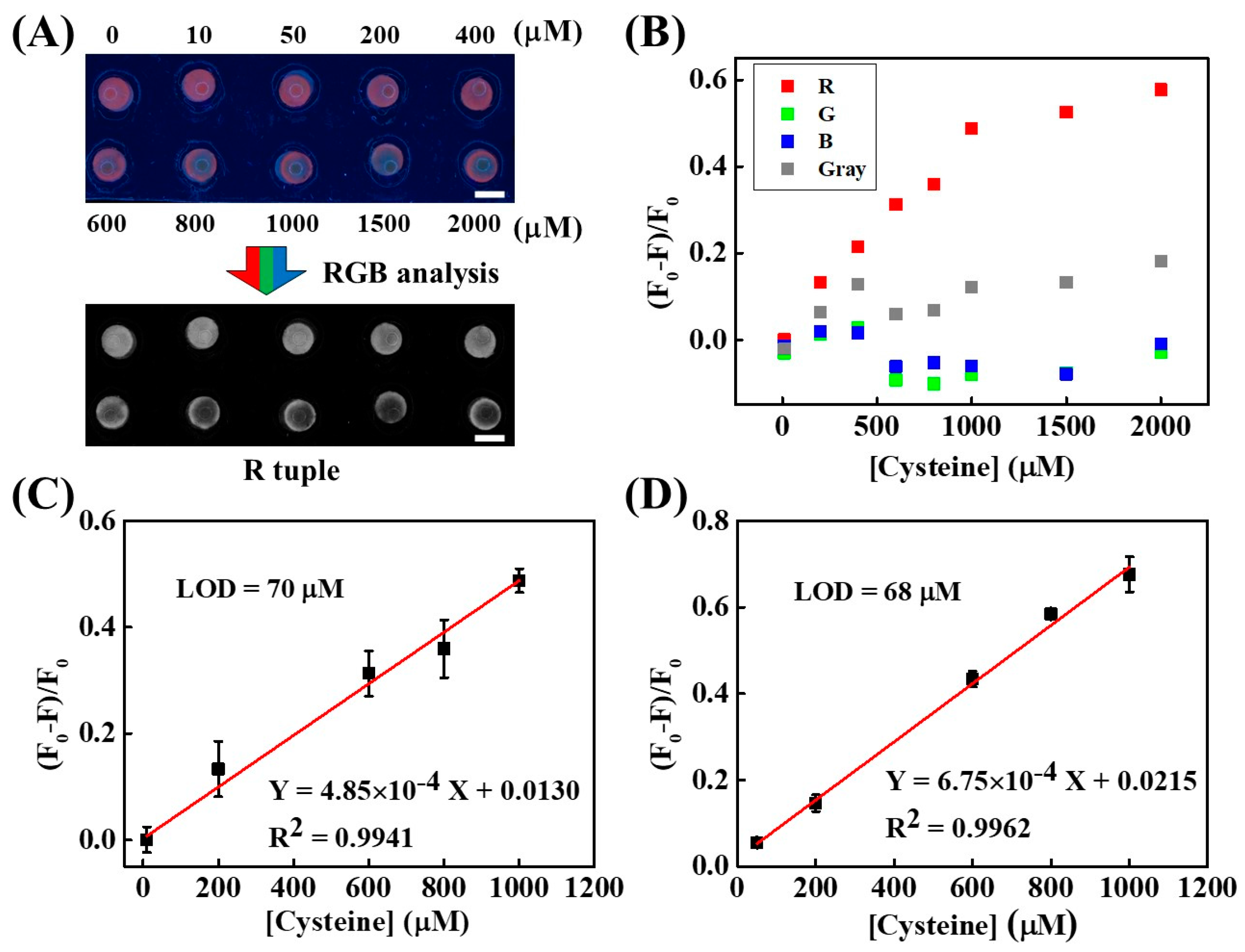
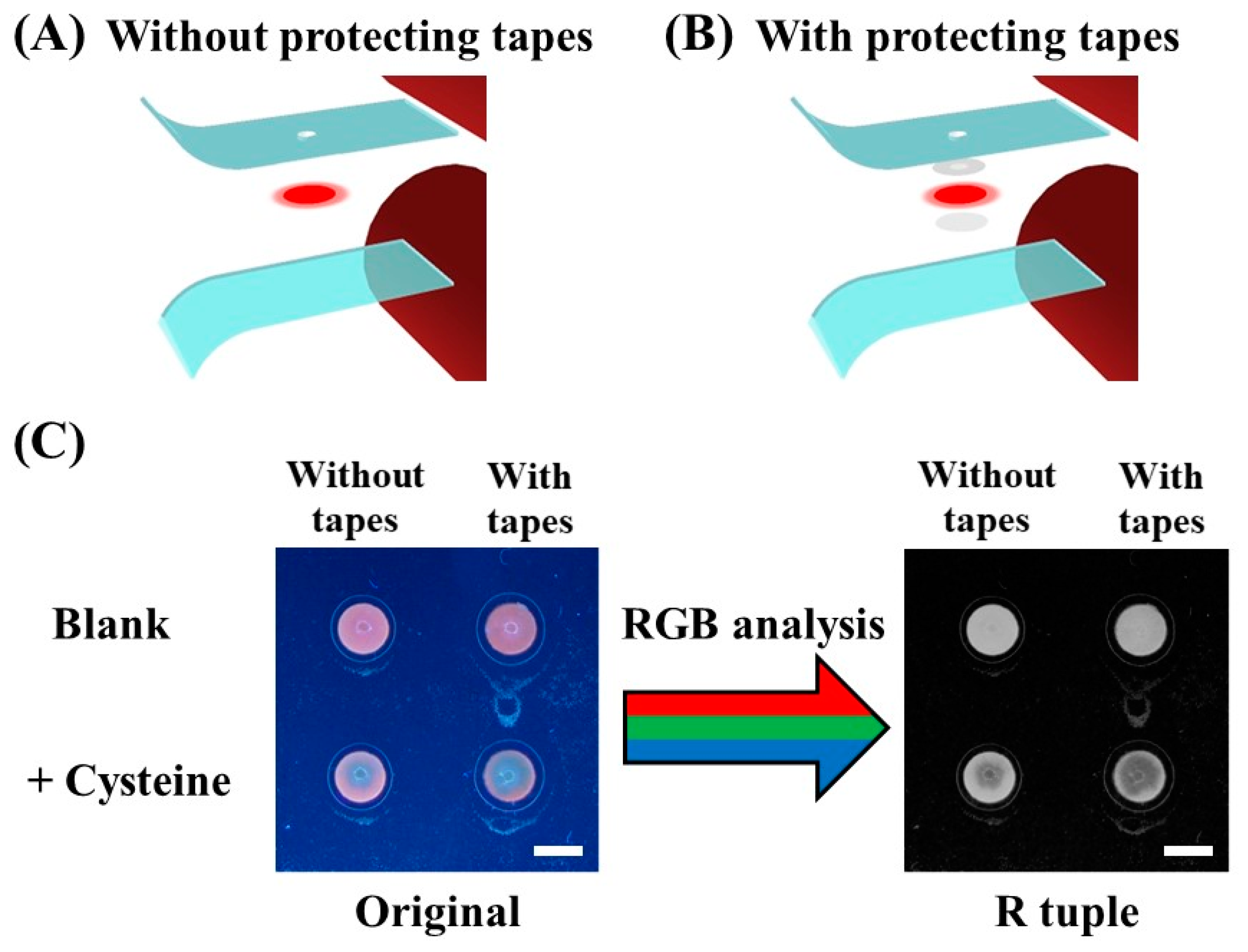
2. Results and Discussion
3. Experimental Section
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, W.A.; van den Berg, A. Lab on Paper. Lab Chip 2008, 8, 1988–1991. [Google Scholar] [PubMed]
- Liana, D.D.; Raguse, B.; Gooding, J.J.; Chow, E. Recent Advances in Paper-Based Sensors. Sensors 2012, 12, 11505–11526. [Google Scholar] [CrossRef] [PubMed]
- Cate, D.M.; Adkins, J.A.; Mettakoonpitak, J.; Henry, C.S. Recent Developments in Paper-Based Microfluidic Devices. Anal. Chem. 2015, 87, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Ju, Q.; Uddayasankar, U.; Krull, U. Paper-Based DNA Detection Using Lanthanide-Doped LiYF4 Upconversion Nanocrystals as Bioprobe. Small 2014, 10, 3912–3917. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the Developing World: Microfluidic Paper-Based Analytical Devices. Anal. Chem. 2010, 82, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-Based Microfluidic Point-of-Care Diagnostic Devices. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, S.; Wang, L.; Li, F.; Pingguan-Murphy, B.; Lu, T.J.; Xu, F. Advances in Paper-Based Point-of-Care Diagnostics. Biosens. Bioelectron. 2014, 54, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.D.; Linder, V.; Sia, S.K. Lab-on-a-chip Devices for Global Health: Past Studies and Future Opportunities. Lab Chip 2007, 7, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.G.; Kim, Y.-G.; Chung, B.G.; Demirci, U.; Khademhosseini, A. Nano/Microfluidics for Diagnosis of Infectious Diseases in Developing Countries. Adv. Drug Deliv. Rev. 2010, 62, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ballerini, D.R.; Shen, W. A Perspective on Paper-Based Microfluidics: Current Status and Future Trends. Biomicrofluidics 2012, 6, 011301. [Google Scholar] [CrossRef] [PubMed]
- Scida, K.; Li, B.; Ellington, A.D.; Crooks, R.M. DNA Detection Using Origami Paper Analytical Devices. Anal. Chem. 2013, 85, 9713–9720. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.P.; Zheng, Y.G.; Ying, J.Y. Protein-Directed Synthesis of Highly Fluorescent Gold Nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-A.J.; Yang, T.-Y.; Lee, C.-H.; Huang, S.H.; Sperling, R.A.; Zanella, M.; Li, J.K.; Shen, J.-L.; Wang, H.-H.; Yeh, H.-I.; et al. Synthesis, Characterization, and Bioconjugation of Fluorescent Gold Nanoclusters toward Biological Labeling Applications. ACS Nano 2009, 3, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Huang, Y.; Li, W.; Cai, Z.; Luo, F.; Yang, C.J.; Chen, X. Facile Synthesis of Red-Emitting Lysozyme-Stabilized Ag Nanoclusters. Nanoscale 2012, 4, 5312–5315. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.-C.; Sharma, J.; Han, J.J.; Martinez, J.S.; Werner, J.H. A DNA-Silver Nanocluster Probe that Fluoresces upon Hybridization. Nano Lett. 2010, 10, 3106–3110. [Google Scholar] [CrossRef] [PubMed]
- Goswami, N.; Giri, A.; Bootharaju, M.S.; Xavier, P.L.; Pradeep, T.; Pal, S.K. Copper Quantum Clusters in Protein Matrix: Potential Sensor of Pb2+ Ion. Anal. Chem. 2011, 83, 9676–9680. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Li, J.; Han, L.; Ren, J.; Yang, X.; Wang, E. DNA-Hosted Copper Nanoclusters for Fluorescent Identification of Single Nucleotide Polymorphisms. ACS Nano 2012, 6, 3311–3317. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Si, L.; Bao, J.; Dai, Z. A Reusable MicroRNA Sensor Based on the Electrocatalytic Property of Heteroduplex-Templated Copper Nanoclusters. Chem. Commun. 2015, 51, 6305–6307. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Liu, S.; Yang, C.; Liu, F.; Wang, K.; Chen, G. Colorimetric Detection of Hepatitis B Virus (HBV) DNA Based on DNA-Templated Copper Nanoclusters. Anal. Chim. Acta 2016, 909, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yuan, Y.; Zhang, L.; Zhao, J.; Majeed, S.; Xu, G. Copper Nanoclusters as Peroxidase Mimetics and Their Applications to H2O2 and Glucose Detection. Anal. Chim. Acta 2013, 762, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Ju, Y.; Liu, J.; Zhang, H.; Chen, X. Polyethyleneimine-Templated Copper Nanoclusters via Ascorbic Acid Reduction Approach as Ferric Ion Sensor. Anal. Chim. Acta 2015, 854, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, C.; Xu, L.; Cheng, H.; Lin, Q.; Zhang, C. Protein-Directed Synthesis of pH-Responsive Red Fluorescent Copper Nanoclusters and Their Applications in Cellular Imaging and Catalysis. Nanoscale 2014, 6, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Leng, F.; Zhan, L.; Chang, Y.; Yang, X.X.; Lan, J.; Huang, C.Z. One-Step Prepared Fluorescent Copper Nanoclusters for Reversible pH-Sensing. Analyst 2014, 139, 2990–2993. [Google Scholar] [CrossRef] [PubMed]
- Bhamore, J.R.; Jha, S.; Mungara, A.K.; Singhal, R.K.; Sonkeshariya, D.; Kailasa, S.K. One-Step Green Synthetic Approach for the Preparation of Multicolor Emitting Copper Nanoclusters and Their Applications in Chemical Species Sensing and Bioimaging. Biosens. Bioelectron. 2016, 80, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Sahoo, A.K.; Ghosh, S.S.; Paul, A.; Chattopadhyay, A. Blue-Emitting Copper Nanoclusters Synthesized in the Presence of Lysozyme as Candidates for Cell Labeling. ACS Appl. Mater. Interfaces 2014, 6, 3822–3828. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, H.; Wang, A.-J.; Zhong, S.-X.; Fang, K.-M.; Feng, J.-J. Green Synthesis of Peptide-Templated Fluorescent Copper Nanoclusters for Temperature Sensing and Cellular Imaging. Analyst 2014, 139, 6536–6541. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; He, X.-W.; Li, W.-Y.; Zhang, Y.-K. Transferrin-Directed Preparation of Red-Emitting Copper Nanoclusters for Targeted Imaging of Transferrin Receptor Over-Expressed Cancer Cells. J. Mater. Chem. B 2015, 3, 2388–2394. [Google Scholar] [CrossRef]
- Diez, I.; Ras, R.H.A. Fluorescent Silver Nanoclusters. Nanoscale 2011, 3, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Y.; Wang, C.-W.; Yuan, Z.; Chang, H.-T. Fluorescent Gold Nanoclusters: Recent Advances in Sensing and Imaging. Anal. Chem. 2015, 87, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Le Guevel, X.; Hotzer, B.; Jung, G.; Hollemeyer, K.; Trouillet, V.; Schneider, M. Formation of Fluorescent Metal (Au, Ag) Nanoclusters Capped in Bovine Serum Albumin Followed by Fluorescence and Spectroscopy. J. Phys. Chem. C 2011, 115, 10955–10963. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, C.; Yu, M.; Liu, J. Different Sized Luminescent Gold Nanoparticles. Nanoscale 2012, 4, 4073–4083. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Chen, Y.-C.; Li, H.-W.; Chang, H.-T. Fluorescent Silver Nanoclusters Stabilized by DNA Scaffolds. Chem. Commun. 2014, 50, 9800–9815. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.-X.; Zhang, M.; Zhu, A.; Shi, G. Terbium(iii)/gold nanocluster conjugates: The development of a novel ratiometric fluorescent probe for mercury(ii) and a paper-based visual sensor. Analyst 2015, 140, 5656–5661. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.Q.; Neoh, K.-G.; Kang, E.-T.; Fu, G.D. Rhodamine derivative-modified filter papers for colorimetric and fluorescent detection of Hg2+ in aqueous media. J. Mater. Chem. A 2013, 1, 2526–2532. [Google Scholar] [CrossRef]
- Chen, P.-C.; Li, Y.-C.; Ma, J.-Y.; Huang, J.-Y.; Chen, C.-F.; Chang, H.-T. Size-tunable copper nanocluster aggregates and their application in hydrogen sulfide sensing on paper-based devices. Sci. Rep. 2016, 6, 24882. [Google Scholar] [CrossRef] [PubMed]
- Vrekoussis, T.; Chaniotis, V.; Navrozoglou, I.; Dousias, V.; Pavlakis, K.; Stathopoulos, E.N.; Zoras, O. Image Analysis of Breast Cancer Immunohistochemistry-stained Sections Using ImageJ: An RGB-based Model. Anticancer Res. 2009, 29, 4995–4998. [Google Scholar] [PubMed]
- Wang, X.; Gorris, H.H.; Stolwijk, J.A.; Meier, R.J.; Groegel, D.B.M.; Wegener, J.; Wolfbeis, O.S. Self-referenced RGB Colour Imaging of Intracellular Oxygen. Chem. Sci. 2011, 2, 901–906. [Google Scholar] [CrossRef]
- Soga, T.; Jimbo, Y.; Suzuki, K.; Citterio, D. Inkjet-Printed Paper-Based Colorimetric Sensor Array for the Discrimination of Volatile Primary Amines. Anal. Chem. 2013, 85, 8973–8978. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Ruiz, N.; Curto, V.F.; Erenas, M.M.; Benito-Lopez, F.; Diamond, D.; Palma, A.J.; Capitan-Vallvey, L.F. Smartphone-Based Simultaneous pH and Nitrite Colorimetric Determination for Paper Microfluidic Devices. Anal. Chem. 2014, 86, 9554–9562. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Mohammadi, S.; Busa, L.S.A.; Maeki, M.; Ishida, A.; Tani, H.; Tokeshi, M. Image analysis for a microfluidic paper-based analytical device using the CIE L*a*b* color system. Analyst 2016, 141, 6507–6509. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, A.; Khajehzadeh, A.; Ghaffarinejad, A. A Simple and Cost-Effective Method, as an Appropriate Alternative for Visible Spectrophotometry: Development of a Dopamine Biosensor. Analyst 2009, 134, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Cui, X.; DeModena, J.; Wang, Y.M.; Sternberg, P.; Yang, C. Implementation of a Color-Capable Optofluidic Microscope on a RGB CMOS Color Sensor Chip Substrate. Lab Chip 2010, 10, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.W.G. The Reproduction of Colour, 6th ed.; John Wiely & Sons, Ltd.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Liu, Y.; Ling, J.; Huang, C.Z. Individually Color-Coded Plasmonic Nanoparticles for RGB Analysis. Chem. Commun. 2011, 47, 8121–8123. [Google Scholar] [CrossRef] [PubMed]
- Schwaebel, T.; Trapp, O.; Bunz, U.H.F. Digital Photography for the Analysis of Fluorescence Responses. Chem. Sci. 2013, 4, 273–281. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Chen, P.-C.; Yuan, Z.; Ma, J.-Y.; Chang, H.-T. The Isomeric Effect of Mercaptobenzoic Acids on the Preparation and Fluorescence Properties of Copper Nanoclusters. Chem. Commun. 2015, 51, 11983–11986. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Lu, Y.; Chen, W.; Chen, S. One-Pot Synthesis, Photoluminescence, and Electrocatalytic Properties of Subnanometer-Sized Copper Clusters. J. Am. Chem. Soc. 2011, 133, 2060–2063. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-Y.; Chen, C.-Y.; Wang, C.-M.; Tan, Y.Z.; Liao, W.-S. Multicolor Functional Carbon Dots via One-Step Refluxing Synthesis. ACS Sens. 2017, 2, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.H.; Zhao, J.; Hicks, E.M.; Schatz, G.C.; Van Duyne, R.P. Plasmonic Properties of Copper Nanoparticles Fabricated by Nanosphere Lithography. Nano Lett. 2007, 7, 1947–1952. [Google Scholar] [CrossRef]
- Hambrock, J.; Becker, R.; Birkner, A.; Weiss, J.; Fischer, R.A. A Non-Aqueous Organometallic Route to Highly Monodispersed Copper Nanoparticles Using [Cu(OCH(Me)CH2NMe2)2]. Chem. Commun. 2002, 68–69. [Google Scholar] [CrossRef]
- Qian, Q.; Deng, J.; Wang, D.; Yang, L.; Yu, P.; Mao, L. Aspartic Acid-Promoted Highly Selective and Sensitive Colorimetric Sensing of Cysteine in Rat Brain. Anal. Chem. 2012, 84, 9579–9584. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhian, S. Lead Phthalocyanine as a Selective Carrier for Preparation of a Cysteine-Selective Electrode. Anal. Chem. 2001, 73, 5972–5978. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Rusin, O.; Xu, X.Y.; Kim, K.K.; Escobedo, J.O.; Fakayode, S.O.; Fletcher, K.A.; Lowry, M.; Schowalter, C.M.; Lawrence, C.M.; et al. Detection of Homocysteine and Cysteine. J. Am. Chem. Soc. 2005, 127, 15949–15958. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Tay, Y.; Dou, X.; Luo, Z.; Leong, D.T.; Xie, J. Glutathione-Protected Silver Nanoclusters as Cysteine-Selective Fluorometric and Colorimetric Probe. Anal. Chem. 2013, 85, 1913–1919. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.C.; Adkins, J.A.; Bisha, B.; Mentele, M.M.; Goodridge, L.D.; Henry, C.S. Development of a Paper-Based Analytical Device for Colorimetric Detection of Select Foodborne Pathogens. Anal. Chem. 2012, 84, 2900–2907. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shi, W.W.; Qin, J.H.; Lin, B.C. Fabrication and Characterization of Paper-Based Microfluidics Prepared in Nitrocellulose Membrane by Wax Printing. Anal. Chem. 2010, 82, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Carrilho, E.; Thomas, S.W.; Sindi, H.; Whitesides, G.M. Simple Telemedicine for Developing Regions: Camera Phones and Paper-Based Microfluidic Devices for Real-Time, Off-Site Diagnosis. Anal. Chem. 2008, 80, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Moerner, W.E.; Fromm, D.P. Methods of single-molecule fluorescence spectroscopy and microscopy. Rev. Sci. Instrum. 2003, 74, 3597–3619. [Google Scholar] [CrossRef]
- Miller, J.N. Basic statistical methods for Analytical Chemistry. Part 2. Calibration and regression methods. A review. Analyst 1991, 116, 3–14. [Google Scholar] [CrossRef]
- Selvarajan, S.; Alluri, N.R.; Chandrasekhar, A.; Kim, S.-J. Direct detection of cysteine using functionalized BaTiO3 nanoparticles film based self-powered biosensor. Biosens. Bioelectron. 2017, 91, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Song, H.; Song, J.; He, C.; Ni, J.; Zhao, Y.; Wang, X. Development of Triphenylamine Functional Dye for Selective Photoelectrochemical Sensing of Cysteine. Anal. Chem. 2014, 86, 5922–5928. [Google Scholar] [CrossRef] [PubMed]
- Kaniowska, E.; Chwatko, G.; Głowacki, R.; Kubalczyk, P.; Bald, E. Urinary excretion measurement of cysteine and homocysteine in the form of their S-pyridinium derivatives by high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. A 1998, 798, 27–35. [Google Scholar] [CrossRef]
- Wang, C.-M.; Chen, C.-Y.; Liao, W.-S. Paper-polymer composite devices with minimal fluorescence background. Anal. Chim. Acta 2017, 963, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cassano, C.L.; Xu, X.; Fan, Z.H. Laminated paper-based analytical devices (LPAD) with origami-enabled chemiluminescence immunoassay for cotinine detection in mouse serum. Anal. Chem. 2013, 85, 10270–10276. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, P.; Stevens, B.; Devlaming, I.; Grunlan, J.C. Polymer-Graphene Oxide Quadlayer Thin-Film Assemblies with Improved Gas Barrier. Langmuir 2015, 31, 5919–5927. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Fu, E.; Lutz, B.; Yager, P. Long-term dry storage of an enzyme-based reagent system for ELISA in point-of-care devices. Analyst 2014, 139, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Kannan, B.; Jahanshahi-Anbuhi, S.; Pelton, R.H.; Li, Y.; Filipe, C.D.M.; Brennan, J.D. Printed Paper Sensors for Serum Lactate Dehydrogenase using Pullulan-Based Inks to Immobilize Reagents. Anal. Chem. 2015, 87, 9288–9293. [Google Scholar] [CrossRef] [PubMed]

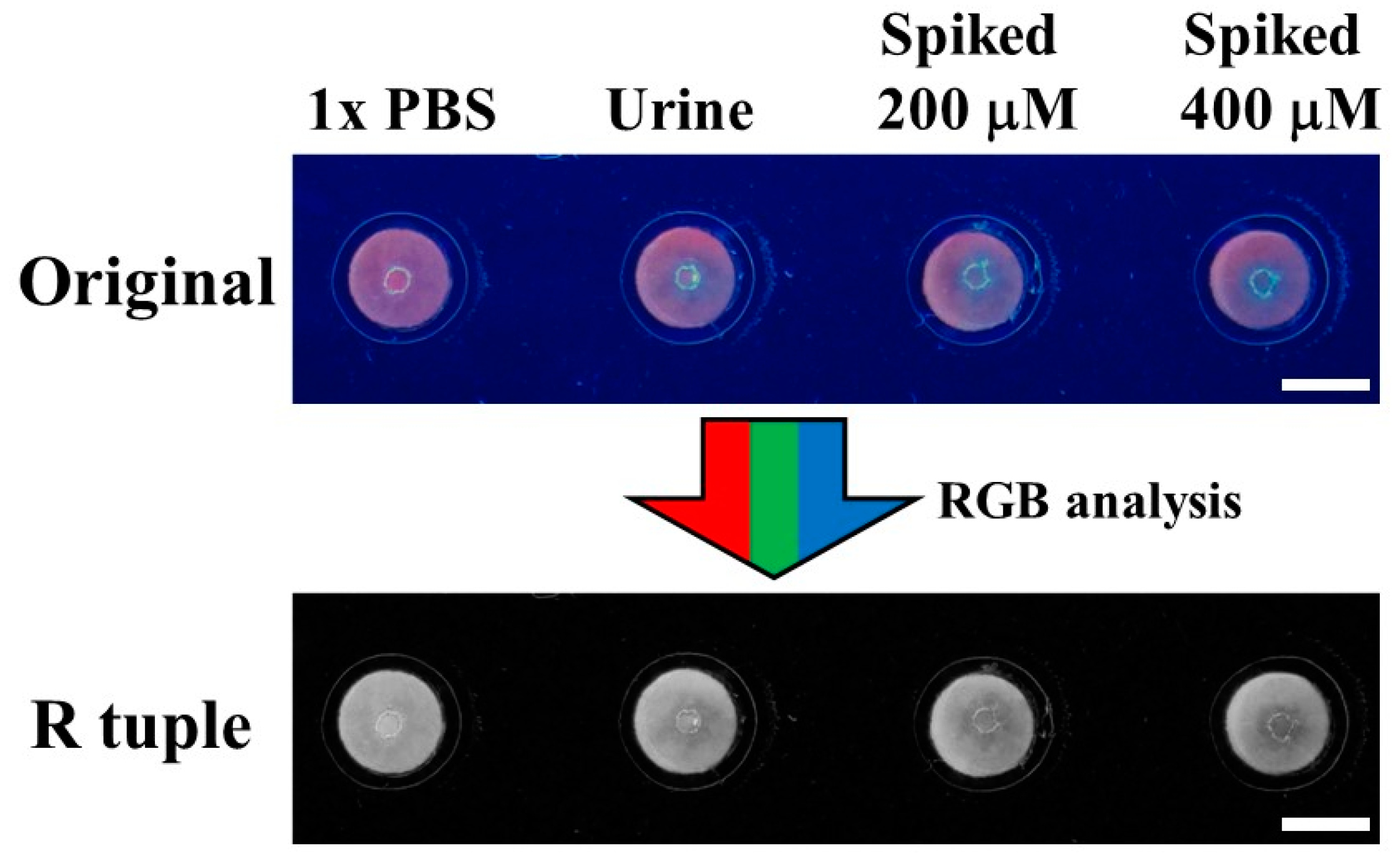

| Spiked (μM) | Found a (μM) | Recovery a (%) |
|---|---|---|
| 0 | 130.90 ± 9.18 | - |
| 200 | 204.71 ± 11.18 | 102.36 ± 5.59 |
| 400 | 416.21 ± 39.17 | 104.05 ± 9.79 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-Y.; Chen, C.-L.; Wang, C.-M.; Liao, W.-S. Laminated Copper Nanocluster Incorporated Antioxidative Paper Device with RGB System-Assisted Signal Improvement. Nanomaterials 2018, 8, 97. https://doi.org/10.3390/nano8020097
Chen C-Y, Chen C-L, Wang C-M, Liao W-S. Laminated Copper Nanocluster Incorporated Antioxidative Paper Device with RGB System-Assisted Signal Improvement. Nanomaterials. 2018; 8(2):97. https://doi.org/10.3390/nano8020097
Chicago/Turabian StyleChen, Chong-You, Chia-Lin Chen, Chang-Ming Wang, and Wei-Ssu Liao. 2018. "Laminated Copper Nanocluster Incorporated Antioxidative Paper Device with RGB System-Assisted Signal Improvement" Nanomaterials 8, no. 2: 97. https://doi.org/10.3390/nano8020097