Rapidly Measuring Scattered Polarization Parameters of the Individual Suspended Particle with Continuously Large Angular Range
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Experimental Setup
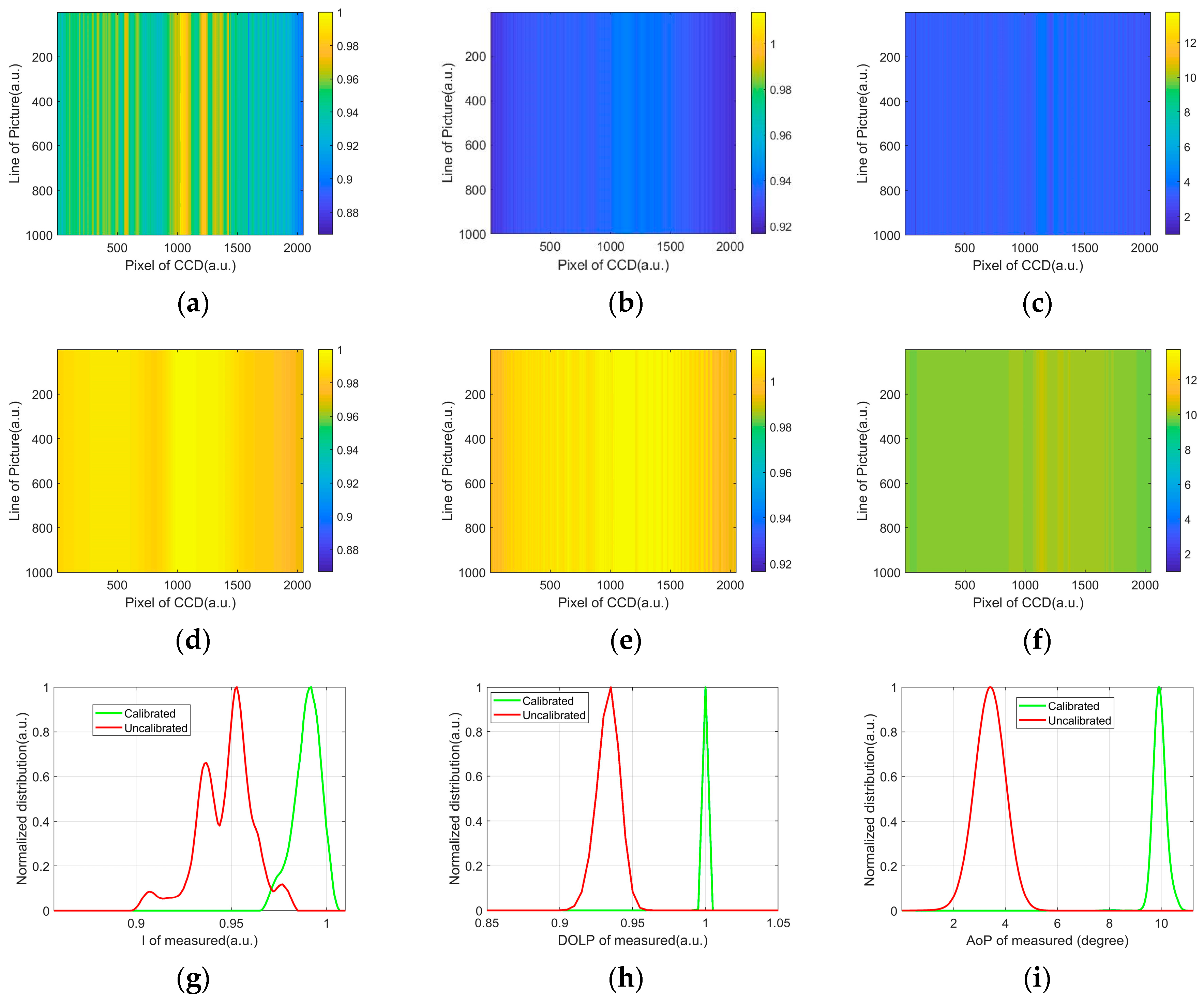
2.3. Calibration
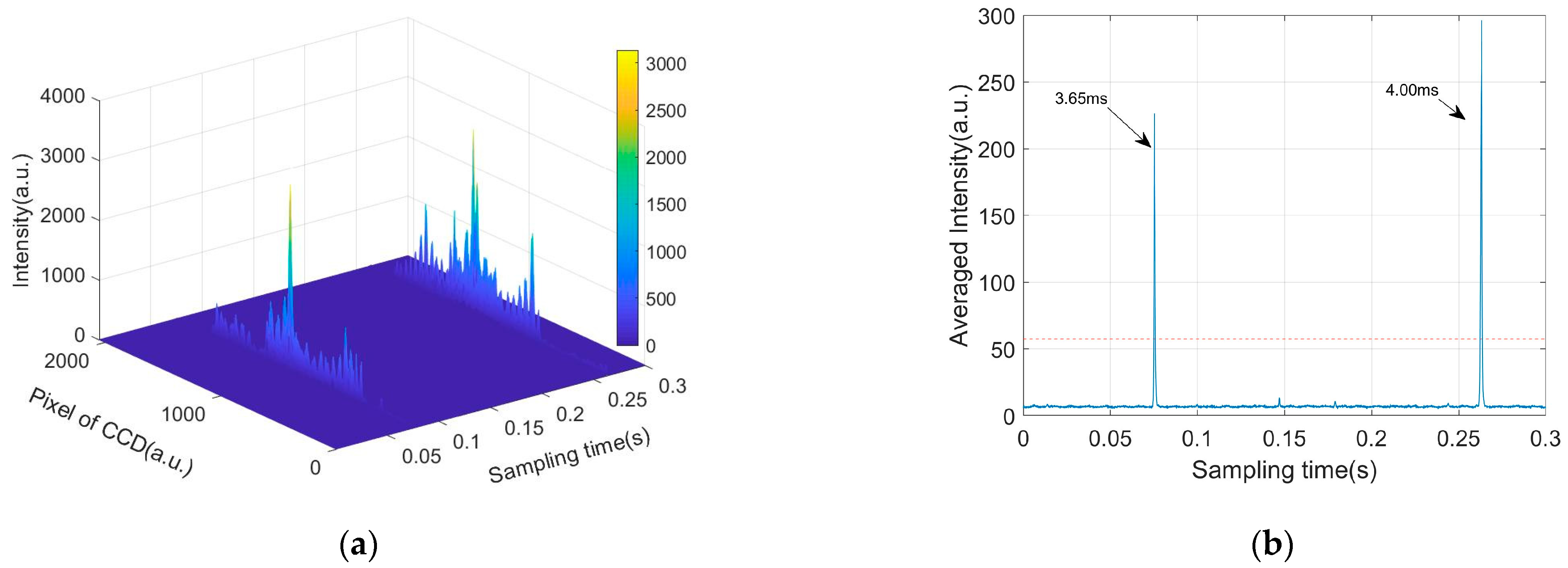
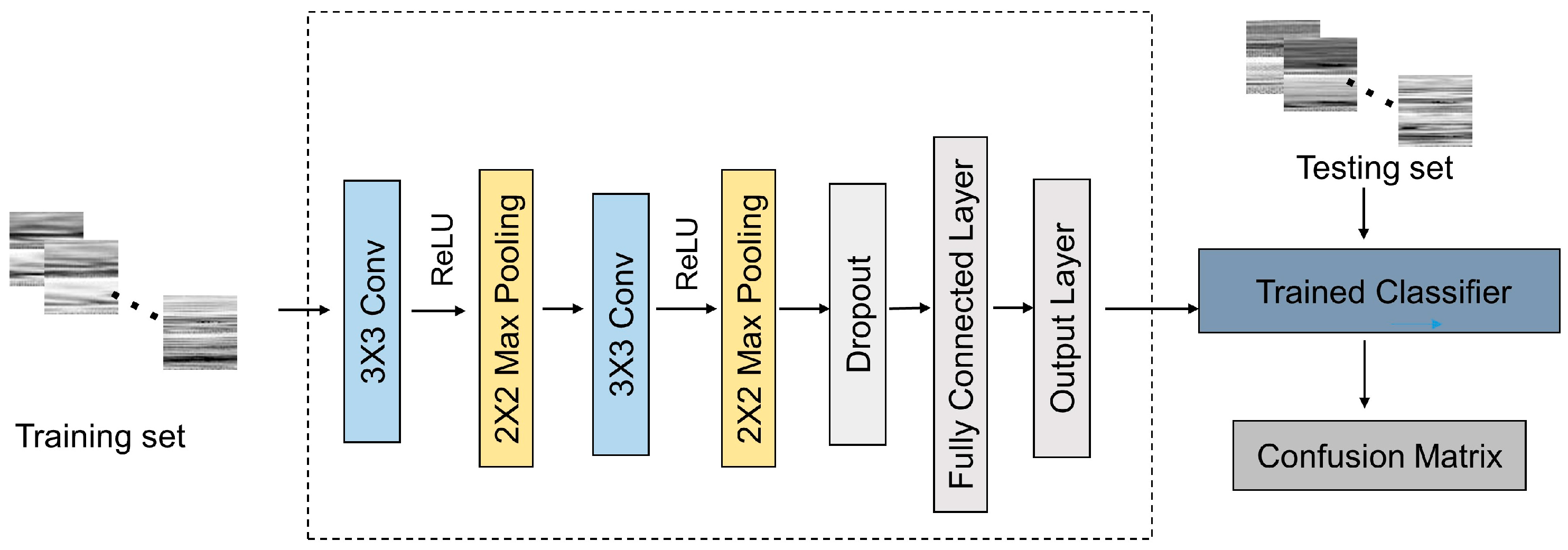
2.4. Signal Processing
3. Results
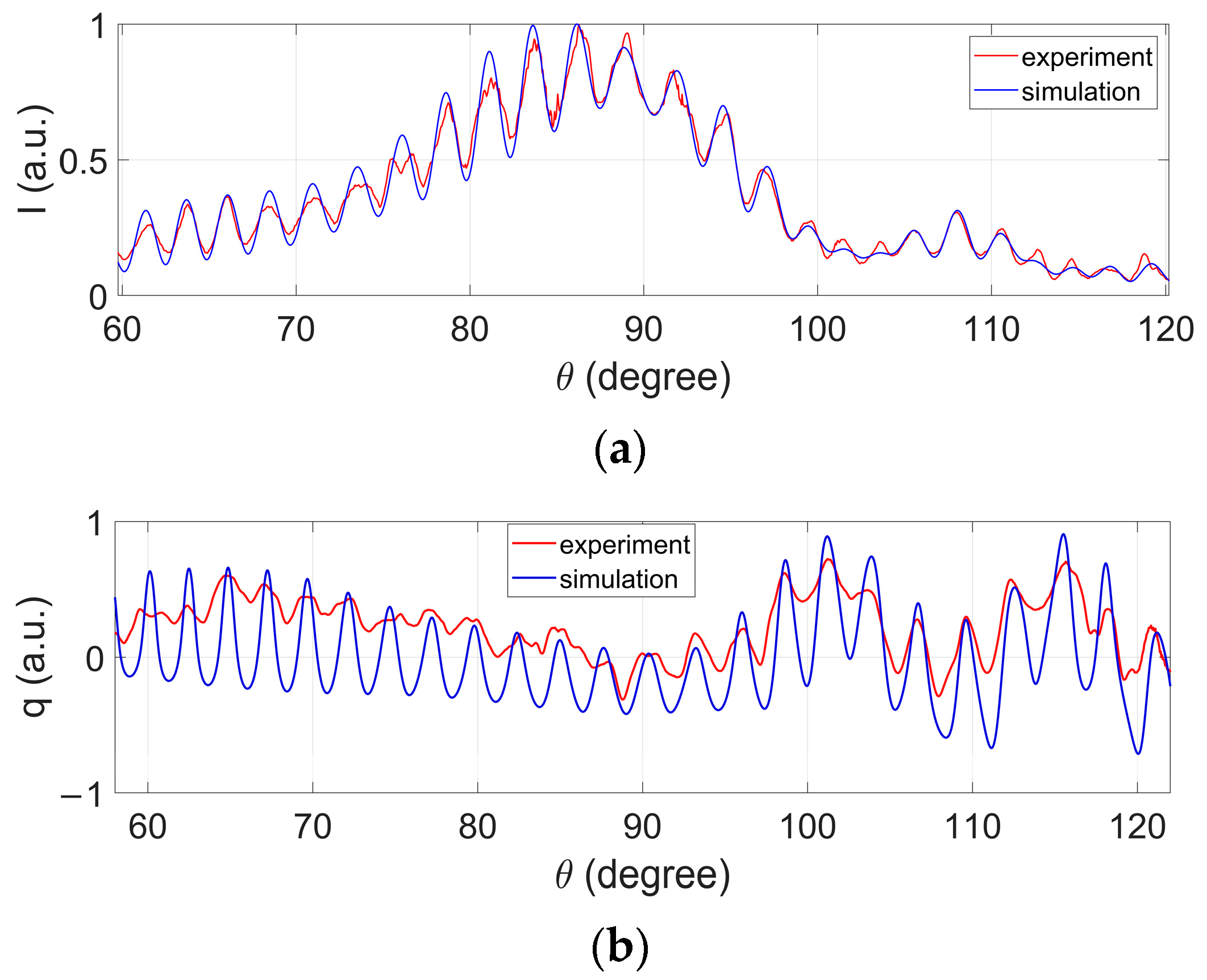
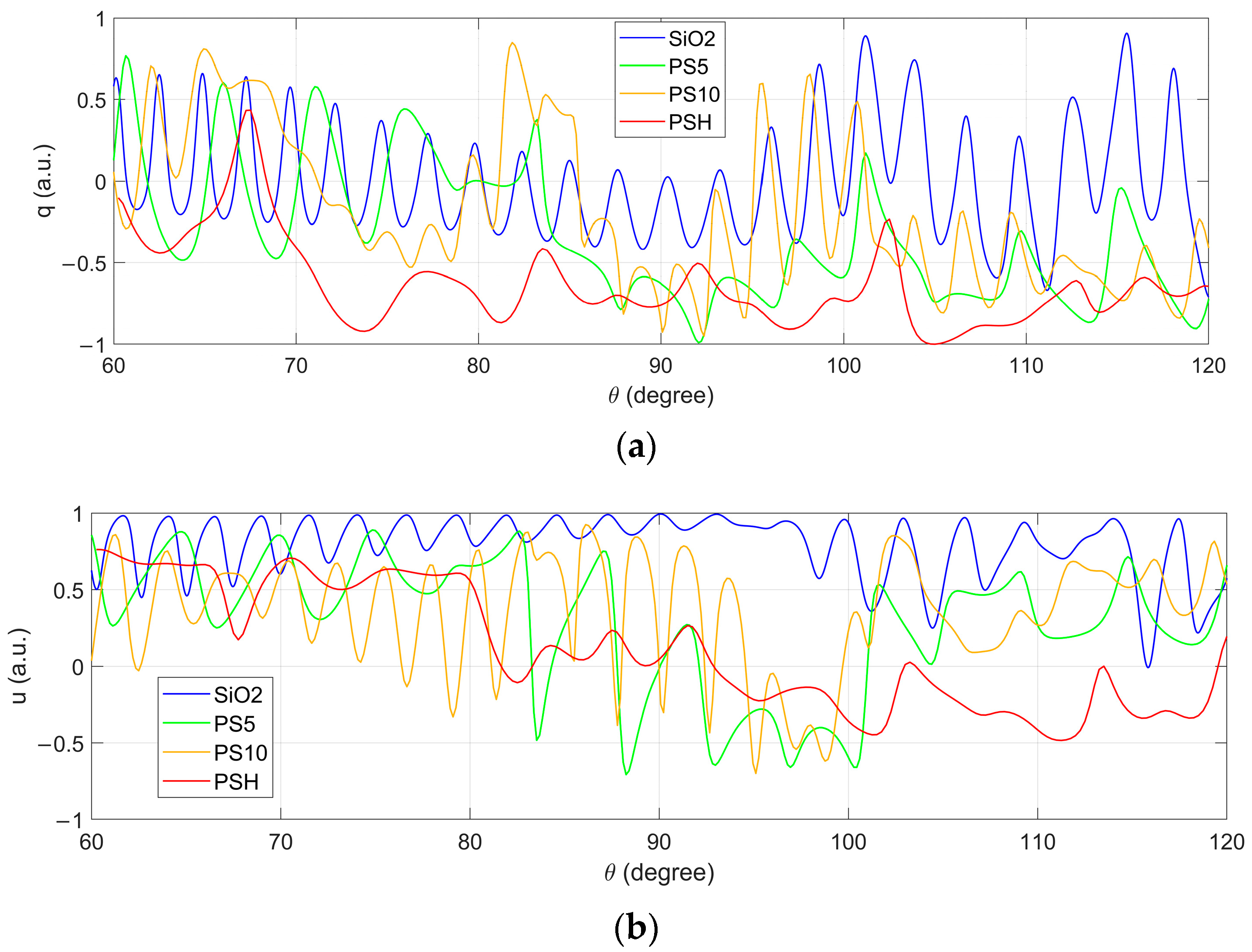
3.1. Comparison of Measured PCLAR with Those Simulated by Mie Theory
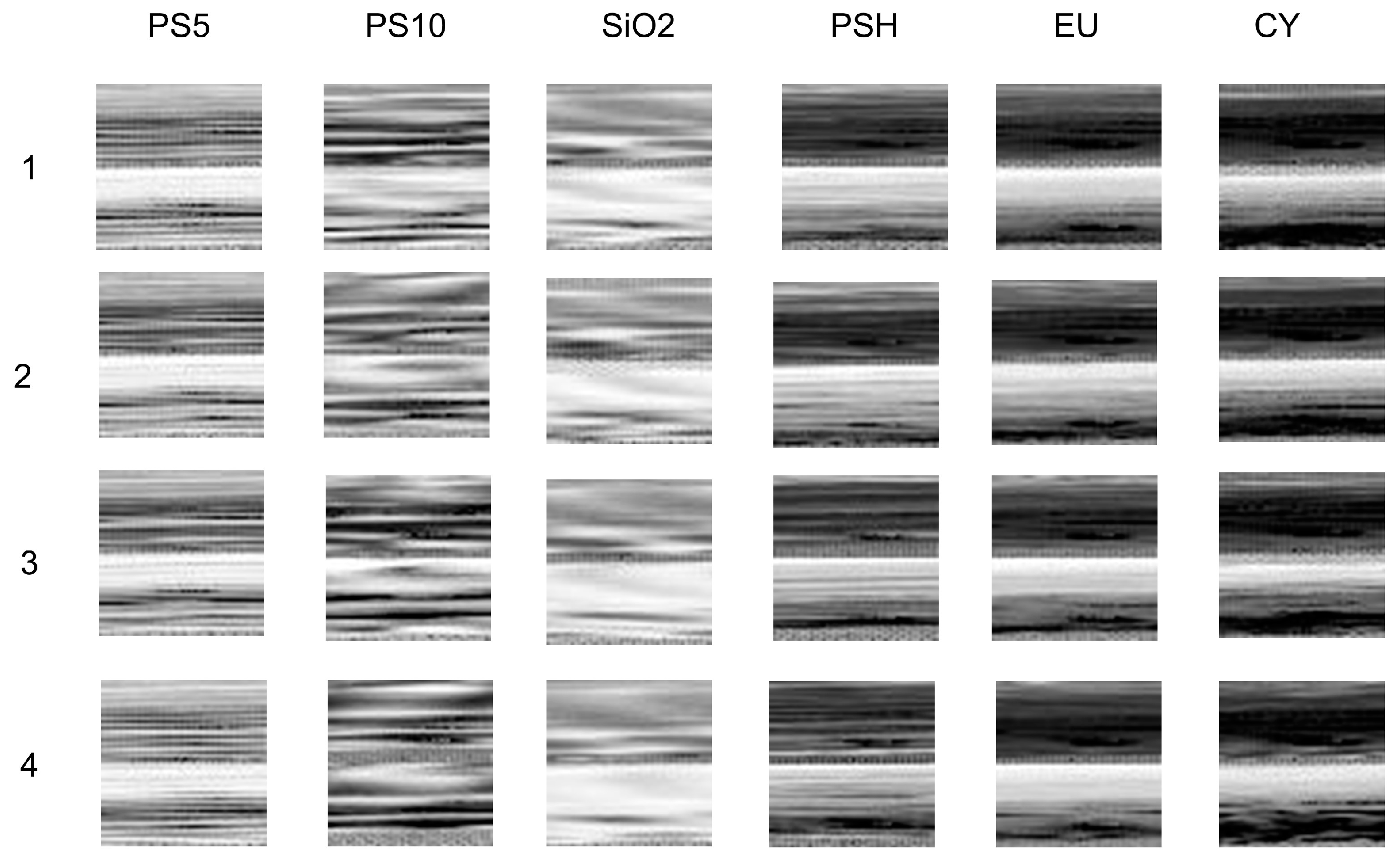
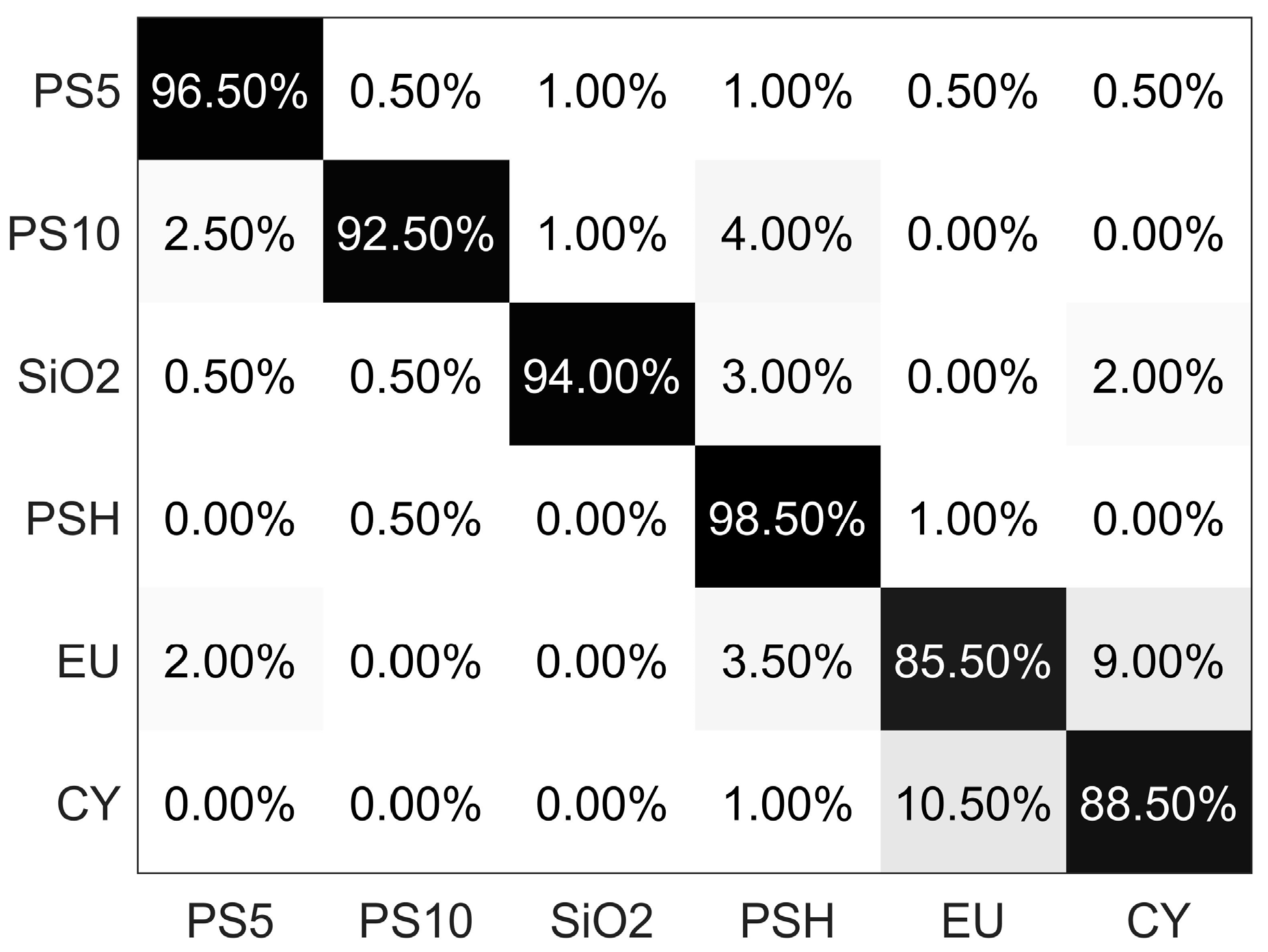
3.2. Classification of Six Categories of Particles
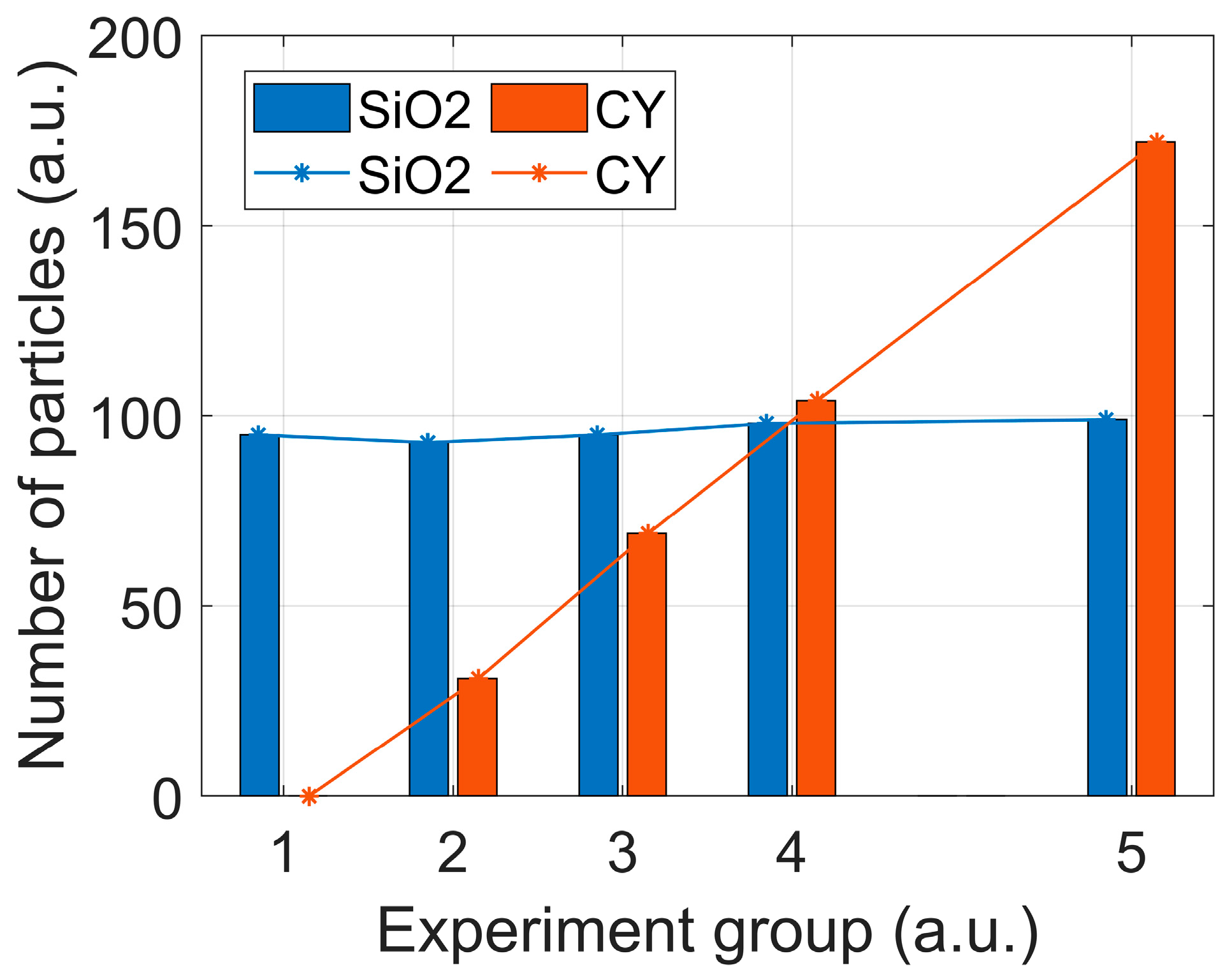
3.3. Identifying the Particulate Compositions in Mixtures
4. Discussion
4.1. Performance Comparisons of Different Anglular Selectin Strategies
4.2. Simulated PCLAR of the Four Non-Biological Microspheres
4.3. Effective Size and Refractive Index of Cells Retrieved from PCLAR
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Windom, H.L. Elemental composition of suspended particles across the southeastern continental shelf off the coast of North Florida and South Georgia: Provenance, transport, fate and implications to mid-outer shelf water column processes. Cont. Shelf. Res. 2019, 178, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Wang, P. Effects of rising atmospheric CO2 levels on physiological response of cyanobacteria and cyanobacterial bloom development: A review. Sci. Total Environ. 2021, 754, 141889. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, Z.T.; Wolanski, E.; Jorge, G.A.; Lewis, S.E.; Brodie, J.E. Fine sediment and nutrient dynamics elated to particle size and floc formation in a Burdekin River flood plume, Australia. Mar. Pollut. Bull. 2012, 65, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Tan, Z.; Wang, J.; Peng, J.; Li, M.; Zhan, Z. Extraction, enumeration and identification methods for monitoring microplastics in the environment. Estuar. Coast. Shelf Sci. 2016, 176, 102–109. [Google Scholar] [CrossRef]
- Lauridsen, T.L.; Schluter, L.; Johansson, L.S. Determining algal assemblages in oligotrophic lakes and streams: Comparing information from newly developed pigment/chlorophyll a ratios with direct microscopy. Freshw. Biol. 2011, 56, 8. [Google Scholar] [CrossRef]
- Heidi, M.S.; Robert, J.O. Automated taxonomic classification of phytoplankton sampled with imaging-in-flow cytometry. Limno Oceanogr-Meth. 2007, 5, 204–216. [Google Scholar]
- Li, J.; Chen, T.; Yang, Z.; Chen, L.; Liu, P.; Zhang, Y.; Yu, G.; Chen, J.; Li, H.; Sun, X. Development of a Buoy-borne underwater imaging system for in situ mesoplankton monitoring of coastal waters. IEEE J. Ocean. Eng. 2022, 47, 88–110. [Google Scholar] [CrossRef]
- Göröcs, Z.; Tamamitsu, M.; Bianco, V.; Wolf, P.; Roy, S.; Shindo, K.; Yanny, K.; Wu, Y.; Koydemir, H.C.; Rivenson, Y.; et al. A deep learning-enabled portable imaging flow cytometer for cost-effective, high-throughput, and label-free analysis of natural water samples. Light Sci. Appl. 2018, 7, 66. [Google Scholar] [CrossRef]
- Orenstein, E.C.; Ratelle, D.; BriseñoAvena, C.; Carter, M.L.; Franks, P.J.S.; Jaffe, J.S.; Roberts, P.L.D. The scripps plankton camera system: A framework and platform for in situ microscopy. Limnol. Oceanogr. Meth. 2020, 18, 11. [Google Scholar] [CrossRef]
- Jaffe, J.S. Underwater optical imaging: The past, the present, and the prospects. IEEE J. Ocean. Eng. 2015, 40, 683–700. [Google Scholar] [CrossRef]
- Johan, F.; Jafri, M.; Lim, H.; Omar, W. Laboratory measurement: Chlorophyll-a concentration measurement with acetone method using spectrophotometer. In Proceedings of the IEEE International Conference on Industrial Engineering and Engineering Management, Singapore, 14–17 December 2014; pp. 744–748. [Google Scholar]
- Porat, R.; Teltsch, B.; Mosse, R.; Dubinsky, Z.; Walsby, A. Turbidity changes caused by collapse of cyanobacterial gas vesicles in water pumped from lake Kinneret into the Israeli national water carrier. Water Res. 1999, 33, 1634–1644. [Google Scholar] [CrossRef]
- Wang, H.; Liao, R.; Xiong, Z.; Wang, Z.; Li, J.; Zhou, Q.; Tao, Y.; Ma, H. Simultaneously acquiring optical and acoustic properties of individual microalgae cells suspended in water. Biosensors 2022, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; Wiley: New York, NY, USA, 1983. [Google Scholar]
- Piedra, P.; Kalume, A.; Zubko, E.; Mackowski, D.; Pan, Y.L.; Videen, G. Particle shape classification using light scattering: An exercise in deep learning. J. Quant. Spectrosc. Radiat. Transf. 2019, 231, 140–156. [Google Scholar] [CrossRef]
- Xu, R. Light scattering: A review of particle characterization applications. Particuology 2014, 18, 11–21. [Google Scholar] [CrossRef]
- He, C.; He, H.; Chang, J.; Chen, B.; Ma, H.; Martin, J.B. Polarisation optics for biomedical and clinical applications: A review. Light Sci. Appl. 2021, 10, 194. [Google Scholar] [CrossRef]
- Chami, M.; Platel, M.D. Sensitivity of the retrieval of the inherent optical properties of marine particles in coastal waters to the directional variations and the polarization of the reflectance. J. Geophys. Res. 2007, 112, C05037. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liao, R.; Dai, J.; Liu, Z.; Xiong, Z.; Zhang, T.; Chen, H.; Hui, M. Differentiation of suspended particles by polarized light scattering at 120°. Opt. Express. 2018, 26, 22419–22431. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Liao, R.; Tao, Y.; Peng, L.; Li, H.; Deng, H.; Ma, H. Early warning of cyanobacterial blooms based on polarized light scattering powered by machine learning. Measurement 2021, 184, 109902. [Google Scholar] [CrossRef]
- Li, J.; Zou, C.; Liao, R.; Peng, L.; Wang, H.; Guo, Z.; Ma, H. Characterization of intracellular structure changes of microcystis under sonication treatment by polarized light scattering. Biosensors 2021, 11, 279. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Liao, R.; Wang, Y.; Liu, Z.; Zhuo, Z.; Guo, Z.; Ma, H. Statistical Mueller matrix driven discrimination of suspended particles. Opt. Lett. 2021, 46, 3645–3648. [Google Scholar] [CrossRef]
- Liao, R.; Zeng, N.; Zeng, M.; Ma, H. Estimation and extraction of the aerosol complex refractive index based on Stokes vector measurements. Opt. Lett. 2019, 44, 4877–4880. [Google Scholar] [CrossRef]
- Xu, Q.; Zeng, N.; Guo, W.; Guo, J.; He, Y.; Ma, M. Real time and online aerosol identification based on deep learning of multi-angle synchronous polarization scattering indexes. Opt. Express 2021, 29, 18540–18564. [Google Scholar] [CrossRef]
- Walters, S.; Zalliea, J.; Seymour, G.; Pan, Y.; Videen, G.; Aptowicz, K.B. Characterizing the size and absorption of single nonspherical aerosol particles from angularly-resolved elastic light scattering. J. Quant. Spectrosc. Radiat. Transf. 2019, 224, 439–444. [Google Scholar] [CrossRef]
- Huang, T.; Meng, R.; Qi, J.; Liu, Y.; Wang, X.; Chen, Y.; Liao, R.; Ma, H. Fast Mueller matrix microscope based on dual DoFP polarimeters. Opt. Lett. 2021, 46, 1676–1679. [Google Scholar] [CrossRef]
- Lechner M, D. Influence of Mie scattering on nanoparticles with different particle sizes and shapes: Photometry and analytical ultracentrifugation with absorption optics. J. Serb. Chem. Soc. 2005, 70, 361–369. [Google Scholar] [CrossRef]
- Sun, D.; Li, Y.; Le, C.; Shi, K.; Huang, C.; Gong, S.; Yin, B. A semi-analytical approach for detecting suspended particulate composition in complex turbid inland waters (China). Remote Sens. Environ. 2013, 134, 92–99. [Google Scholar] [CrossRef]
- Forero López, A.D.; Truchet, D.M.; Rimondino, G.N.; Maisano, L.; Spetter, C.V.; Buzzi, N.S.; Nazzarro, M.S.; Malanca, F.E.; Furlong, O.; Ferná ndez Severini, M.D. Microplastics and suspended particles in a strongly impacted coastal environment: Composition, abundance, surface texture, and interaction with metal ions. Sci. Total Environ. 2021, 754, 142413. [Google Scholar] [CrossRef]
- Zhuo, Z.; Wang, H.; Liao, R.; Ma, H. Machine learning powered microalgae classification by use of polarized light scattering data. Appl. Sci. 2022, 12, 3422. [Google Scholar] [CrossRef]
- Guo, W.; Zeng, N.; Liao, R.; Xu, Q.; Guo, J.; He, Y.; Di, H.; Hua, D.; Ma, H. Simultaneous retrieval of aerosol size and composition by multi-angle polarization scattering measurements. Opt. Lasers Eng. 2022, 149, 106799. [Google Scholar] [CrossRef]
- Lu, Y.; Mao, J.; Zhang, Y.; Zhao, H.; Zhou, C.; Gong, X.; Wang, Q.; Zhang, Y. Simulation and Analysis of Mie-Scattering Lidar-Measuring Atmospheric Turbulence Profile. Sensors 2022, 22, 2333. [Google Scholar] [CrossRef] [PubMed]
- Evgenij, Z.; Dmitry, P.; Yevgen, G.; Yuriy, S.; Haijime, O.; Karri, M.; Yimo, N.; Hiroshi, K.; Tetsuo, Y.; Gorden, V. Validity criteria of the discrete dipole approximation. Appl. Opt. 2010, 49, 1267–1279. [Google Scholar]
- Li, Y.; Guo, F.; Wei, W.; Qu, J.; Ma, G.; Zhou, W. Pore size of macroporous polystyrene microspheres affects lipase immobilization. J. Mol. Catal. B Enzym. 2010, 66, 182–189. [Google Scholar] [CrossRef]
- Kitchen, J.C.; Zaneveld, J.R.V. A three-layered sphere model of the optical properties of phytoplankton. Limnol. Oceanogr. 1992, 37, 1680–1690. [Google Scholar] [CrossRef]
- Karmakar, B.; De, G.; Ganguli, D. Dense silica microspheres from organic and inorganic acid hydrolysis of TEOS. J. Non-Cryst. Solids 2000, 272, 119–126. [Google Scholar] [CrossRef]


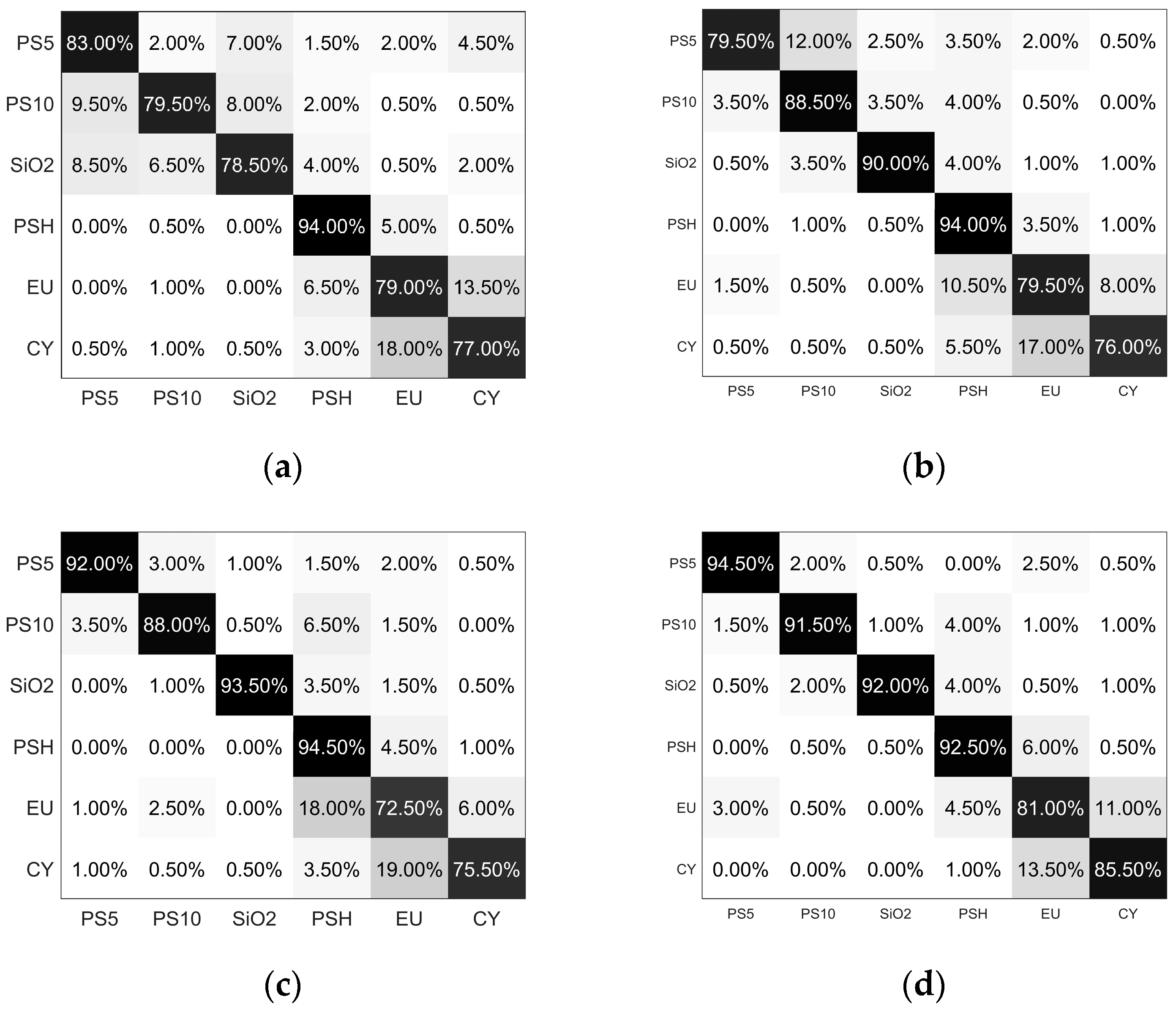


| Angular Selection Strategy | Single Angle: 120° | Discrete Angles: 60°, 90°, 120° | Forward Continuous Angles, Range from 60° to 90° | Backward Continuous Angles, Range from 90° to 120° | Continuous Angles, Range from 60° to 120° |
|---|---|---|---|---|---|
| Overall accuracy | 81.83% | 84.58% | 85.91% | 89.50% | 92.58% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, H.; Liao, R.; Li, H.; Wang, Y.; Zhou, H.; Li, J.; Huang, T.; Zhang, X.; Ma, H. Rapidly Measuring Scattered Polarization Parameters of the Individual Suspended Particle with Continuously Large Angular Range. Biosensors 2022, 12, 321. https://doi.org/10.3390/bios12050321
Chen Y, Wang H, Liao R, Li H, Wang Y, Zhou H, Li J, Huang T, Zhang X, Ma H. Rapidly Measuring Scattered Polarization Parameters of the Individual Suspended Particle with Continuously Large Angular Range. Biosensors. 2022; 12(5):321. https://doi.org/10.3390/bios12050321
Chicago/Turabian StyleChen, Yan, Hongjian Wang, Ran Liao, Hening Li, Yihao Wang, Hu Zhou, Jiajin Li, Tongyu Huang, Xu Zhang, and Hui Ma. 2022. "Rapidly Measuring Scattered Polarization Parameters of the Individual Suspended Particle with Continuously Large Angular Range" Biosensors 12, no. 5: 321. https://doi.org/10.3390/bios12050321





