Recent Advances in Metal Chalcogenides (MX; X = S, Se) Nanostructures for Electrochemical Supercapacitor Applications: A Brief Review
Abstract
:1. Introduction
- (i)
- Multiple oxidation states
- (ii)
- Superior electrical conductivity
- (iii)
- High surface area & chemical stability
- (iv)
- Electrochemical activity (electrolyte ions can freely interact into the electrode surface)
- (i)
- Doping of the metals to increase the conductivity and redox activity
- (ii)
- A wide potential window
- (iii)
- High surface area for the redox reaction
- (iv)
- High charge/discharge rate
2. Metal Chalcogenides for Electrochemical SCs
3. Transition Metal Sulfides
3.1. Nickel Sulfides
3.2. Copper Sulfide
3.3. Cobalt Sulfides
3.4. Binary Metal Sulfides
3.5. Molybdenum Disulfide
3.6. Other Transition Metal Sulfides
4. Transition Metal Selenides
4.1. Nickel Selenide
4.2. Copper Selenide
4.3. Molybdenum Diselenide
4.4. Cobalt Selenides
4.5. Binary Metal Selenides
5. Summary and Outlook
- (i)
- Energy density: For practical application, high energy density electrochemical system is required. In view of this, the energy density of electrochemical supercapacitors is less than less than of batteries.
- (ii)
- Cost efficiency: The commonly employed electrode materials such as high porous surface area carbon materials and RuO2 are more expensive. Also, the cost of organic electrolytes is far from negligible.
- (iii)
- Self-discharge rate: Electrochemical supercapacitors have high in self discharge rate 10–40%/day.
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SCs | Supercapacitors |
| EDLCs | Electric double layer capacitors |
| CNTs | Carbon nanotubes |
| MCs | Metal chalcogenides |
| KOH | Potassium hydroxide |
| 3-D-GN | Three dimensional graphene nanosheet |
| AC | Activated carbon |
| rGO | Reduced graphene oxide |
| MWCNT | Multi-walled carbon nanotubes |
| PANI | Poly aniline |
| SILAR | Successive ionic layer adsorption and reaction |
| LiClO4 | Lithium perchlorate |
| PC | Propylene carbonate |
| TMCs | Transition metal carbides |
| EC | Ethylene carbonate |
| CD | Charge-discharge |
References
- Theerthagiri, J.; Senthil, R.; Senthilkumar, B.; Polu, A.R.; Madhavan, J.; Ashokkumar, M. Recent advances in MoS2 nanostructured materials for energy and environmental applications—A review. J. Solid State Chem. 2017, 252, 43–71. [Google Scholar] [CrossRef]
- Thiagarajan, K.; Theerthagiri, J.; Senthil, R.; Arunachalam, P.; Madhavan, J.; Ghanem, M.A. Synthesis of Ni3V2O8@graphene oxide nanocomposite as an efficient electrode material for supercapacitor applications. J. Solid State Electrochem. 2017, 22, 527–536. [Google Scholar] [CrossRef]
- Arunachalam, P.; Shaddad, M.N.; Alamoudi, A.S.; Ghanem, M.A.; Al-Mayouf, A.M. Microwave-assisted synthesis of Co3(PO4)2 nanospheres for electrocatalytic oxidation of methanol in alkaline media. Catalysts 2017, 7, 119. [Google Scholar] [CrossRef]
- Arunachalam, P.; Ghanem, M.A.; Al-Mayouf, A.M.; Al-shalwi, M. Enhanced electrocatalytic performance of mesoporous nickel-cobalt oxide electrode for methanol oxidation in alkaline solution. Mater. Lett. 2017, 196, 365–368. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Sudha, R.; Premnath, K.; Arunachalam, P.; Madhavan, J.; Al-Mayouf, A.M. Growth of iron diselenide nanorods on graphene oxide nanosheets as advanced electrocatalyst for hydrogen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 13020–13030. [Google Scholar] [CrossRef]
- Ramesh, S.; Karuppasamy, K.; Msolli, S.; Kim, H.-S.; Kim, H.S.; Kim, J.-H. A nanocrystalline structured NiO/MnO2@nitrogen-doped graphene oxide hybrid nanocomposite for high performance supercapacitors. New J. Chem. 2017, 41, 15517–15527. [Google Scholar] [CrossRef]
- Thiagarajan, K.; Theerthagiri, J.; Senthil, R.; Madhavan, J. Simple and low cost electrode material based on Ca2V2O7/PANI nanoplatelets for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2017, 28, 17354–17362. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef] [PubMed]
- Karuppasamy, K.; Prasanna, K.; Kim, D.; Kang, Y.H.; Rhee, H.W. Headway in rhodanide anion based ternary gel polymer electrolytes (TILGPEs) for applications in rechargeable lithium ion batteries: An efficient route to achieve high electrochemical and cycling performances. RSC Adv. 2017, 7, 19211–19222. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Rhee, H.W.; Reddy, P.A.; Gupta, D.; Mitu, L.; Polu, A.R.; Shajan, X.S. Ionic liquid incorporated nanocomposite polymer electrolytes for rechargeable lithium ion battery: A way to achieve improved electrochemical and interfacial properties. J. Ind. Eng. Chem. 2016. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Kim, H.-S.; Kim, D.; Vikraman, D.; Prasanna, K.; Kathalingam, A.; Sharma, R.; Rhee, H.W. An enhanced electrochemical and cycling properties of novel boronic ionic liquid based ternary gel polymer electrolytes for rechargeable Li/LiCoO2 cells. Sci. Rep. 2017, 7, 11103. [Google Scholar] [CrossRef] [PubMed]
- Karthikprabhu, S.; Karuppasamy, K.; Vikraman, D.; Prasanna, K.; Maiyalagan, T.; Nichelson, A.; Kathalingam, A.; Kim, H.-S. Electrochemical performances of LiNi1−xMnxPO4 (x = 0.05–0.2) olivine cathode materials for high voltage rechargeable lithium ion batteries. Appl. Surf. Sci. 2017. [Google Scholar] [CrossRef]
- Borenstein, A.; Hanna, O.; Attias, R.; Luski, S.; Brousse, T.; Aurbach, D. Carbon-based composite materials for supercapacitor electrodes: A review. J. Mater. Chem. A 2017, 5, 12653–12672. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, S.; Lou, G.; Shen, Y.; Chen, H.; Shen, Z.; Zhao, S.; Zhang, J.; Chai, S.; Zou, Q. Review of macroporous materials as electrochemical supercapacitor electrodes. J. Mater. Sci. 2017, 52, 11201–11228. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Materiomics 2016, 2, 37–54. [Google Scholar] [CrossRef]
- Vadivel, S.; Naveen, A.; Theerthagiri, J.; Madhavan, J.; Priya, T.S.; Balasubramanian, N. Solvothermal synthesis of BiPO4 nanorods/MWCNT (1D-1D) composite for photocatalyst and supercapacitor applications. Ceram. Int. 2016, 42, 14196–14205. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Jiang, L.; Wu, H.; Wu, C.; Su, J. Effect of aqueous electrolytes on the electrochemical behaviors of supercapacitors based on hierarchically porous carbons. J. Power Sources 2012, 216, 290–296. [Google Scholar] [CrossRef]
- Qiao, J.; Zhong, C.; Deng, Y.; Hu, W.; Sun, D.; Han, X.; Zhang, J. Electrolytes for Electrochemical Supercapacitors; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Theerthagiri, J.; Thiagarajan, K.; Senthilkumar, B.; Khan, Z.; Senthil, R.A.; Arunachalam, P.; Madhavan, J.; Ashokkumar, M. Synthesis of hierarchical cobalt phosphate nanoflakes and their enhanced electrochemical performances for supercapacitor applications. ChemistrySelect 2017, 2, 201–210. [Google Scholar] [CrossRef]
- Senthilkumar, B.; Khan, Z.; Park, S.; Kim, K.; Ko, H.; Kim, Y. Highly porous graphitic carbon and Ni2P2O7 for a high performance aqueous hybrid supercapacitor. J. Mater. Chem. A 2015, 3, 21553–21561. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Kötz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Pang, H.; Wei, C.; Li, X.; Li, G.; Ma, Y.; Li, S.; Chen, J.; Zhang, J. Microwave-assisted synthesis of NiS2 nanostructures for supercapacitors and cocatalytic enhancing photocatalytic H2 production. Sci. Rep. 2014, 4, 3577. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Li, L.; Tan, H.; Cai, R.; Shi, W.; Li, C.; Mhaisalkar, S.G.; Srinivasan, M.; Ramakrishna, S.; Yan, Q. MS2 (M = Co and Ni) hollow spheres with tunable interiors for high-performance supercapacitors and photovoltaics. Adv. Funct. Mater. 2014, 24, 2155–2162. [Google Scholar] [CrossRef]
- Chou, S.-W.; Lin, J.-Y. Cathodic deposition of flaky nickel sulfide nanostructure as an electroactive material for high-performance supercapacitors. J. Electrochem. Soc. 2013, 160, D178–D182. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerasubramani, G.K.; Radhakrishnan, S.; Kim, S.J. One pot hydrothermal growth of hierarchical nanostructured Ni3S2 on Ni foam for supercapacitor application. Chem. Eng. J. 2014, 251, 116–122. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Z.; Ren, L.; Shen, Y.; Qi, X.; Zhong, J. One-pot synthesis of hierarchically nanostructured Ni3S2 dendrites as active materials for supercapacitors. Electrochim. Acta 2014, 149, 316–323. [Google Scholar] [CrossRef]
- Huo, H.; Zhao, Y.; Xu, C. 3D Ni3S2 nanosheet arrays supported on Ni foam for high-performance supercapacitor and non-enzymatic glucose detection. J. Mater. Chem. A 2014, 2, 15111–15117. [Google Scholar] [CrossRef]
- Zhou, W.; Cao, X.; Zeng, Z.; Shi, W.; Zhu, Y.; Yan, Q.; Liu, H.; Wang, J.; Zhang, H. One-step synthesis of Ni3S2 nanorod@Ni(OH)2 nanosheet core–shell nanostructures on a three-dimensional graphene network for high-performance supercapacitors. Energy Environ. Sci. 2013, 6, 2216–2221. [Google Scholar] [CrossRef]
- Shehzad, K.; Xu, Y.; Gao, C.; Duan, X. Three-dimensional macro-structures of two-dimensional nanomaterials. Chem. Soc. Rev. 2016, 45, 5541–5588. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Wu, H.B.; Wang, Y.; Xu, R.; Lou, X.W.D. Formation of 1D hierarchical structures composed of Ni3S2 nanosheets on CNTs backbone for supercapacitors and photocatalytic H2 production. Adv. Energy Mater. 2012, 2, 1497–1502. [Google Scholar] [CrossRef]
- Pan, S.; Zhu, J.; Liu, X. Preparation, electrochemical properties, and adsorption kinetics of Ni3S2/graphene nanocomposites using alkyldithiocarbonatio complexes of nickel(ii) as single-source precursors. New J. Chem. 2013, 37, 654–662. [Google Scholar] [CrossRef]
- Ou, X.; Gan, L.; Luo, Z. Graphene-templated growth of hollow Ni3S2 nanoparticles with enhanced pseudocapacitive performance. J. Mater. Chem. A 2014, 2, 19214–19220. [Google Scholar] [CrossRef]
- Ou, X.; Luo, Z. One-step synthesis of Ni3S2 nanoplatelets on graphene for high performance supercapacitors. RSC Adv. 2016, 6, 10280–10284. [Google Scholar] [CrossRef]
- Xing, Z.; Chu, Q.; Ren, X.; Tian, J.; Asiri, A.M.; Alamry, K.A.; Al-Youbi, A.O.; Sun, X. Biomolecule-assisted synthesis of nickel sulfides/reduced graphene oxide nanocomposites as electrode materials for supercapacitors. Electrochem. Commun. 2013, 32, 9–13. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, C.; Min, S.; Qian, X. A facile one-step route to RGO/Ni3S2 for high-performance supercapacitors. Electrochim. Acta 2014, 144, 100–110. [Google Scholar] [CrossRef]
- Zang, X.; Dai, Z.; Yang, J.; Zhang, Y.; Huang, W.; Dong, X. Template-assisted synthesis of nickel sulfide nanowires: Tuning the compositions for supercapacitors with improved electrochemical stability. ACS Appl. Mater. Interfaces 2016, 8, 24645–24651. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Guan, C.; Gui, Y.; Blackwood, D.J. Rational design of self-supported Ni3S2 nanosheets array for advanced asymmetric supercapacitor with a superior energy density. ACS Appl. Mater. Interfaces 2016, 9, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Stender, C.L.; Odom, T.W. Chemical nanofabrication: A general route to surface-patterned and free-standing transition metal chalcogenide nanostructures. J. Mater. Chem. 2007, 17, 1866–1869. [Google Scholar] [CrossRef]
- Yang, S.-L.; Yao, H.-B.; Gao, M.-R.; Yu, S.-H. Monodisperse cubic pyrite NiS2 dodecahedrons and microspheres synthesized by a solvothermal process in a mixed solvent: Thermal stability and magnetic properties. CrystEngComm 2009, 11, 1383–1390. [Google Scholar] [CrossRef]
- Ruan, Y.; Jiang, J.; Wan, H.; Ji, X.; Miao, L.; Peng, L.; Zhang, B.; Lv, L.; Liu, J. Rapid self-assembly of porous square rod-like nickel persulfide via a facile solution method for high-performance supercapacitors. J. Power Sources 2016, 301, 122–130. [Google Scholar] [CrossRef]
- Dai, S.; Zhao, B.; Qu, C.; Chen, D.; Dang, D.; Song, B.; Fu, J.; Hu, C.; Wong, C.-P.; Liu, M. Controlled synthesis of three-phase NixSy/rGO nanoflake electrodes for hybrid supercapacitors with high energy and power density. Nano Energy 2017, 33, 522–531. [Google Scholar] [CrossRef]
- Xiong, X.; Zhao, B.; Ding, D.; Chen, D.; Yang, C.; Lei, Y.; Liu, M. One-step synthesis of architectural Ni3S2 nanosheet-on-nanorods array for use as high-performance electrodes for supercapacitors. NPG Asia Mater. 2016, 8, 300. [Google Scholar] [CrossRef]
- Qu, C.; Zhang, L.; Meng, W.; Liang, Z.; Zhu, B.; Dang, D.; Dai, S.; Zhao, B.; Tabassum, H.; Gao, S. MOF-derived α-NiS nanorods on graphene as an electrode for high-energy-density supercapacitors. J. Mater. Chem. A 2018, 6, 4003–4012. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, L.; Zhang, L.; Yan, J.; Lu, H.; Fan, W.; Liu, T. Immobilization of NiS nanoparticles on n-doped carbon fiber aerogels as advanced electrode materials for supercapacitors. Nano Res. 2016, 9, 2747–2759. [Google Scholar] [CrossRef]
- Yang, J.; Duan, X.; Qin, Q.; Zheng, W. Solvothermal synthesis of hierarchical flower-like β-NiS with excellent electrochemical performance for supercapacitors. J. Mater. Chem. A 2013, 1, 7880–7884. [Google Scholar] [CrossRef]
- Wang, Z.; Nan, C.; Wang, D.; Li, Y. Fabrication of 1D nickel sulfide nanocrystals with high capacitances and remarkable durability. RSC Adv. 2014, 4, 47513–47516. [Google Scholar] [CrossRef]
- Wei, C.; Cheng, C.; Zhao, J.; Wang, Y.; Cheng, Y.; Xu, Y.; Du, W.; Pang, H. NiS hollow spheres for high-performance supercapacitors and non-enzymatic glucose sensors. Chem. Asian J. 2015, 10, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Ma, M.; Yang, J.; Zhang, Y.; Chen, P.; Huang, W.; Dong, X. Phase-controlled synthesis of α-NiS nanoparticles confined in carbon nanorods for high performance supercapacitors. Sci. Rep. 2014, 4, 7054. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yang, B.; Liu, Q.; Liu, J.; Wang, X.; Song, D.; Wang, J.; Jing, X. Interconnected NiS nanosheets supported by nickel foam: Soaking fabrication and supercapacitors application. J. Electroanal. Chem. 2015, 739, 156–163. [Google Scholar] [CrossRef]
- Tran, V.C.; Sahoo, S.; Shim, J.-J. Room-temperature synthesis of NiS hollow spheres on nickel foam for high-performance supercapacitor electrodes. Mater. Lett. 2018, 210, 105–108. [Google Scholar] [CrossRef]
- Li, Z.; Yu, X.; Gu, A.; Tang, H.; Wang, L.; Lou, Z. Anion exchange strategy to synthesis of porous NiS hexagonal nanoplates for supercapacitors. Nanotechnology 2017, 28, 065406. [Google Scholar] [CrossRef] [PubMed]
- Jothi, P.R.; Salunkhe, R.R.; Pramanik, M.; Kannan, S.; Yamauchi, Y. Surfactant-assisted synthesis of nanoporous nickel sulfide flakes and their hybridization with reduced graphene oxides for supercapacitor applications. RSC Adv. 2016, 6, 21246–21253. [Google Scholar] [CrossRef]
- LiáZhang, L. Rigid three-dimensional Ni3S4 nanosheet frames: Controlled synthesis and their enhanced electrochemical performance. RSC Adv. 2015, 5, 8422–8426. [Google Scholar]
- Huang, F.; Yan, A.; Sui, Y.; Wei, F.; Qi, J.; Meng, Q.; He, Y. One-step hydrothermal synthesis of Ni3S4@MoS2 nanosheet on carbon fiber paper as a binder-free anode for supercapacitor. J. Mater. Sci. Mater. Electron. 2017, 28, 12747–12754. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, W.; Rui, X.; Li, B.; Tan, H.T.; Guo, G.; Madhavi, S.; Zong, Y.; Yan, Q. One-pot synthesis of tunable crystalline Ni3S4@amorphous MoS2 core/shell nanospheres for high-performance supercapacitors. Small 2015, 11, 3694–3702. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhang, G.; Cui, Y.; Sun, Y.; Qin, Q.; Zhang, J.; Zheng, W. One-step extended strategy for the ionic liquid-assisted synthesis of Ni3S4–MoS2 heterojunction electrodes for supercapacitors. J. Mater. Chem. A 2017, 5, 11278–11285. [Google Scholar] [CrossRef]
- Li, S.; Chen, T.; Wen, J.; Gui, P.; Fang, G. In situ grown Ni9S8 nanorod/O-MoS2 nanosheet nanocomposite on carbon cloth as a free binder supercapacitor electrode and hydrogen evolution catalyst. Nanotechnology 2017, 28, 445407. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Ramirez, P.V.; Arenas-Arrocena, M.C.; Santos-Cruz, J.; Vega-González, M.; Martínez-Alvarez, O.; Castaño-Meneses, V.M.; Acosta-Torres, L.S.; de la Fuente-Hernández, J. Growth evolution and phase transition from chalcocite to digenite in nanocrystalline copper sulfide: Morphological, optical and electrical properties. Beilstein J. Nanotechnol. 2014, 5, 1542. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.T. Copper coordination in low chalcocite and djurleite and other copper-rich sulfides. Am. Mineral. 1981, 66, 807–818. [Google Scholar]
- Zhu, T.; Xia, B.; Zhou, L.; Lou, X.W.D. Arrays of ultrafine CuS nanoneedles supported on a CNT backbone for application in supercapacitors. J. Mater. Chem. 2012, 22, 7851–7855. [Google Scholar] [CrossRef]
- Ghezelbash, A.; Korgel, B.A. Nickel sulfide and copper sulfide nanocrystal synthesis and polymorphism. Langmuir 2005, 21, 9451–9456. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-J.; Zhang, J.-Z.; Fan, Y. One-step solvothermal synthesis of different morphologies CuS nanosheets compared as supercapacitor electrode materials. J. Alloys Compd. 2015, 625, 158–163. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerasubramani, G.K.; Rao, A.N.; Kim, S.J. One-pot hydrothermal synthesis, characterization and electrochemical properties of CuS nanoparticles towards supercapacitor applications. Mater. Res. Express 2014, 1, 035006. [Google Scholar] [CrossRef]
- Heydari, H.; Moosavifard, S.E.; Elyasi, S.; Shahraki, M. Nanoporous CuS nano-hollow spheres as advanced material for high-performance supercapacitors. Appl. Surf. Sci. 2017, 394, 425–430. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Kim, B.-S.; Hong, J.; Choi, H.; Jang, H.-S.; Hou, B.; Pak, S.; Lee, J.; Lee, S.-H.; Morris, S.M. Hierarchically assembled tubular shell-core-shell heterostructure of hybrid transition metal chalcogenides for high-performance supercapacitors with ultrahigh cyclability. Nano Energy 2017, 37, 15–23. [Google Scholar] [CrossRef]
- Peng, H.; Ma, G.; Mu, J.; Sun, K.; Lei, Z. Controllable synthesis of CuS with hierarchical structures via a surfactant-free method for high-performance supercapacitors. Mater. Lett. 2014, 122, 25–28. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerasubramani, G.K.; Radhakrishnan, S.; Kim, S.J. Preparation of copper sulfide nanoparticles by sonochemical method and study on their electrochemical properties. J. Nanosci. Nanotechnol. 2015, 15, 4409–4413. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-J.; Zhang, J.-Z.; Liu, Y.; Liu, Y.-M. Synthesis of reduced graphene oxide wrapped-copper sulfide hollow spheres as electrode material for supercapacitor. Int. J. Hydrogen Energy 2015, 40, 10158–10167. [Google Scholar] [CrossRef]
- Huang, K.-J.; Zhang, J.-Z.; Xing, K. One-step synthesis of layered CuS/multi-walled carbon nanotube nanocomposites for supercapacitor electrode material with ultrahigh specific capacitance. Electrochim. Acta 2014, 149, 28–33. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, X.; Wang, W.; Cheng, J.; Yan, H.; Tang, C.; Kim, J.-K.; Luo, Y. Hierarchical, porous CuS microspheres integrated with carbon nanotubes for high-performance supercapacitors. Sci. Rep. 2015, 5, 16584. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Ma, G.; Sun, K.; Mu, J.; Wang, H.; Lei, Z. High-performance supercapacitor based on multi-structural CuS@polypyrrole composites prepared by in situ oxidative polymerization. J. Mater. Chem. A 2014, 2, 3303–3307. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Kim, B.-S.; Hong, J.; Lee, J.; Pak, S.; Jang, H.-S.; Whang, D.; Cha, S.; Sohn, J.I.; Kim, J.M. A pseudo-capacitive chalcogenide-based electrode with dense 1-dimensional nanoarrays for enhanced energy density in asymmetric supercapacitors. J. Mater. Chem. A 2016, 4, 10084–10090. [Google Scholar] [CrossRef]
- Huang, K.-J.; Zhang, J.-Z.; Jia, Y.-L.; Xing, K.; Liu, Y.-M. Acetylene black incorporated layered copper sulfide nanosheets for high-performance supercapacitor. J. Alloys Compd. 2015, 641, 119–126. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Q.; Peng, C. Facile synthesis of core-shell structured CuS@PANI microspheres and electrochemical capacitance investigations. Polym. Polym. Compos. 2017, 25, 483–488. [Google Scholar]
- Gopi, C.V.M.; Ravi, S.; Rao, S.S.; Reddy, A.E.; Kim, H.-J. Carbon nanotube/metal-sulfide composite flexible electrodes for high-performance quantum dot-sensitized solar cells and supercapacitors. Sci. Rep. 2017, 7, 46519. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Ji, X.; Jiang, J.; Yu, J.; Miao, L.; Zhang, L.; Bie, S.; Chen, H.; Ruan, Y. Hydrothermal synthesis of cobalt sulfide nanotubes: The size control and its application in supercapacitors. J. Power Sources 2013, 243, 396–402. [Google Scholar] [CrossRef]
- Meng, X.; Sun, H.; Zhu, J.; Bi, H.; Han, Q.; Liu, X.; Wang, X. Graphene-based cobalt sulfide composite hydrogel with enhanced electrochemical properties for supercapacitors. New J. Chem. 2016, 40, 2843–2849. [Google Scholar] [CrossRef]
- Xing, J.-C.; Zhu, Y.-L.; Li, M.-Y.; Jiao, Q.-J. Hierarchical mesoporous CoS2 microspheres: Morphology-controlled synthesis and their superior pseudocapacitive properties. Electrochim. Acta 2014, 149, 285–292. [Google Scholar] [CrossRef]
- Zhang, Y.; Sui, Y.; Qi, J.; Hou, P.; Wei, F.; He, Y.; Meng, Q.; Sun, Z. Facile synthesis of NiCo2S4 spheres with granular core used as supercapacitor electrode materials. J. Mater. Sci. Mater. Electron. 2017, 28, 5686–5695. [Google Scholar] [CrossRef]
- Pu, J.; Cui, F.; Chu, S.; Wang, T.; Sheng, E.; Wang, Z. Preparation and electrochemical characterization of hollow hexagonal NiCo2S4 nanoplates as pseudocapacitor materials. ACS Sustain. Chem. Eng. 2013, 2, 809–815. [Google Scholar] [CrossRef]
- Thangappan, R.; Kalaiselvam, S.; Elayaperumal, A.; Jayavel, R.; Arivanandhan, M.; Karthikeyan, R.; Hayakawa, Y. Graphene decorated with MoS2 nanosheets: A synergetic energy storage composite electrode for supercapacitor applications. Dalton Trans. 2016, 45, 2637–2646. [Google Scholar] [CrossRef] [PubMed]
- Raut, S.S.; Dhobale, J.A.; Sankapal, B.R. Silar deposited Bi2S3 thin film towards electrochemical supercapacitor. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 87, 209–212. [Google Scholar] [CrossRef]
- Fang, L.; Qiu, Y.; Zhai, T.; Wang, F.; Lan, M.; Huang, K.; Jing, Q. Flower-like nanoarchitecture assembled from Bi2S3 nanorod/MoS2 nanosheet heterostructures for high-performance supercapacitor electrodes. Colloids Surf. A Physicochem. Eng. Aspects 2017, 535, 41–48. [Google Scholar] [CrossRef]
- Patil, S.; Kumbhar, V.; Patil, B.; Bulakhe, R.; Lokhande, C. Chemical synthesis of α-La2S3 thin film as an advanced electrode material for supercapacitor application. J. Alloys Compd. 2014, 611, 191–196. [Google Scholar] [CrossRef]
- Ratha, S.; Rout, C.S. Supercapacitor electrodes based on layered tungsten disulfide-reduced graphene oxide hybrids synthesized by a facile hydrothermal method. ACS Appl. Mater. Interfaces 2013, 5, 11427–11433. [Google Scholar] [CrossRef] [PubMed]
- Otero-Leal, M.; Rivadulla, F.; Rivas, J. The magnetic phase transition of CoS2−xSex. IEEE Trans. Magn. 2008, 44, 4503–4505. [Google Scholar] [CrossRef]
- Sadjadi, M.; Pourahmad, A.; Sohrabnezhad, S.; Zare, K. Formation of NiS and CoS semiconductor nanoparticles inside mordenite-type zeolite. Mater. Lett. 2007, 61, 2923–2926. [Google Scholar] [CrossRef]
- Behret, H.; Binder, H.; Sandstede, G. Electrocatalytic oxygen reduction with thiospinels and other sulphides of transition metals. Electrochim. Acta 1975, 20, 111–117. [Google Scholar] [CrossRef]
- Sohrabnezhad, S.; Pourahmad, A.; Radaee, E. Photocatalytic degradation of basic blue 9 by CoS nanoparticles supported on ALMCM-41 material as a catalyst. J. Hazard. Mater. 2009, 170, 184–190. [Google Scholar] [CrossRef] [PubMed]
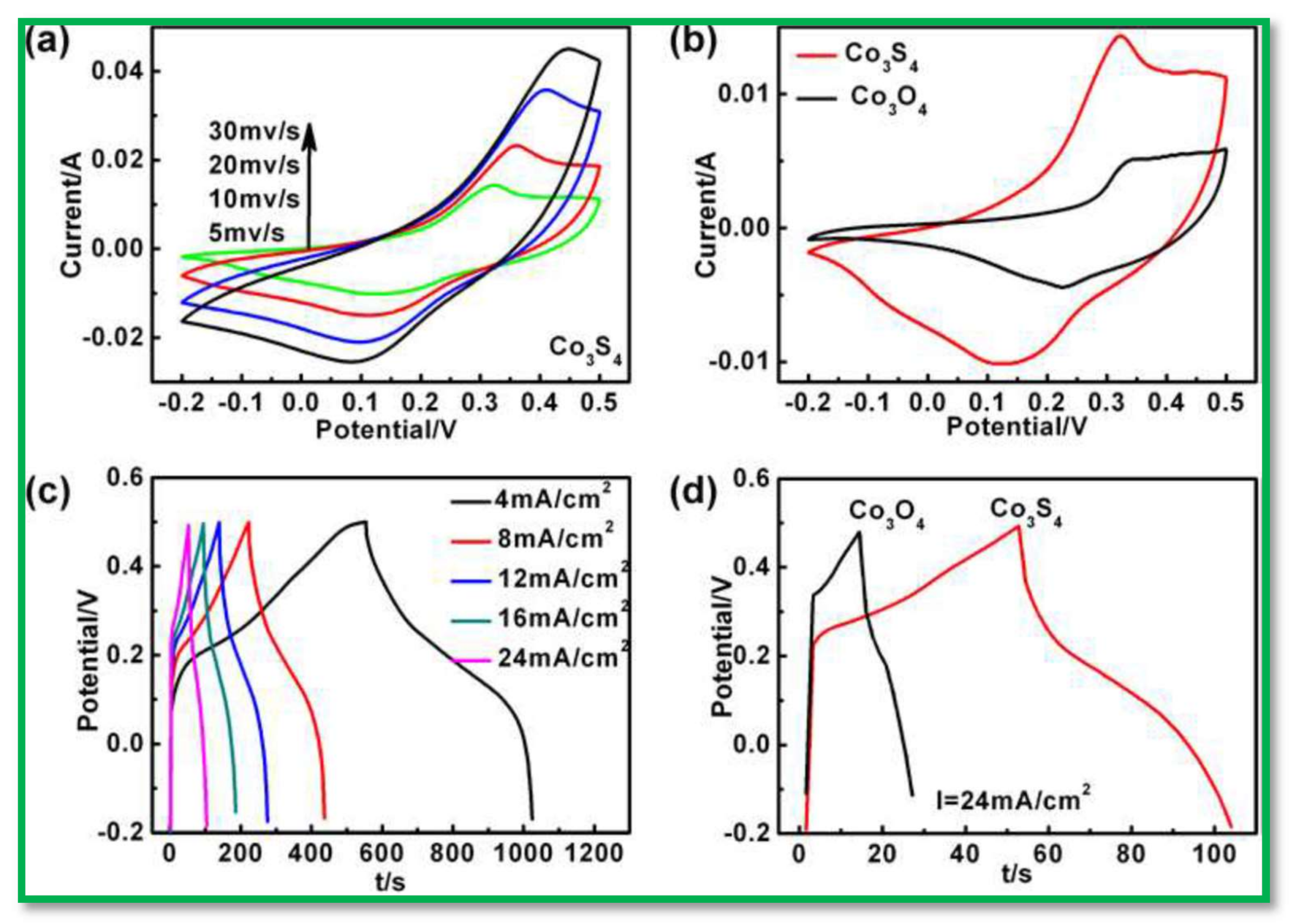
- Chen, Q.; Li, H.; Cai, C.; Yang, S.; Huang, K.; Wei, X.; Zhong, J. In situ shape and phase transformation synthesis of Co3S4 nanosheet arrays for high-performance electrochemical supercapacitors. RSC Adv. 2013, 3, 22922–22926. [Google Scholar] [CrossRef]
- Patil, S.; Kim, J.; Lee, D. Graphene-nanosheet wrapped cobalt sulphide as a binder free hybrid electrode for asymmetric solid-state supercapacitor. J. Power Sources 2017, 342, 652–665. [Google Scholar] [CrossRef]
- Luo, F.; Li, J.; Yuan, H.; Xiao, D. Rapid synthesis of three-dimensional flower-like cobalt sulfide hierarchitectures by microwave assisted heating method for high-performance supercapacitors. Electrochim. Acta 2014, 123, 183–189. [Google Scholar] [CrossRef]
- Huang, K.-J.; Zhang, J.-Z.; Shi, G.-W.; Liu, Y.-M. One-step hydrothermal synthesis of two-dimensional cobalt sulfide for high-performance supercapacitors. Mater. Lett. 2014, 131, 45–48. [Google Scholar] [CrossRef]
- Justin, P.; Rao, G.R. CoS spheres for high-rate electrochemical capacitive energy storage application. Int. J. Hydrogen Energy 2010, 35, 9709–9715. [Google Scholar] [CrossRef]
- Wang, Q.; Jiao, L.; Han, Y.; Du, H.; Peng, W.; Huan, Q.; Song, D.; Si, Y.; Wang, Y.; Yuan, H. CoS2 hollow spheres: Fabrication and their application in lithium-ion batteries. J. Phys. Chem. C 2011, 115, 8300–8304. [Google Scholar] [CrossRef]
- Ranaweera, C.; Wang, Z.; Alqurashi, E.; Kahol, P.; Dvornic, P.; Gupta, B.K.; Ramasamy, K.; Mohite, A.D.; Gupta, G.; Gupta, R.K. Highly stable hollow bifunctional cobalt sulfides for flexible supercapacitors and hydrogen evolution. J. Mater. Chem. A 2016, 4, 9014–9018. [Google Scholar] [CrossRef]
- Subramani, K.; Sudhan, N.; Divya, R.; Sathish, M. All-solid-state asymmetric supercapacitors based on cobalt hexacyanoferrate-derived CoS and activated carbon. RSC Adv. 2017, 7, 6648–6659. [Google Scholar] [CrossRef]
- Ray, R.S.; Sarma, B.; Jurovitzki, A.L.; Misra, M. Fabrication and characterization of titania nanotube/cobalt sulfide supercapacitor electrode in various electrolytes. Chem. Eng. J. 2015, 260, 671–683. [Google Scholar] [CrossRef]
- Wang, Q.; Jiao, L.; Du, H.; Yang, J.; Huan, Q.; Peng, W.; Si, Y.; Wang, Y.; Yuan, H. Facile synthesis and superior supercapacitor performances of three-dimensional cobalt sulfide hierarchitectures. CrystEngComm 2011, 13, 6960–6963. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Tai, S.-Y.; Chou, S.-W. Bifunctional one-dimensional hierarchical nanostructures composed of cobalt sulfide nanoclusters on carbon nanotubes backbone for dye-sensitized solar cells and supercapacitors. J. Phys. Chem. C 2013, 118, 823–830. [Google Scholar] [CrossRef]
- Liu, S.; Mao, C.; Niu, Y.; Yi, F.; Hou, J.; Lu, S.; Jiang, J.; Xu, M.; Li, C. Facile synthesis of novel networked ultralong cobalt sulfide nanotubes and its application in supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 25568–25573. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, B.; Wang, L.; Yuan, Y.; Wang, D. A facile hydrothermal synthesis of a reduced graphene oxide modified cobalt disulfide composite electrode for high-performance supercapacitors. RSC Adv. 2016, 6, 7129–7138. [Google Scholar] [CrossRef]
- Pujari, R.; Lokhande, A.; Kim, J.; Lokhande, C. Bath temperature controlled phase stability of hierarchical nanoflakes CoS2 thin films for supercapacitor application. RSC Adv. 2016, 6, 40593–40601. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.B.; Lou, X.W.D. Unusual CoS2 ellipsoids with anisotropic tube-like cavities and their application in supercapacitors. Chem. Commun. 2012, 48, 6912–6914. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Faber, M.S.; Dziedzic, R.; Wen, Z.; Jin, S.; Mao, S.; Chen, J. Metallic CoS2 nanowire electrodes for high cycling performance supercapacitors. Nanotechnology 2015, 26, 494001. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.-C.; Zhu, Y.-L.; Zhou, Q.-W.; Zheng, X.-D.; Jiao, Q.-J. Fabrication and shape evolution of CoS2 octahedrons for application in supercapacitors. Electrochim. Acta 2014, 136, 550–556. [Google Scholar] [CrossRef]
- Zeng, X.; Yang, B.; Li, X.; Yu, R. Three-dimensional hollow CoS2 nanoframes fabricated by anion replacement and their enhanced pseudocapacitive performances. Electrochim. Acta 2017, 240, 341–349. [Google Scholar] [CrossRef]
- Wei, T.Y.; Chen, C.H.; Chien, H.C.; Lu, S.Y.; Hu, C.C. A cost-effective supercapacitor material of ultrahigh specific capacitances: Spinel nickel cobaltite aerogels from an epoxide-driven sol–gel process. Adv. Mater. 2010, 22, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Shen, J.; Li, N.; Ye, M. A free template strategy for the synthesis of CoS2-reduced graphene oxide nanocomposite with enhanced electrode performance for supercapacitors. Ceram. Int. 2014, 40, 15411–15419. [Google Scholar] [CrossRef]
- Su, C.; Xiang, J.; Wen, F.; Song, L.; Mu, C.; Xu, D.; Hao, C.; Liu, Z. Microwave synthesized three-dimensional hierarchical nanostructure CoS2/MoS2 growth on carbon fiber cloth: A bifunctional electrode for hydrogen evolution reaction and supercapacitor. Electrochim. Acta 2016, 212, 941–949. [Google Scholar] [CrossRef]
- Ko, Y.N.; Choi, S.H.; Park, S.B.; Kang, Y.C. Preparation of yolk-shell and filled Co9S8 microspheres and comparison of their electrochemical properties. Chem. Asian J. 2014, 9, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, D.; Xu, H.; Liu, S.; Yang, J.; Qian, Y. Multiwalled carbon nanotube@a-C@Co9S8 nanocomposites: A high-capacity and long-life anode material for advanced lithium ion batteries. Nanoscale 2015, 7, 3520–3525. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Du, G.; Zhang, J.; Zhong, Y.; Xu, B.; Yang, Y.; Neupane, S.; Li, W. In situ transmission electron microscopy observation of electrochemical sodiation of individual Co9S8-filled carbon nanotubes. ACS Nano 2014, 8, 3620–3627. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, D.; Xu, H.; Feng, J.; Jiang, X.; Yue, J.; Yang, J.; Qian, Y. Hollow nanospheres of mesoporous Co9S8 as a high-capacity and long-life anode for advanced lithium ion batteries. Nano Energy 2015, 12, 528–537. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.; Meng, L.; Wang, H.; Bi, W.; Peng, Y.; Yao, T.; Wei, S.; Xie, Y. In-plane coassembly route to atomically thick inorganic–organic hybrid nanosheets. ACS Nano 2013, 7, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ye, M.; Zhang, N.; Wen, X.; Zheng, D.; Lin, C. Preparation of hollow Co9S8 nanoneedle arrays as effective counter electrodes for quantum dot-sensitized solar cells. J. Mater. Chem. A 2015, 3, 6311–6314. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, D.; Xiong, X.; Song, B.; Hu, R.; Zhang, Q.; Rainwater, B.H.; Waller, G.H.; Zhen, D.; Ding, Y. A high-energy, long cycle-life hybrid supercapacitor based on graphene composite electrodes. Energy Storage Mater. 2017, 7, 32–39. [Google Scholar] [CrossRef]
- Deng, X.; Zhao, B.; Zhu, L.; Shao, Z. Molten salt synthesis of nitrogen-doped carbon with hierarchical pore structures for use as high-performance electrodes in supercapacitors. Carbon 2015, 93, 48–58. [Google Scholar] [CrossRef]
- Li, H.; Gao, Y.; Shao, Y.; Su, Y.; Wang, X. Vapor-phase atomic layer deposition of Co9S8 and its application for supercapacitors. Nano Lett. 2015, 15, 6689–6695. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Saranya, M.; Santhosh, C.; Velmurugan, V.; Raghupathy, B.P.; Jeong, S.K.; Grace, A.N. Co9S8 nanoflakes on graphene (Co9S8/G) nanocomposites for high performance supercapacitors. RSC Adv. 2014, 4, 21151–21162. [Google Scholar] [CrossRef]
- Masikhwa, T.M.; Madito, M.J.; Bello, A.; Lekitima, J.; Manyala, N. Microwave-assisted synthesis of cobalt sulphide nanoparticle clusters on activated graphene foam for electrochemical supercapacitors. RSC Adv. 2017, 7, 20231–20240. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Zhao, C.; Min, S.; Qian, X. One-step hydrothermal synthesis of 3D petal-like Co9S8/RGO/Ni3S2 composite on nickel foam for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 4861–4868. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Pan, L.; Hu, H.; Zhao, S. Co9S8 nanotubes synthesized on the basis of nanoscale kirkendall effect and their magnetic and electrochemical properties. CrystEngComm 2010, 12, 1899–1904. [Google Scholar] [CrossRef]
- Du, W.; Zhu, Z.; Wang, Y.; Liu, J.; Yang, W.; Qian, X.; Pang, H. One-step synthesis of CoNi2S4 nanoparticles for supercapacitor electrodes. RSC Adv. 2014, 4, 6998–7002. [Google Scholar] [CrossRef]
- Xia, C.; Alshareef, H.N. Self-templating scheme for the synthesis of nanostructured transition-metal chalcogenide electrodes for capacitive energy storage. Chem. Mater. 2015, 27, 4661–4668. [Google Scholar] [CrossRef]
- Hua, H.; Liu, S.; Chen, Z.; Bao, R.; Shi, Y.; Hou, L.; Pang, G.; Hui, K.N.; Zhang, X.; Yuan, C. Self-sacrifice template formation of hollow hetero-Ni7S6/Co3S4 nanoboxes with intriguing pseudo-capacitance for high-performance electrochemical capacitors. Sci. Rep. 2016, 6, 20973. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Pu, X.; Ji, X.; Zhu, Y.; Jing, M.; Chen, Q.; Jiao, F. High energy density asymmetric supercapacitors from mesoporous NiCo2S4 nanosheets. Electrochim. Acta 2015, 174, 238–245. [Google Scholar] [CrossRef]
- Sun, M.; Tie, J.; Cheng, G.; Lin, T.; Peng, S.; Deng, F.; Ye, F.; Yu, L. In situ growth of burl-like nickel cobalt sulfide on carbon fibers as high-performance supercapacitors. J. Mater. Chem. A 2015, 3, 1730–1736. [Google Scholar] [CrossRef]
- Chen, Y.M.; Li, Z.; Lou, X.W.D. General formation of MxCo3−xS4 (M = Ni, Mn, Zn) hollow tubular structures for hybrid supercapacitors. Angew. Chem. 2015, 127, 10667–10670. [Google Scholar] [CrossRef]
- Sahoo, S.; Rout, C.S. Facile electrochemical synthesis of porous manganese-cobalt-sulfide based ternary transition metal sulfide nanosheets architectures for high performance energy storage applications. Electrochim. Acta 2016, 220, 57–66. [Google Scholar] [CrossRef]
- Yu, M.; Li, X.; Ma, Y.; Liu, R.; Liu, J.; Li, S. Nanohoneycomb-like manganese cobalt sulfide/three dimensional graphene-nickel foam hybid electrodes for high-rate capability supercapacitors. Appl. Surf. Sci. 2017, 396, 1816–1824. [Google Scholar] [CrossRef]
- Liu, S.; Kim, K.H.; Yun, J.M.; Kundu, A.; Sankar, K.V.; Patil, U.M.; Ray, C.; Jun, S.C. 3D yolk–shell NiGa2S4 microspheres confined with nanosheets for high performance supercapacitors. J. Mater. Chem. A 2017, 5, 6292–6298. [Google Scholar] [CrossRef]
- Chen, J.; Li, S.-L.; Xu, Q.; Tanaka, K. Synthesis of open-ended MoS2 nanotubes and the application as the catalyst of methanation. Chem. Commun. 2002, 0, 1722–1723. [Google Scholar] [CrossRef]
- Chen, J.; Kuriyama, N.; Yuan, H.; Takeshita, H.T.; Sakai, T. Electrochemical hydrogen storage in MoS2 nanotubes. J. Am. Chem. Soc. 2001, 123, 11813–11814. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Chen, J.S.; Lou, X.W.D. Glucose-assisted growth of MoS2 nanosheets on CNT backbone for improved lithium storage properties. Chem. Eur. J. 2011, 17, 13142–13145. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Peng, H.; Mu, J.; Huang, H.; Zhou, X.; Lei, Z. In situ intercalative polymerization of pyrrole in graphene analogue of MoS2 as advanced electrode material in supercapacitor. J. Power Sources 2013, 229, 72–78. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, H.; Qu, J.; Li, J. Two-dimensional layered MoS2: Rational design, properties and electrochemical applications. Energy Environ. Sci. 2016, 9, 1190–1209. [Google Scholar] [CrossRef]
- Zheng, N.; Bu, X.; Feng, P. Synthetic design of crystalline inorganic chalcogenides exhibiting fast-ion conductivity. Nature 2003, 426, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, H.; Li, J. Graphene and graphene-like layered transition metal dichalcogenides in energy conversion and storage. Small 2014, 10, 2165–2181. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Yang, S.; Gao, W.; Liu, Z.; Gong, Y.; Ma, L.; Shi, G.; Lei, S.; Zhang, Y.; Zhang, S. Direct laser-patterned micro-supercapacitors from paintable MoS2 films. Small 2013, 9, 2905–2910. [Google Scholar] [CrossRef] [PubMed]
- Ramadoss, A.; Kim, T.; Kim, G.-S.; Kim, S.J. Enhanced activity of a hydrothermally synthesized mesoporous MoS2 nanostructure for high performance supercapacitor applications. New J. Chem. 2014, 38, 2379–2385. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerasubramani, G.K.; Radhakrishnan, S.; Kim, S.J. Supercapacitive properties of hydrothermally synthesized sphere like MoS2 nanostructures. Mater. Res. Bull. 2014, 50, 499–502. [Google Scholar] [CrossRef]
- Ilanchezhiyan, P.; Kumar, G.M.; Kang, T. Electrochemical studies of spherically clustered MoS2 nanostructures for electrode applications. J. Alloys Compd. 2015, 634, 104–108. [Google Scholar] [CrossRef]
- Acerce, M.; Voiry, D.; Chhowalla, M. Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials. Nat. Nanotechnol. 2015, 10, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Soon, J.M.; Loh, K.P. Electrochemical double-layer capacitance of MoS2 nanowall films. Electrochem. Solid-State Lett. 2007, 10, A250–A254. [Google Scholar] [CrossRef]
- Pujari, R.; Lokhande, A.; Shelke, A.; Kim, J.; Lokhande, C. Chemically deposited nano grain composed MoS2 thin films for supercapacitor application. J. Colloid Interface Sci. 2017, 496, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Pazhamalai, P.; Veerasubramani, G.K.; Kim, S.J. Mechanically delaminated few layered MoS2 nanosheets based high performance wire type solid-state symmetric supercapacitors. J. Power Sources 2016, 321, 112–119. [Google Scholar] [CrossRef]
- Huang, K.-J.; Wang, L.; Liu, Y.-J.; Wang, H.-B.; Liu, Y.-M.; Wang, L.-L. Synthesis of polyaniline/2-dimensional graphene analog MoS2 composites for high-performance supercapacitor. Electrochim. Acta 2013, 109, 587–594. [Google Scholar] [CrossRef]
- Falola, B.D.; Wiltowski, T.; Suni, I.I. Electrodeposition of MoS2 for charge storage in electrochemical supercapacitors. J. Electrochem. Soc. 2016, 163, D568–D574. [Google Scholar] [CrossRef]
- Huang, K.-J.; Wang, L.; Liu, Y.-J.; Liu, Y.-M.; Wang, H.-B.; Gan, T.; Wang, L.-L. Layered MoS2–graphene composites for supercapacitor applications with enhanced capacitive performance. Int. J. Hydrogen Energy 2013, 38, 14027–14034. [Google Scholar] [CrossRef]
- Bissett, M.A.; Kinloch, I.A.; Dryfe, R.A. Characterization of MoS2–graphene composites for high-performance coin cell supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 17388–17398. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, K.; Pramoda, K.; Maitra, U.; Mahima, U.; Shah, M.; Rao, C. Performance of MoS2-reduced graphene oxide nanocomposites in supercapacitors and in oxygen reduction reaction. Nanomater. Energy 2015, 4, 9–17. [Google Scholar] [CrossRef]
- Xie, B.; Chen, Y.; Yu, M.; Sun, T.; Lu, L.; Xie, T.; Zhang, Y.; Wu, Y. Hydrothermal synthesis of layered molybdenum sulfide/n-doped graphene hybrid with enhanced supercapacitor performance. Carbon 2016, 99, 35–42. [Google Scholar] [CrossRef]
- Masikhwa, T.M.; Madito, M.J.; Bello, A.; Dangbegnon, J.K.; Manyala, N. High performance asymmetric supercapacitor based on molybdenum disulphide/graphene foam and activated carbon from expanded graphite. J. Colloid Interface Sci. 2017, 488, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Patil, U.M.; Nam, M.S.; Kang, S.; Sohn, J.S.; Sim, H.B.; Kang, S.; Jun, S.C. Fabrication of ultra-high energy and power asymmetric supercapacitors based on hybrid 2D MoS2/graphene oxide composite electrodes: A binder-free approach. RSC Adv. 2016, 6, 43261–43271. [Google Scholar] [CrossRef]
- Clerici, F.; Fontana, M.; Bianco, S.; Serrapede, M.; Perrucci, F.; Ferrero, S.; Tresso, E.; Lamberti, A. In situ MoS2 decoration of laser-induced graphene as flexible supercapacitor electrodes. ACS Appl. Mater. Interfaces 2016, 8, 10459–10465. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.; Ghosh, D.; Kalra, S.; Das, C. High performance supercapacitor electrode material based on flower like MoS2/reduced graphene oxide nanocomposite. Int. J. Lat. Res. Sci. Technol. 2014, 3, 65. [Google Scholar]
- Huang, K.-J.; Wang, L.; Zhang, J.-Z.; Wang, L.-L.; Mo, Y.-P. One-step preparation of layered molybdenum disulfide/multi-walled carbon nanotube composites for enhanced performance supercapacitor. Energy 2014, 67, 234–240. [Google Scholar] [CrossRef]
- Hu, B.; Qin, X.; Asiri, A.M.; Alamry, K.A.; Al-Youbi, A.O.; Sun, X. Synthesis of porous tubular C/MoS2 nanocomposites and their application as a novel electrode material for supercapacitors with excellent cycling stability. Electrochim. Acta 2013, 100, 24–28. [Google Scholar] [CrossRef]
- Fan, L.-Q.; Liu, G.-J.; Zhang, C.-Y.; Wu, J.-H.; Wei, Y.-L. Facile one-step hydrothermal preparation of molybdenum disulfide/carbon composite for use in supercapacitor. Int. J. Hydrogen Energy 2015, 40, 10150–10157. [Google Scholar] [CrossRef]
- Kumuthini, R.; Ramachandran, R.; Therese, H.; Wang, F. Electrochemical properties of electrospun MoS2@C nanofiber as electrode material for high-performance supercapacitor application. J. Alloys Compd. 2017, 705, 624–630. [Google Scholar] [CrossRef]
- Nam, M.S.; Patil, U.; Park, B.; Sim, H.B.; Jun, S.C. A binder free synthesis of 1D PANI and 2D MoS2 nanostructured hybrid composite electrodes by the electrophoretic deposition (EPD) method for supercapacitor application. RSC Adv. 2016, 6, 101592–101601. [Google Scholar] [CrossRef]
- Thakur, A.K.; Choudhary, R.B.; Majumder, M.; Gupta, G.; Shelke, M.V. Enhanced electrochemical performance of polypyrrole coated MoS2 nanocomposites as electrode material for supercapacitor application. J. Electroanal. Chem. 2016, 782, 278–287. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerasubramani, G.K.; Pazhamalai, P.; Kim, S.J. Designing two dimensional nanoarchitectured MoS2 sheets grown on Mo foil as a binder free electrode for supercapacitors. Electrochim. Acta 2016, 190, 305–312. [Google Scholar] [CrossRef]
- Wu, T.; Zhou, X.; Zhang, H.; Zhong, X. Bi2S3 nanostructures: A new photocatalyst. Nano Res. 2010, 3, 379–386. [Google Scholar] [CrossRef]
- Zhao, H.; Tian, F.; Wang, R.; Chen, R. A review on bismuth-related nanomaterials for photocatalysis. Rev. Adv. Sci. Eng. 2014, 3, 3–27. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, Q.; Zhang, Q.; Ding, M.; Huang, J.; Liu, X.; Liu, Y.; Wu, X.; Xu, X. Controlled assembly of Bi2S3 architectures as Schottky diode, supercapacitor electrodes and highly efficient photocatalysts. RSC Adv. 2014, 4, 41636–41641. [Google Scholar] [CrossRef]
- Patil, S.J.; Lokhande, C.D. Fabrication and performance evaluation of rare earth lanthanum sulfide film for supercapacitor application: Effect of air annealing. Mater. Des. 2015, 87, 939–948. [Google Scholar] [CrossRef]
- Han, D.; Jing, X.; Wang, J.; Yang, P.; Song, D.; Liu, J. Porous lanthanum doped NiO microspheres for supercapacitor application. J. Electroanal. Chem. 2012, 682, 37–44. [Google Scholar] [CrossRef]
- Bagde, G.; Sartale, S.; Lokhande, C. Deposition and annealing effect on lanthanum sulfide thin films by spray pyrolysis. Thin Solid Films 2003, 445, 1–6. [Google Scholar] [CrossRef]
- Patil, S.; Lokhande, A.; Lokhande, C. Effect of aqueous electrolyte on pseudocapacitive behavior of chemically synthesized La2S3 electrode. Mater. Sci. Semicond. Process. 2016, 41, 132–136. [Google Scholar] [CrossRef]
- Tu, C.-C.; Lin, L.-Y.; Xiao, B.-C.; Chen, Y.-S. Highly efficient supercapacitor electrode with two-dimensional tungsten disulfide and reduced graphene oxide hybrid nanosheets. J. Power Sources 2016, 320, 78–85. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Ambrosi, A.; Eng, A.Y.S.; Sofer, Z.; Pumera, M. Transition metal dichalcogenides (MoS2, MoSe2, WS2 and WSe2) exfoliation technique has strong influence upon their capacitance. Electrochem. Commun. 2015, 56, 24–28. [Google Scholar] [CrossRef]
- Bissett, M.A.; Worrall, S.D.; Kinloch, I.A.; Dryfe, R.A. Comparison of two-dimensional transition metal dichalcogenides for electrochemical supercapacitors. Electrochim. Acta 2016, 201, 30–37. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.-Z.; Fu, Z.-W. Lithium electrochemistry of NiSe2: A new kind of storage energy material. Electrochem. Commun. 2006, 8, 1855–1862. [Google Scholar] [CrossRef]
- Wang, S.; Li, W.; Xin, L.; Wu, M.; Long, Y.; Huang, H.; Lou, X. Facile synthesis of truncated cube-like NiSe2 single crystals for high-performance asymmetric supercapacitors. Chem. Eng. J. 2017, 330, 1334–1341. [Google Scholar] [CrossRef]
- Arul, N.S.; Han, J.I. Facile hydrothermal synthesis of hexapod-like two dimensional dichalcogenide NiSe2 for supercapacitor. Mater. Lett. 2016, 181, 345–349. [Google Scholar] [CrossRef]
- Yu, B.; Liu, W.; Chen, S.; Wang, H.; Wang, H.; Chen, G.; Ren, Z. Thermoelectric properties of copper selenide with ordered selenium layer and disordered copper layer. Nano Energy 2012, 1, 472–478. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, K.; Srivastava, O. Template free-solvothermaly synthesized copper selenide (CuSe, Cu2−xSe, β-Cu2Se and Cu2Se) hexagonal nanoplates from different precursors at low temperature. J. Cryst. Growth 2010, 312, 2804–2813. [Google Scholar] [CrossRef]
- Pazhamalai, P.; Krishnamoorthy, K.; Kim, S.J. Hierarchical copper selenide nanoneedles grown on copper foil as a binder free electrode for supercapacitors. Int. J. Hydrogen Energy 2016, 41, 14830–14835. [Google Scholar] [CrossRef]
- Li, L.; Gong, J.; Liu, C.; Tian, Y.; Han, M.; Wang, Q.; Hong, X.; Ding, Q.; Zhu, W.; Bao, J. Vertically oriented and interpenetrating CuSe nanosheet films with open channels for flexible all-solid-state supercapacitors. ACS Omega 2017, 2, 1089–1096. [Google Scholar] [CrossRef]
- Shinde, S.; Ghodake, G.; Dubal, D.; Patel, R.V.; Saratale, R.; Kim, D.-Y.; Maile, N.; Koli, R.; Dhaygude, H.; Fulari, V. Electrochemical synthesis: Monoclinic Cu2Se nano-dendrites with high performance for supercapacitors. J. Taiwan Inst. Chem. Eng. 2017, 75, 271–279. [Google Scholar] [CrossRef]
- Balasingam, S.K.; Lee, J.S.; Jun, Y. Molybdenum diselenide/reduced graphene oxide based hybrid nanosheets for supercapacitor applications. Dalton Trans. 2016, 45, 9646–9653. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-J.; Zhang, J.-Z.; Cai, J.-L. Preparation of porous layered molybdenum selenide-graphene composites on Ni foam for high-performance supercapacitor and electrochemical sensing. Electrochim. Acta 2015, 180, 770–777. [Google Scholar] [CrossRef]
- Huang, K.-J.; Zhang, J.-Z.; Fan, Y. Preparation of layered MoSe2 nanosheets on Ni-foam substrate with enhanced supercapacitor performance. Mater. Lett. 2015, 152, 244–247. [Google Scholar] [CrossRef]
- Karade, S.S.; Sankapal, B.R. Two dimensional cryptomelane like growth of MoSe2 over mwcnts: Symmetric all-solid-state supercapacitor. J. Electroanal. Chem. 2017, 802, 131–138. [Google Scholar] [CrossRef]
- Wang, Z.; Sha, Q.; Zhang, F.; Pu, J.; Zhang, W. Synthesis of polycrystalline cobalt selenide nanotubes and their catalytic and capacitive behaviors. CrystEngComm 2013, 15, 5928–5934. [Google Scholar] [CrossRef]
- Kong, D.; Wang, H.; Lu, Z.; Cui, Y. CoSe2 nanoparticles grown on carbon fiber paper: An efficient and stable electrocatalyst for hydrogen evolution reaction. J. Am. Chem. Soc. 2014, 136, 4897–4900. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Bhatnagar, S.; Upadhyay, K.K.; Yadav, P.; Ogale, S. Hollow Co0.85Se nanowire array on carbon fiber paper for high rate pseudocapacitor. ACS Appl. Mater. Interfaces 2014, 6, 18844–18852. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Ma, G.; Sun, K.; Zhang, Z.; Li, J.; Zhou, X.; Lei, Z. A novel aqueous asymmetric supercapacitor based on petal-like cobalt selenide nanosheets and nitrogen-doped porous carbon networks electrodes. J. Power Sources 2015, 297, 351–358. [Google Scholar] [CrossRef]
- Liu, C.-C.; Song, J.-M.; Zhao, J.-F.; Li, H.-J.; Qian, H.-S.; Niu, H.-L.; Mao, C.-J.; Zhang, S.-Y.; Shen, Y.-H. Facile synthesis of tremelliform Co0.85Se nanosheets: An efficient catalyst for the decomposition of hydrazine hydrate. Appl. Catal. B Environ. 2012, 119, 139–145. [Google Scholar] [CrossRef]
- Zhao, X.; Li, X.; Zhao, Y.; Su, Z.; Wang, R. Facile synthesis of tremelliform Co0.85Se nanosheets for supercapacitor. J. Alloys Compd. 2017, 697, 124–131. [Google Scholar] [CrossRef]
- Gong, C.; Huang, M.; Zhou, P.; Sun, Z.; Fan, L.; Lin, J.; Wu, J. Mesoporous Co0.85Se nanosheets supported on Ni foam as a positive electrode material for asymmetric supercapacitor. Appl. Surf. Sci. 2016, 362, 469–476. [Google Scholar] [CrossRef]
- Bhat, K.S.; Shenoy, S.; Nagaraja, H.; Sridharan, K. Porous cobalt chalcogenide nanostructures as high performance pseudo-capacitor electrodes. Electrochim. Acta 2017, 248, 188–196. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, A.; Wang, Y.; Cao, X.; Zhou, Z.; Zhu, T.; Liang, S.; Cao, G. Self-templated synthesis of n-doped CoSe2/C double-shelled dodecahedra for high-performance supercapacitors. Energy Storage Mater. 2017, 8, 28–34. [Google Scholar] [CrossRef]
- Zhang, C.; Yin, H.; Han, M.; Dai, Z.; Pang, H.; Zheng, Y.; Lan, Y.-Q.; Bao, J.; Zhu, J. Two-dimensional tin selenide nanostructures for flexible all-solid-state supercapacitors. ACS Nano 2014, 8, 3761–3770. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Cui, S.; Hou, H.; Chen, W.; Mi, L. Hierarchical ternary Ni–Co–Se nanowires for high-performance supercapacitor device design. Dalton Trans. 2016, 45, 19458–19465. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Zhu, M.-Q.; Chen, D. Flexible all-solid-state asymmetric supercapacitors with three-dimensional CoSe2/carbon cloth electrodes. J. Mater. Chem. A 2015, 3, 7910–7918. [Google Scholar] [CrossRef]
- Patil, S.J.; Bulakhe, R.N.; Lokhande, C.D. Nanoflake-modulated La2Se3 thin films prepared for an asymmetric supercapacitor device. ChemPlusChem 2015, 80, 1478–1487. [Google Scholar] [CrossRef]
- Xia, C.; Jiang, Q.; Zhao, C.; Beaujuge, P.M.; Alshareef, H.N. Asymmetric supercapacitors with metal-like ternary selenides and porous graphene electrodes. Nano Energy 2016, 24, 78–86. [Google Scholar] [CrossRef]
- Peng, H.; Zhou, J.; Sun, K.; Ma, G.; Zhang, Z.; Feng, E.; Lei, Z. High-performance asymmetric supercapacitor designed with a novel NiSe@MoSe2 nanosheet arrays and nitrogen-doped carbon nanosheet. ACS Sustain. Chem. Eng. 2017, 5, 5951–5963. [Google Scholar] [CrossRef]
- Peng, H.; Wei, C.; Wang, K.; Meng, T.; Ma, G.; Lei, Z.; Gong, X. Ni0.85Se@MoSe2 nanosheet arrays as the electrode for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 17067–17075. [Google Scholar] [CrossRef] [PubMed]
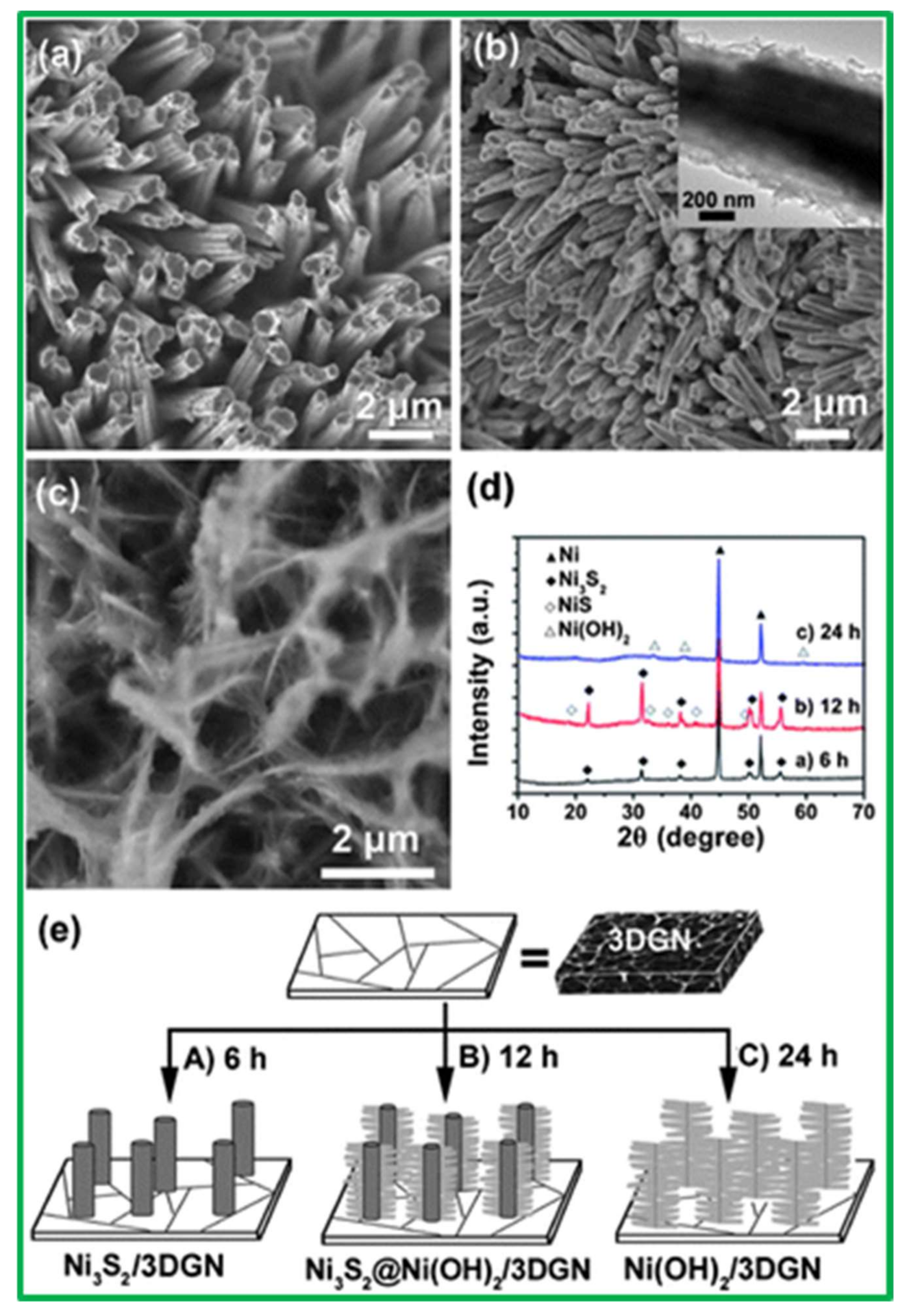
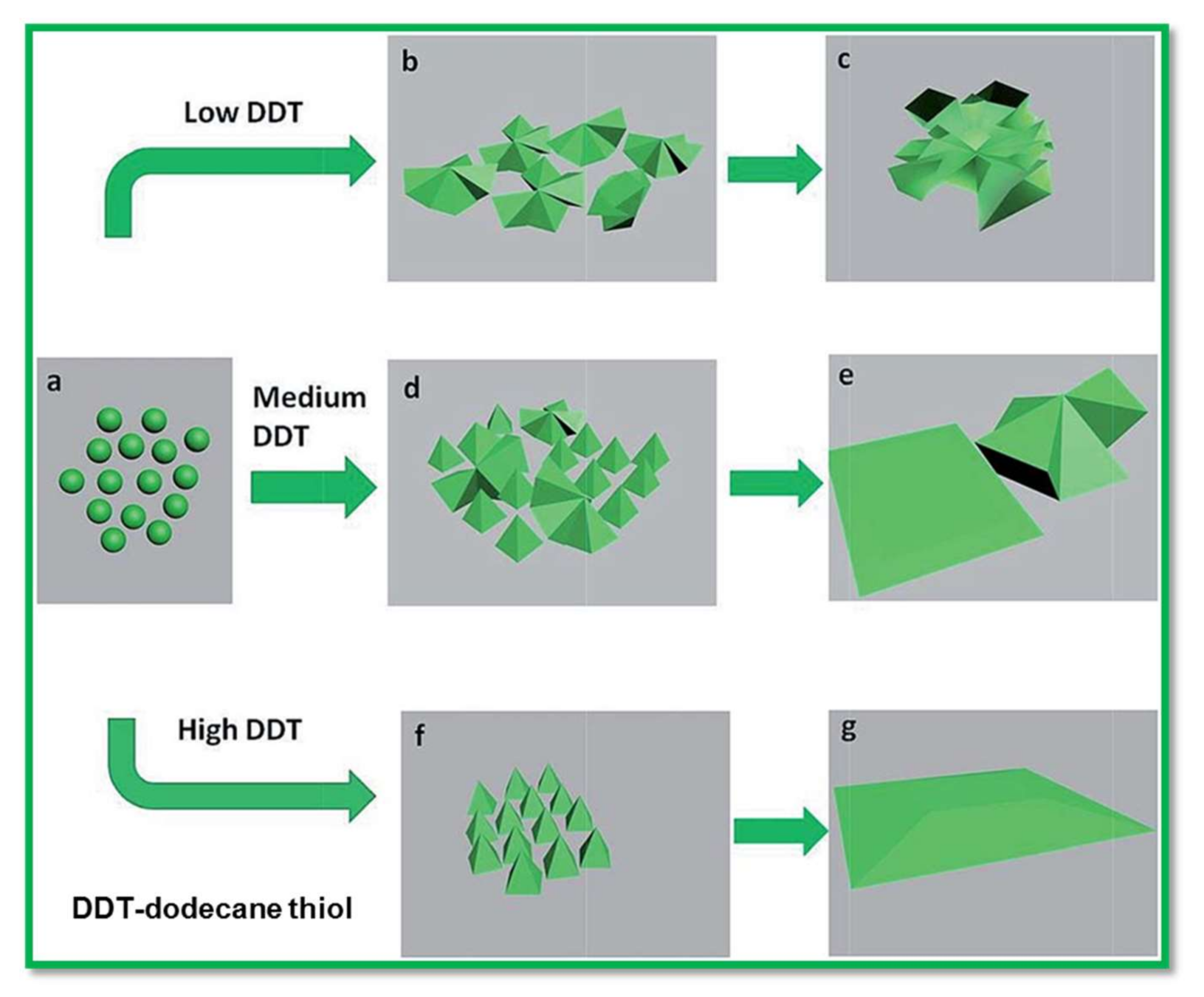
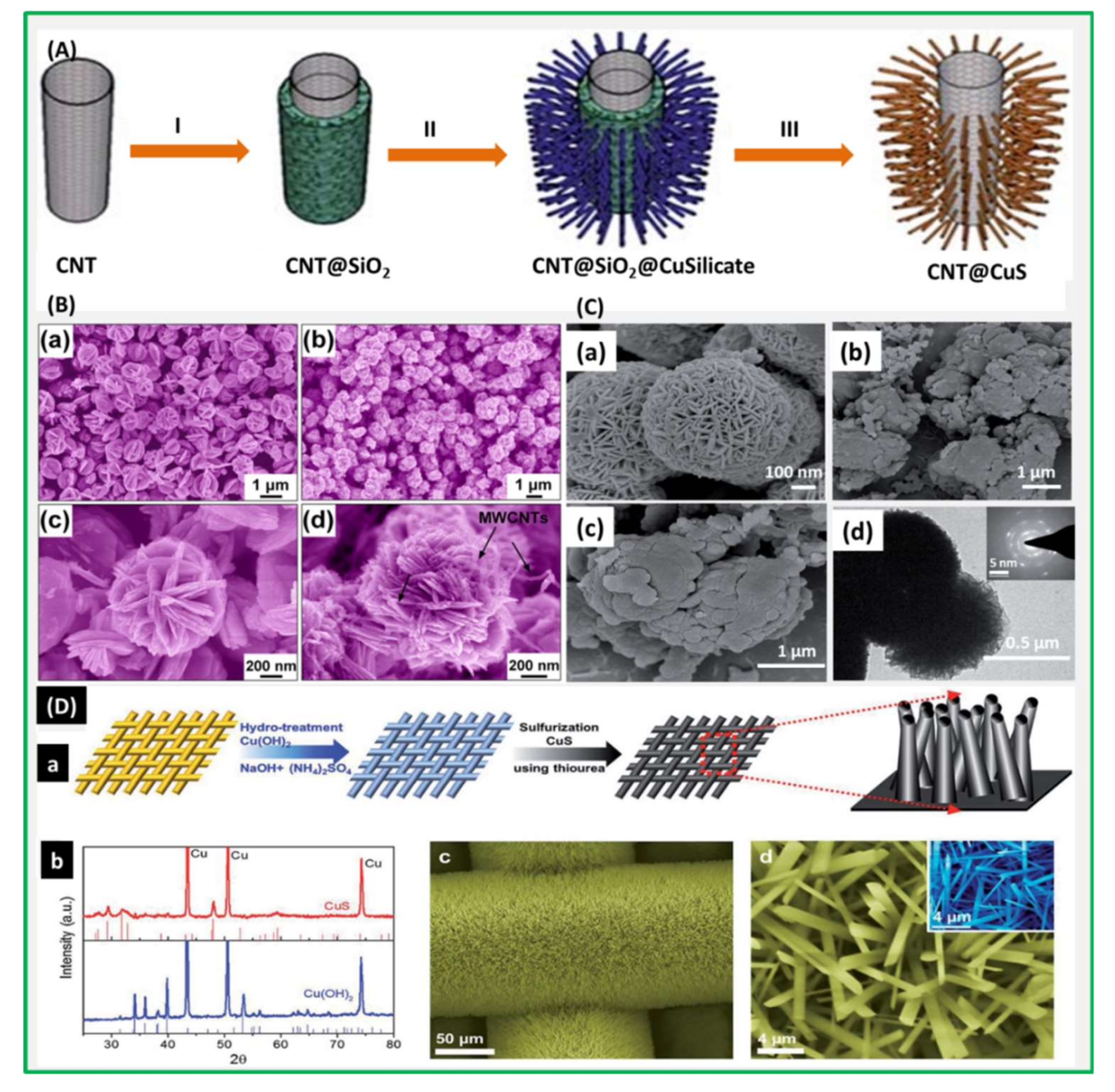
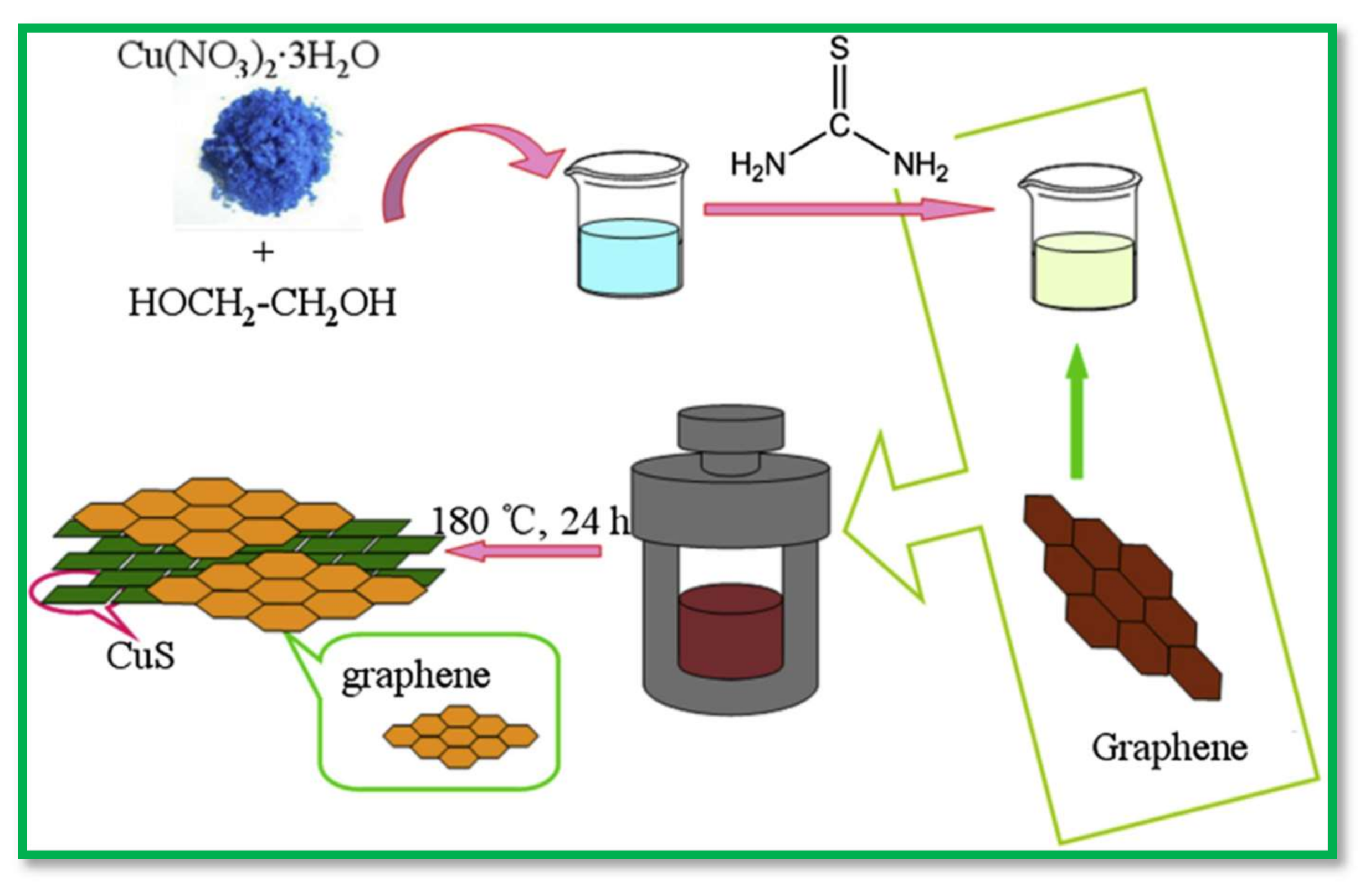
| Electrodes | Capacitance (F·g−1) | Current Density (A·g−1) | Electrolytes | % of Capacity Retention (>1000 Cycles) | Ref. |
|---|---|---|---|---|---|
| Ni3S2 | 717 | 2 | 1 M KOH | 91.0 | [26] |
| Ni3S2@Ni(OH)2/3D graphene nanosheet | 1037.5 | 5.1 | 3 M KOH | 99.1 | [30] |
| Ni3S2/graphene | 875.6 | 1 | 2 M KOH | 93.6 | [34] |
| β-NiS | 857.76 | 2 | 2 M KOH | 99.0 | [44] |
| Ni3S4@amorphous MoS2 | 1440.9 | 2 | 6 M KOH | 90.7 | [57] |
| CuS nano-hollow spheres | 948 | 1 | 6 M KOH | 90.0 | [51] |
| CuS@PANI | 308.1 | 0.5 | 0.1 M Li2SO4 | 71.6 | [76] |
| CoS | 285 | 0.5 | 6 M KOH | 99.0 | [78] |
| CoS/graphene | 435.7 | 0.5 | 6 M KOH | 82.3 | [79] |
| CoS2 microsphere | 718.7 | 1 | 6 M KOH | 93.0 | [80] |
| NiCo2S4 nanosphere | 1156 | 1 | 1 M KOH | 82.0 | [81] |
| NiCo2S4 nanoplates | 437 | 1 | 3 M KOH | 81.0 | [82] |
| MoS2 | 162 | 0.1 | 1 M Na2SO4 | 93.0 | [83] |
| MoS2/graphene | 270 | 0.1 | 1 M Na2SO4 | 89.6 | [83] |
| Bi2S3 | 289 | (5 mV/s) | 1 M Na2SO4 | 60.0 | [84] |
| Bi2S3 | 1007 | 1 | 6 M KOH | 92.0 | [85] |
| Bi2S3/MoS2 | 3040 | 1 | 6 M KOH | 92.6 | [85] |
| MoS2 nanosphere | 1565 | 1 | 6 M KOH | 92.0 | [85] |
| a-La2S3 | 256 | (5 mV/s) | 1M LiClO4/PC | 85.0 | [86] |
| WS2 | 70 | (5 mV/s) | 1 M Na2SO4 | ----- | [87] |
| WS2/RGO | 350 | (5 mV/s) | 1 M Na2SO4 | 99.9 | [87] |
| Electrodes | Capacitance (F·g−1) | Current Density (A·g−1) | Electrolytes | % of Capacity Retention (>1000 Cycles) | Ref. |
|---|---|---|---|---|---|
| NiSe2 single crystal | 1044 | 3 | 4 M KOH | 87.4 | [180] |
| CuSe2/Cu | 1037.5 | (0.25 mA·cm−2) | 1 M NaOH | 104.3 | [184] |
| CuSe nanosheet | 209 | 0.2 | 1 M Na2SO4 | 90.0 | [185] |
| Cu2Se | 688 | (5 mV/s) | 1 M Na2SO4 | 86.0 | [186] |
| MoSe2 nanosheet | 1114.3 | 1 | 6 M KOH | 104.7 | [189] |
| MoSe2/MWCNT | 232 | 1.4 | 1 M KOH | 93.0 | [190] |
| Porous CoSe2 | 951 | (5 mV/s) | 1 M KOH | 52.0 | [198] |
| Co0.85Se nanosheet | 1378 | 1 | 3 M KOH | 95.5 | [199] |
| CoSe2/C dodecahedra | 726 | 2 | 2 M KOH | 48.3 | [199] |
| SnSe2 nanodisks | 168 | 0.5 | 6 M KOH | --- | [200] |
| SnSe nanosheets | 228 | 0.5 | 6 M KOH | --- | [200] |
| Ni-Co-Se | 86 | 1 | 2 M KOH | 100.0 | [201] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theerthagiri, J.; Karuppasamy, K.; Durai, G.; Rana, A.U.H.S.; Arunachalam, P.; Sangeetha, K.; Kuppusami, P.; Kim, H.-S. Recent Advances in Metal Chalcogenides (MX; X = S, Se) Nanostructures for Electrochemical Supercapacitor Applications: A Brief Review. Nanomaterials 2018, 8, 256. https://doi.org/10.3390/nano8040256
Theerthagiri J, Karuppasamy K, Durai G, Rana AUHS, Arunachalam P, Sangeetha K, Kuppusami P, Kim H-S. Recent Advances in Metal Chalcogenides (MX; X = S, Se) Nanostructures for Electrochemical Supercapacitor Applications: A Brief Review. Nanomaterials. 2018; 8(4):256. https://doi.org/10.3390/nano8040256
Chicago/Turabian StyleTheerthagiri, Jayaraman, K. Karuppasamy, Govindarajan Durai, Abu Ul Hassan Sarwar Rana, Prabhakarn Arunachalam, Kirubanandam Sangeetha, Parasuraman Kuppusami, and Hyun-Seok Kim. 2018. "Recent Advances in Metal Chalcogenides (MX; X = S, Se) Nanostructures for Electrochemical Supercapacitor Applications: A Brief Review" Nanomaterials 8, no. 4: 256. https://doi.org/10.3390/nano8040256






