Tracking a Major Egg Allergen to Assess Commercial Food Label Compliance: Towards a Simple and Fast Immunosensing Device
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus and Equipment
2.2. Reagents and Solutions
2.3. Food Sample Preparation and Allergen Extraction
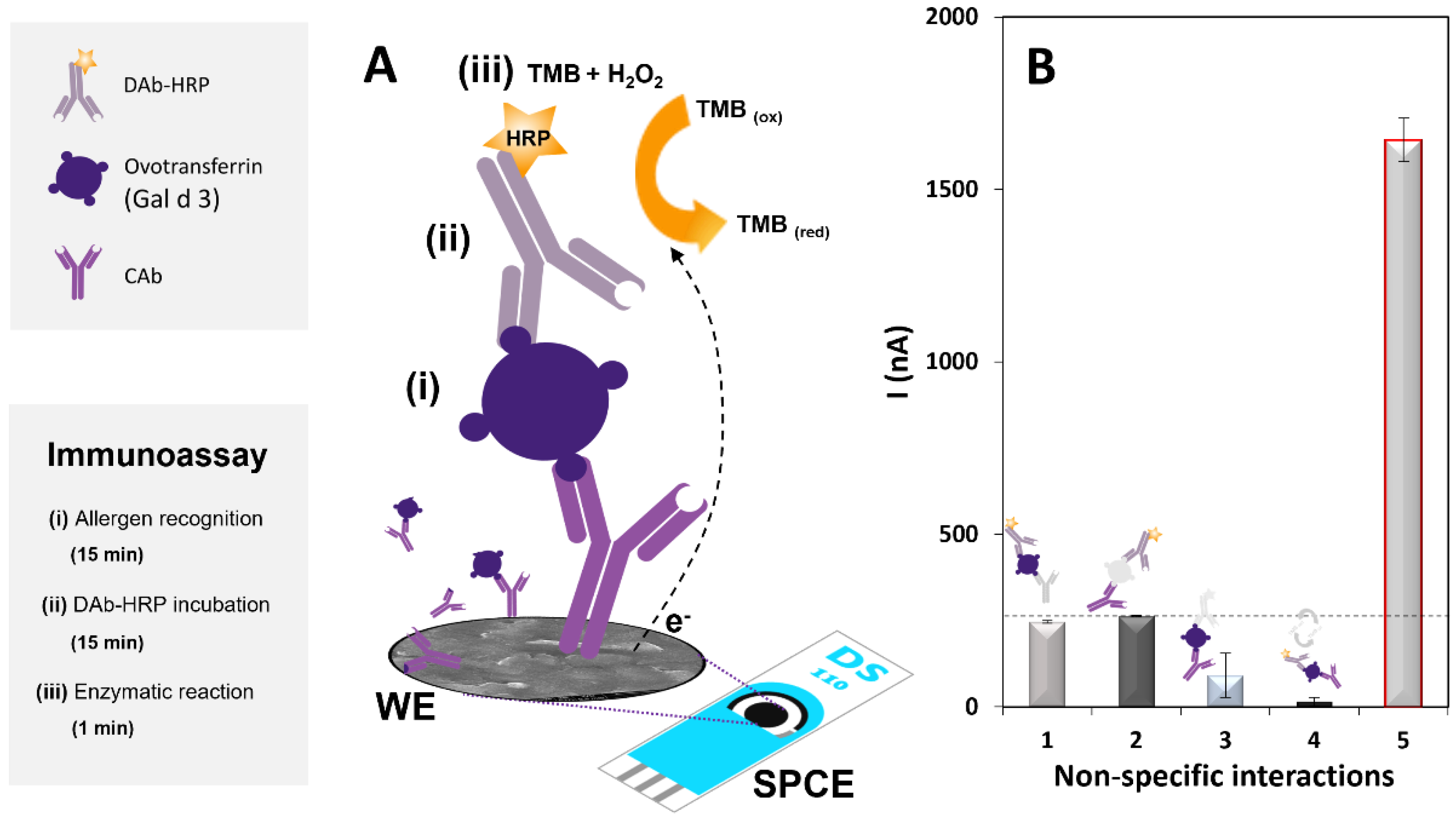
2.4. Immunosensor Construction and Detection Strategy
3. Results and Discussion
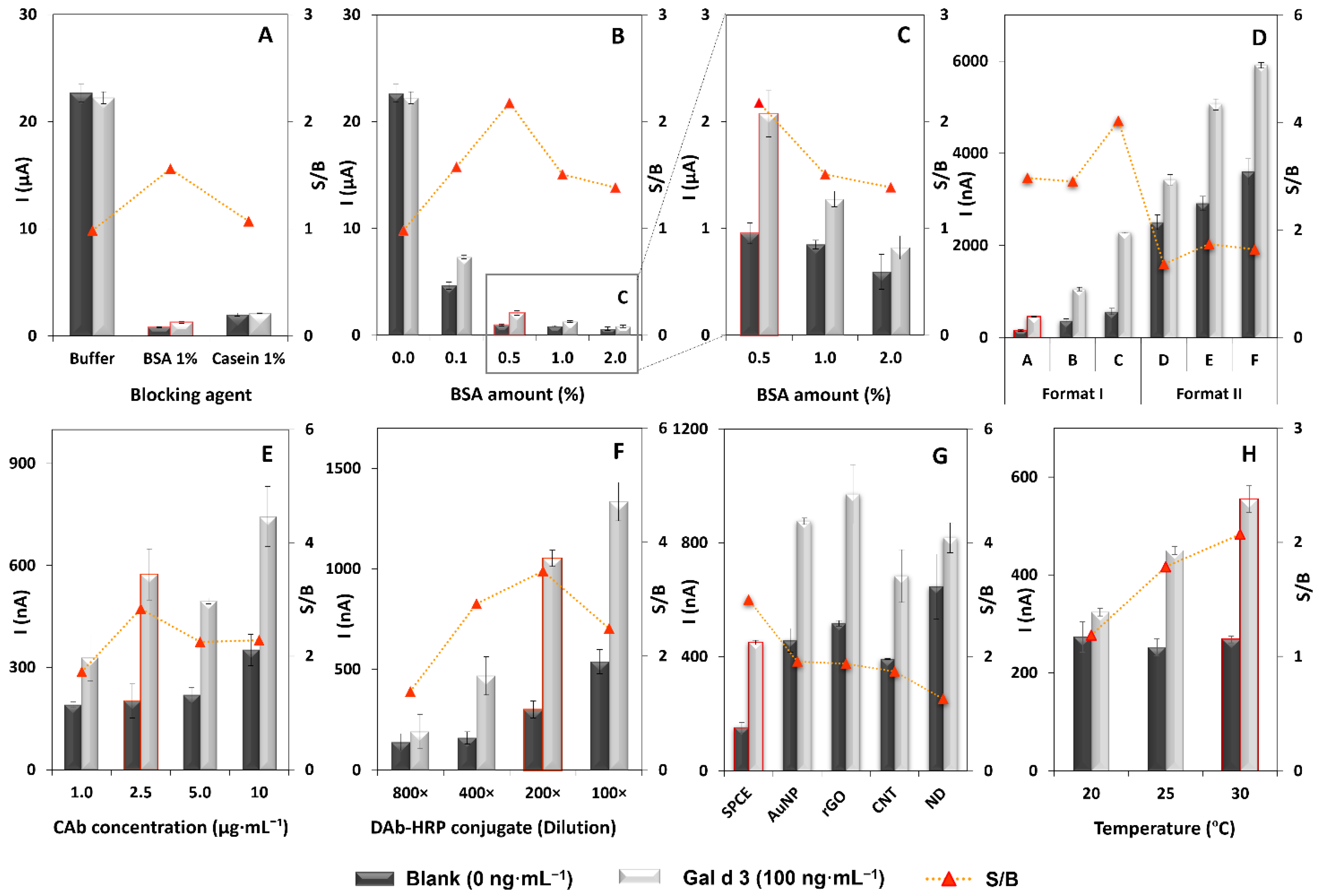
3.1. Optimization of the Immunoassay
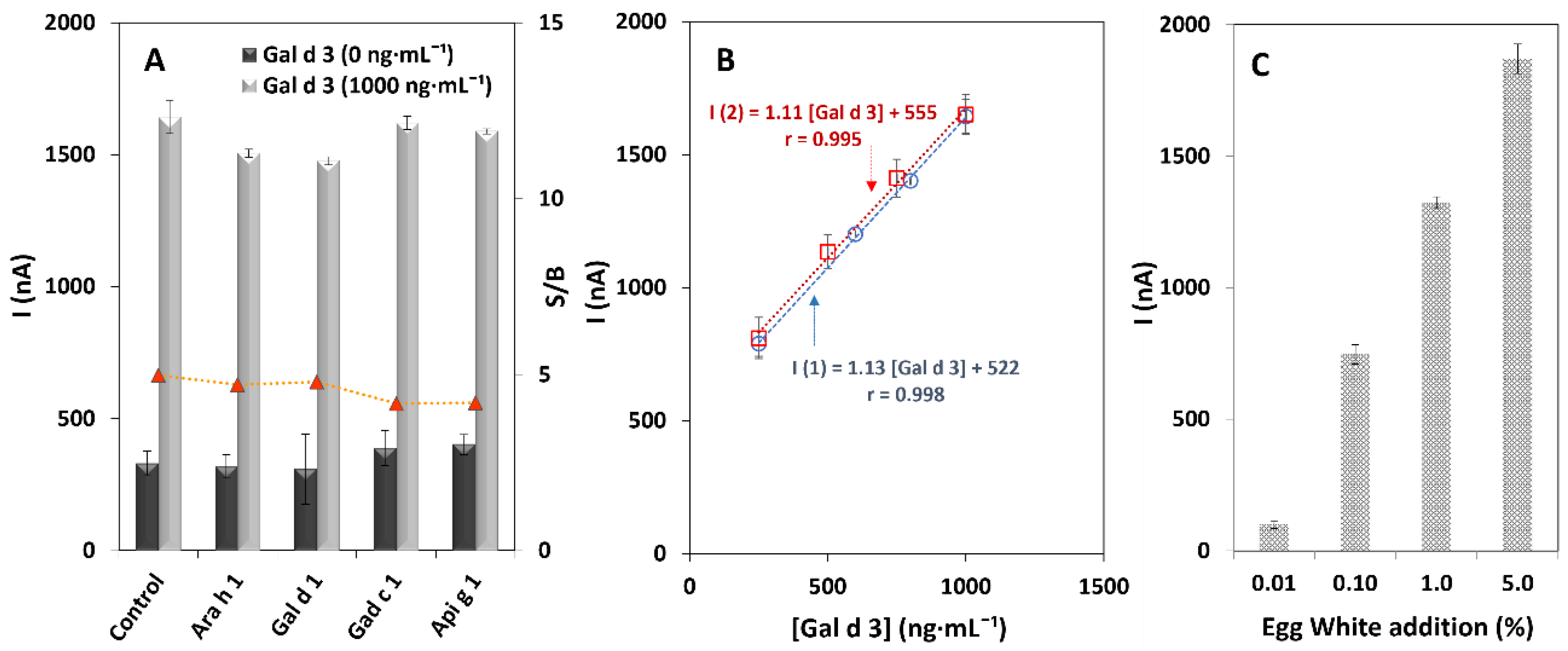
3.2. Analytical Characteristics and Stability of the Immunosensor
3.3. Selectivity and Applicability of the Developed Immunosensor
3.4. Analysis and Quantification of Gal d 3 in Food Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Onyenweaku, E.O.; Akah, L.U.; Kesa, H.; Alawa, D.A.; Ebai, P.A.; Kalu, U.U.; Ajigo, I.; Owan, V.J. Protein Quality Evaluation of Some Commonly Consumed Bird Egg Varieties Using Amino Acid Scores. Biochem. Res. Int. 2022, 2022, 6536826. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.G.; Legette, L.L.; Ikonte, C.J.; Mitmesser, S.H. Choline and DHA in Maternal and Infant Nutrition: Synergistic Implications in Brain and Eye Health. Nutrients 2019, 11, 1125. [Google Scholar] [CrossRef] [Green Version]
- EC. Regulation (EU) No 1169/2011 on the Provision of Food Information to Consumers. Commission. 2011. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02011R1169-20180101 (accessed on 16 July 2022).
- Moghtaderi, M.; Nabavizadeh, S.H.; Hosseini Teshnizi, S. The frequency of cross-reactivity with various avian eggs among children with hen’s egg allergy using skin prick test results: Fewer sensitizations with pigeon and goose egg. Allergol. Immunopathol. 2020, 48, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.; Williams, K.M.; Ferguson, M.; Lee, D.; Sharma, G.M.; Do, A.B.; Khuda, S.E. Enhanced quantitation of egg allergen in foods using incurred standards and antibodies against processed egg in a model ELISA. Anal. Chim. Acta 2019, 1081, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Maggadottir, S.M.; Hakonarson, H. Are genetic tests informative in predicting food allergy? Curr. Opin. Allergy Clin. Immunol. 2016, 16, 257–264. [Google Scholar] [CrossRef]
- Alves, R.C.; Barroso, M.F.; González-García, M.B.; Oliveira, M.B.P.P.; Delerue-Matos, C. New Trends in Food Allergens Detection: Toward Biosensing Strategies. Crit. Rev. Food Sci. Nutr. 2016, 56, 2304–2319. [Google Scholar] [CrossRef]
- Freitas, M.; Neves, M.M.P.S.; Nouws, H.P.A.; Delerue-Matos, C. Electrochemical Immunosensor for the Simultaneous Determination of Two Main Peanut Allergenic Proteins (Ara h 1 and Ara h 6) in Food Matrices. Foods 2021, 10, 1718. [Google Scholar] [CrossRef]
- Freitas, M.; Nouws, H.P.A.; Delerue-Matos, C. Voltammetric Immunosensor to Track a Major Peanut Allergen (Ara h 1) in Food Products Employing Quantum Dot Labels. Biosensors 2021, 11, 426. [Google Scholar] [CrossRef]
- Freitas, M.; Carvalho, A.; Nouws, H.P.A.; Delerue-Matos, C. Tracking Arachis hypogaea Allergen in Pre-Packaged Foodstuff: A Nanodiamond-Based Electrochemical Biosensing Approach. Biosensors 2022, 12, 429. [Google Scholar] [CrossRef]
- Torre, R.; Freitas, M.; Costa-Rama, E.; Nouws, H.P.A.; Delerue-Matos, C. Food allergen control: Tropomyosin analysis through electrochemical immunosensing. Food Chem. 2022, 396, 133659. [Google Scholar] [CrossRef]
- Benedé, S.; Ruiz-Valdepeñas Montiel, V.; Povedano, E.; Villalba, M.; Mata, L.; Galán-Malo, P.; Torrente-Rodríguez, R.M.; Vargas, E.; Reviejo, A.J.; Campuzano, S.; et al. Fast amperometric immunoplatform for ovomucoid traces determination in fresh and baked foods. Sensors Actuators B Chem. 2018, 265, 421–428. [Google Scholar] [CrossRef]
- Mohamad, A.; Rizwan, M.; Keasberry, N.; Ahmed, M. Fabrication of label-free electrochemical food biosensor for the sensitive detection of ovalbumin on nanocomposite-modified graphene electrode. Biointerface Res. Appl. Chem. 2019, 9, 4655–4662. [Google Scholar] [CrossRef]
- Eissa, S.; L’Hocine, L.; Siaj, M.; Zourob, M. A graphene-based label-free voltammetric immunosensor for sensitive detection of the egg allergen ovalbumin. Analyst 2013, 138, 4378–4384. [Google Scholar] [CrossRef] [PubMed]
- Čadková, M.; Metelka, R.; Holubová, L.; Horák, D.; Dvořáková, V.; Bílková, Z.; Korecká, L. Magnetic beads-based electrochemical immunosensor for monitoring allergenic food proteins. Anal. Biochem. 2015, 484, 4–8. [Google Scholar] [CrossRef]
- Sugawara, K.; Kuramitz, H.; Shinohara, H. Fabrication of micromagnetic beads with molecular recognition/electron-transfer peptides for the sensing of ovalbumin. Anal. Chim. Acta 2017, 958, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Baldo, T.A.; dos Anjos Proença, C.; da Silva Felix, F.; Freitas, T.A.; Sakata, S.K.; Angnes, L.; Faria, R.C. Disposable electrochemical microfluidic device for ultrasensitive detection of egg allergen in wine samples. Talanta 2021, 232, 122447. [Google Scholar] [CrossRef]
- Zhu, Y.; Vanga, S.K.; Wang, J.; Raghavan, V. Impact of food processing on the structural and allergenic properties of egg white. Trends Food Sci. Technol. 2018, 78, 188–196. [Google Scholar] [CrossRef]
- Freitas, M.; Sá Couto, M.; Barroso, M.F.; Pereira, C.; de-los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J.; Delerue-Matos, C. Highly Monodisperse Fe3O4@Au Superparamagnetic Nanoparticles as Reproducible Platform for Genosensing Genetically Modified Organisms. ACS Sensors 2016, 1, 1044–1053. [Google Scholar] [CrossRef]
- Neves, M.M.P.S.; Nouws, H.P.A.; Santos-Silva, A.; Delerue-Matos, C. Neutrophil gelatinase-associated lipocalin detection using a sensitive electrochemical immunosensing approach. Sensors Actuators B Chem. 2020, 304, 127285. [Google Scholar] [CrossRef]
- Silva, N.F.D.; Neves, M.M.P.S.; Magalhães, J.M.C.S.; Freire, C.; Delerue-Matos, C. Electrochemical immunosensor towards invasion-associated protein p60: An alternative strategy for Listeria monocytogenes screening in food. Talanta 2020, 216, 120976. [Google Scholar] [CrossRef]
- Escamilla-Gómez, V.; Campuzano, S.; Pedrero, M.; Pingarrón, J.M. Electrochemical immunosensor designs for the determination of Staphylococcus aureus using 3,3-dithiodipropionic acid di(N-succinimidyl ester)-modified gold electrodes. Talanta 2008, 77, 876–881. [Google Scholar] [CrossRef]
- Barhoumi, L.; Bellagambi, F.G.; Vivaldi, F.M.; Baraket, A.; Clément, Y.; Zine, N.; Ben Ali, M.; Elaissari, A.; Errachid, A. Ultrasensitive Immunosensor Array for TNF-α Detection in Artificial Saliva using Polymer-Coated Magnetic Microparticles onto Screen-Printed Gold Electrode. Sensors 2019, 19, 692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Salcedo, R.; Miranda-Castro, R.; de-los-Santos-Álvarez, N.; Lobo-Castañón, M.J. Dual electrochemical genosensor for early diagnosis of prostate cancer through lncRNAs detection. Biosens. Bioelectron. 2021, 192, 113520. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.; Nouws, H.P.A.; Keating, E.; Delerue-Matos, C. High-performance electrochemical immunomagnetic assay for breast cancer analysis. Sensors Actuators B Chem. 2020, 308, 127667. [Google Scholar] [CrossRef]
- Grandke, J.; Resch-Genger, U.; Bremser, W.; Garbe, L.-A.; Schneider, R.J. Quality assurance in immunoassay performance-temperature effects. Anal. Methods 2012, 4, 901–905. [Google Scholar] [CrossRef]
- Wachholz, P.A.; Dearman, R.J.; Kimber, I. Detection of Allergen-Specific IgE Antibody Responses. J. Immunotoxicol. 2005, 1, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Eckl-Dorna, J.; Villazala-Merino, S.; Linhart, B.; Karaulov, A.V.; Zhernov, Y.; Khaitov, M.; Niederberger-Leppin, V.; Valenta, R. Allergen-Specific Antibodies Regulate Secondary Allergen-Specific Immune Responses. Front. Immunol. 2019, 9, 3131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, P.-W.; Tu, Z.-C.; Hu, Y.-M.; Wang, H. Effects of Superheated Steam Treatment on the Allergenicity and Structure of Chicken Egg Ovomucoid. Foods 2022, 11, 238. [Google Scholar] [CrossRef]
- Tong, P.; Gao, J.; Chen, H.; Li, X.; Zhang, Y.; Jian, S.; Wichers, H.; Wu, Z.; Yang, A.; Liu, F. Effect of heat treatment on the potential allergenicity and conformational structure of egg allergen ovotransferrin. Food Chem. 2012, 131, 603–610. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, T.; Li, Q.; Wang, F.; Li, J. A microfluidic approach for rapid and continuous synthesis of glycoprotein-imprinted nanospheres. Talanta 2022, 239, 123084. [Google Scholar] [CrossRef]


| Food/Ingredient | Gal d 3 (µg·g−1) | ||||
|---|---|---|---|---|---|
| Immunosensor | CV (%) | ELISA | CV (%) | ||
| Egg-Free | 1-Whole grain cereal | <LOQ | – | <LOQ | – |
| 2-Wafer | 38.5 ± 3.2 | 8.4 | <LOQ | – | |
| 3-Wheat flour | <LOQ | – | <LOQ | – | |
| 4-Celery | <LOQ | – | <LOQ | – | |
| 5-Sesame seed | <LOQ | – | <LOQ | – | |
| 6-Soybean | <LOQ | – | <LOQ | – | |
| 7-Peanut | <LOQ | – | <LOQ | – | |
| 8-Hazelnut | <LOQ | – | <LOQ | – | |
| 9-Oat | <LOQ | – | <LOQ | – | |
| 10-Royal pudding | <LOQ | – | <LOQ | – | |
| 11-Powdered flan pudding | <LOQ | – | <LOQ | – | |
| May Contain Egg | 12-Peanut and pineapple biscuit | 108.4 ± 10.1 | 9.3 | <LOQ | – |
| 13-Water and salt crackers | 80.1 ± 5.8 | 7.2 | <LOQ | – | |
| 14-Portuguese cookie (maria) | 112.4 ± 3.0 | 2.7 | <LOQ | – | |
| 15-Vegetable broth | <LOQ | – | <LOQ | – | |
| 16-Powdered “Pão de Ló” | 115.0 ± 4.2 | 3.7 | <LOQ | – | |
| Contain Egg and/or Egg White | 17-Biscuits (egg) | <LOQ | – | 12.9 ± 1.7 | 13.1 |
| 18-Muffins (egg 13%, egg white 8%) | <LOQ | – | <LOQ | – | |
| 19-Sponge cake (egg 5%, egg white 8%) | <LOQ | – | <LOQ | – | |
| 20-Pasta (60% of wheat semolina + egg white) | 48.7 ± 3.7 | 7.6 | <LOQ | – | |
| 21-Cake roll (egg 7%, egg white 3%) | 362.8 ± 21.5 | 8.7 | 307.1 ± 4.2 | 1.4 | |
| 22-Flan pudding (egg 17.8%) | 112.8 ± 4.9 | 4.3 | 112.1 ± 2.3 | 2.1 | |
| 23-Egg pudding (egg 5%) | 2376 ± 15.8 | 0.7 | 2242 ± 4.5 | 0.2 | |
| 24-“Pão de Ló” (powdered egg) | 2646 ± 23.8 | 0.9 | 2436 ± 18.2 | 0.7 | |
| 25-“Doce de Ovos” (egg 13%) | 1995 ± 42.7 | 2.1 | 1755 ± 10.1 | 0.6 | |
| Analyte | Methodology | Assay Time | Analytical Features | Application | Ref | ||
|---|---|---|---|---|---|---|---|
| Method | Technique | Linear Range | LOD | ||||
| Gal d 3 | Immunosensor | Electrochemistry | 30 min | 55–1000 ng·mL−1 | 16 ng·mL−1 | Yes | This work |
| MIP | Microfluidics | <2 h | n.r. | n.r. | No | [31] | |
| ELISA | Spectrometry | >3 h | n.r. | n.r. | Yes | [30] | |
| Egg-white protein | ELISA | Spectrometry | >2 h | 0–24 µg·g−1 | 0.25 µg·g−1 | Yes | [5] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, M.; del Rio, M.; Nouws, H.P.A.; Delerue-Matos, C. Tracking a Major Egg Allergen to Assess Commercial Food Label Compliance: Towards a Simple and Fast Immunosensing Device. Biosensors 2022, 12, 1109. https://doi.org/10.3390/bios12121109
Freitas M, del Rio M, Nouws HPA, Delerue-Matos C. Tracking a Major Egg Allergen to Assess Commercial Food Label Compliance: Towards a Simple and Fast Immunosensing Device. Biosensors. 2022; 12(12):1109. https://doi.org/10.3390/bios12121109
Chicago/Turabian StyleFreitas, Maria, Mariana del Rio, Henri P. A. Nouws, and Cristina Delerue-Matos. 2022. "Tracking a Major Egg Allergen to Assess Commercial Food Label Compliance: Towards a Simple and Fast Immunosensing Device" Biosensors 12, no. 12: 1109. https://doi.org/10.3390/bios12121109
APA StyleFreitas, M., del Rio, M., Nouws, H. P. A., & Delerue-Matos, C. (2022). Tracking a Major Egg Allergen to Assess Commercial Food Label Compliance: Towards a Simple and Fast Immunosensing Device. Biosensors, 12(12), 1109. https://doi.org/10.3390/bios12121109







