Low Platinum-Content Electrocatalysts for Highly Sensitive Detection of Endogenously Released H2O2
Abstract
:1. Introduction
2. Hydrogen Peroxide Electrochemical Detection
2.1. H2O2 Sensing Analytical Methods
2.2. H2O2 Electrochemical Detection by Enzyme-Based Modified Electrodes
2.3. H2O2 Electrochemical Detection by Metallic Nanoparticles-Based Electrodes
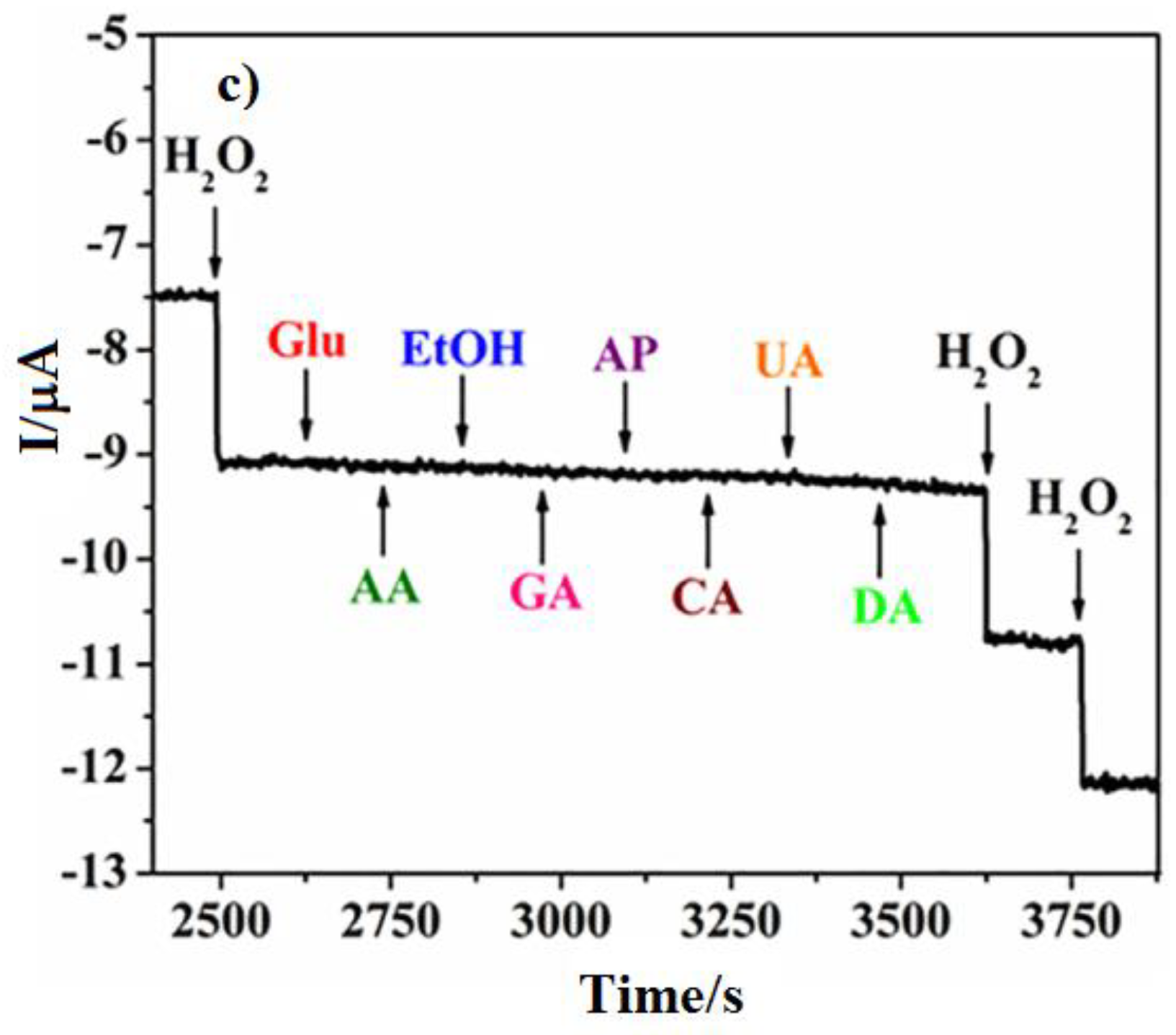
2.3.1. Pt-Based Nanocomposite Structures
2.3.2. Pt-Based@ Carbonaceous Structures
2.3.3. Pt-Based@ Highly Porous Structures
2.3.4. Pt-Based@ Conductive Polymer Structures
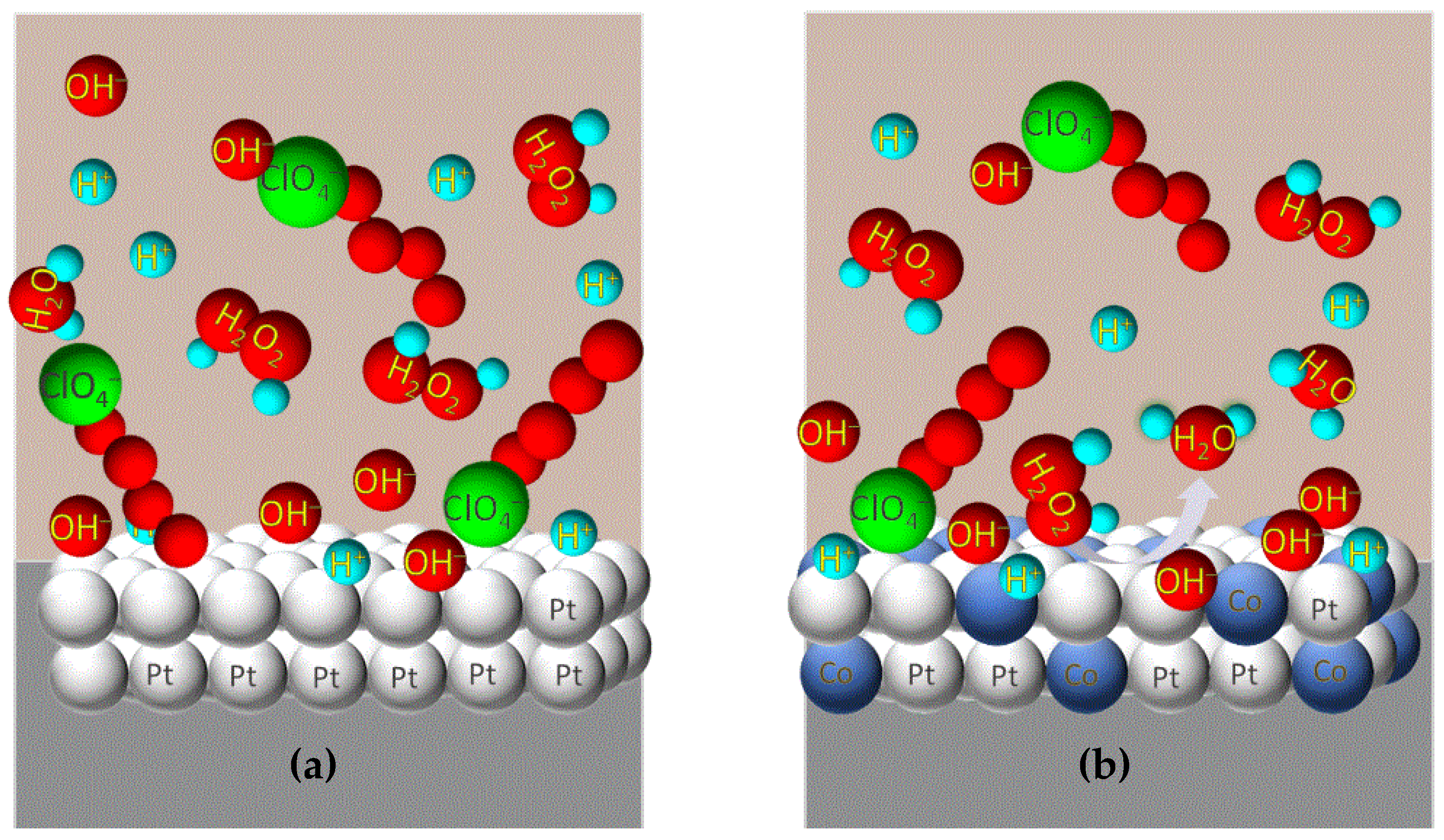
2.3.5. Pt-Based Bimetallic Alloys
| Electrocatalyst | Cyclic Voltammetry | Amperometry | Ref. | |||||
|---|---|---|---|---|---|---|---|---|
| Epc (V) | Ipc (μA) | Eworking (V) | Kcat (mol−1 cm3 s−1) | Linear Range (mM) | Sensitivity (μA mM−1 cm−2) | Detection Limit (μM) | ||
| SPGFE/Pt-PdBNC | −0.05/Ag/AgCl | 400.0 (10.0 mM H2O2) | −0.40 | 0.0050–6.00 | 804.0 | 0.870 | [74] | |
| Fe@Pt/C | −0.55/Ag/AgCl | 300.0 (20.0 mM H2O2) | −0.40 | 0.0025–0.0416 | 218.9 | 0.750 | [75] | |
| Pt0.75Ni0.25/C | 0.53/Ag/AgCl | 1075.2 (30.0 mM H2O2) | −0.20 | 11.2 × 103 (30.0 mM H2O2) | [18] | |||
| Pt0.75Co0.25/C | 0.37/Ag/AgCl | 1274.0 (30.0 mM H2O2) | −0.20 | 11.8 × 103 (30.0 mM H2O2) | ||||
| Pt-Sm | −0.40/RHE | 2025.0 (50.0 mM H2O2) | −0.40 | [89] | ||||
| Pt/Fe3O4/rGO | 0.00/SCE | 20.0 (0.2 mM H2O2) | 0.00 | 0.1000–2.40 | 0.973 | 1.580 | [79] | |
| PtNi/NCNFs(3:1) | −0.50/SCE | 350.6 (20.0 mM H2O2) | −0.10 | 31.3 × 103 (1.0 mM H2O2) | 0.0005–8.00 | 248.5 | 0.0375 | [90] |
| PtNi/CeO2/NCNFs | −0.40/SCE | 506.5 (20.0 mM H2O2) | −0.10 | 35.2 × 103 (1.0 mM H2O2) | 0.0005–15.00 | 345.0 | 0.025 | [91] |
| CNT/SiO2/(Au/Pt) | −0.15/Ag/AgCl | 23.0 (1.5 mM H2O2) | −0.10 | 0.0005–1.67 | 0.300 | [82] | ||
| Pt NF-N-Gr | −0.40/Ag/AgCl | −0.40 | 0.0010–1.00 | 61.23 | 0.340 | [80] | ||
| Pt/PPy-C35% | 0.80/RHE | 11,600.0 (50.0 mM H2O2) | 0.90 | [85] | ||||
| PtNi/MWCNTs | −0.40/SCE | 275.0 (10.0 mM H2O2) | −0.45 | 0.0002–24.60 | 2123.1 | 0.060 | [87] | |
| Cu@Pt/C | −0.30/SCE | 310.0 (20.0 mM H2O2) | −0.30 | 0.0005–32.56 | 351.3 | 0.150 | [68] | |
| np-PtCu | 0.30/RHE | 0.70 | 0.0100–1.70 | 64.7 | 0.100 | [92] | ||
| PPy/Pt | −0.175/Ag/AgCl | 180.0 (4.0 mM H2O2) | −0.175 | 0.0250–0.50 | 305.45 | 0.600 | [86] | |
| Cu@PtPd/C | −0.10/Ag/AgCl | −0.10 | 0.0050–0.25 | 530.0 | 0.370 | [88] | ||
| Pt-Au/rGSs | 0.10/SCE | 35.0 (0.1 mM H2O2) | 0.00 | 0.0010–1.78 | 0.735 | 0.310 | [69] | |
| Pt-Pd/CFME | −0.50/Ag/AgCl | 7.7 (5.0 mM H2O2) | −0.40 | 0.0050–3.92 | 11,600.0 | 0.420 | [93] | |
| PtNPs@GR/GLN | 0.16/Ag/AgCl | 0.14 | 0.00005–0.871 | 5643.0 | 0.037 | [83] | ||
3. Electrocatalysts’ Intrinsic Catalytic Activity toward H2O2 Reduction
- Catalyst’ active surface area;
- Synergistic effects of catalyst mixture.
3.1. Catalyst’ Active Surface Area
3.2. Synergistic Effects of Catalyst-Blend Mixture
- (i).
- Geometric factor: A change in the surface geometry of the catalyst surface as a result of distance from its nearest neighbour atom (strain or ensemble effects);
- (ii).
- Electronic effect: A change in the reactivity of the catalyst as a result of electron transfer or polarisation between the two adjacent metals, which leads to a change in the width of the surface d-band and a shift in the binding energy aimed at constant band filling (ligand or electronic effect);
- (iii).
- Co-catalytic effect: Upon adding a second metal element in the Pt lattice, it can provide a reinforced adsorption site for some intermediate species or reactants, thereby enhancing their interaction with the catalyst. It is referred to as a synergistic effect because the combined action of both metals fosters improved adsorption to the catalyst. This way reinforces the catalytic ability of the bimetallic NPs.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marzo, N.D.; Chisci, E.; Giovannoni, R. The Role of Hydrogen Peroxide in Redox-Dependent Signalling: Homeostatic and Pathological Responses in Mammalian Cells. Cells 2018, 7, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pędziwiatr, P.; Mikołajczyk, F.; Zawadzki, D.; Mikołajczyk, K.; Bedka, A. Decomposition of hydrogen peroxide-kinetics and review of chosen catalysts. Acta Innov. 2018, 26, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Winterbourn, C. Biological Production, Detection and Fate of Hydrogen Peroxide. Antioxid. Redox Signal. 2017, 29, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.H.; Li, B.S.; Dai, Z.J. Oxidative Desulfurization of Fuel Oil with Hydrogen Peroxide Catalyzed by Keggin-type Polyoxotungstate in a DC Magnetic Field. Pet. Sci. Technol. 2010, 28, 700–711. [Google Scholar] [CrossRef]
- Li, L.; Lee, S.; Lae, H.L.; Youn, H.J. Hydrogen peroxide bleaching of hardwood kraft pulp with adsorbed birch xylan and its effect on paper properties. BioResources 2011, 6, 721–736. [Google Scholar] [CrossRef]
- Hachem, C.; Bocquillon, F.; Zahraa, O.; Bouchy, M. Decolourization of textile industry wastewater by the photocatalytic degradation process. Dye. Pigm. 2001, 49, 117–125. [Google Scholar] [CrossRef]
- Targhan, H.; Evans, P.; Bahrami, K. A review of the role of hydrogen peroxide in organic transformations. J. Ind. Eng. Chem. 2021, 104, 295–332. [Google Scholar] [CrossRef]
- Pravda, J. Hydrogen peroxide and disease: Towards a unified system of pathogenesis and therapeutics. Mol. Med. 2020, 26, 41. [Google Scholar] [CrossRef]
- Tredwin, C.J.; Naik, S.; Lewis, N.J.; Scully, C. Hydrogen peroxide tooth-whitening (bleaching) products: Review of adverse effects and safety issues. Br. Dent. J. 2006, 200, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Dutta, A.; Das, S.; Samanta, P.; Roy, S.; Adhikary, B.; Biswas, P. Non–enzymatic amperometric sensing of hydrogen peroxide at a CuS modified electrode for the determination of urine H2O2. Electrochim. Acta 2014, 144, 282–287. [Google Scholar] [CrossRef]
- Ksibi, M. Chemical oxidation with hydrogen peroxide for domestic wastewater treatment. Chem. Eng. J. 2006, 119, 161–165. [Google Scholar] [CrossRef]
- Anojčić, J.; Guzsvany, V.; Vajdle, O.; Kónya, Z.; Kalcher, K. Rapid amperometric determination of H2O2 by a Pt nanoparticle/Vulcan XC72 composite-coated carbon paste electrode in disinfection and contact lens solutions. Monatsh. Chem. 2018, 149, 1727–1738. [Google Scholar] [CrossRef]
- Al-Attabi, Z.H.; D’Arcy, B.R.; Deeth, H.C. Effect of hydrogen peroxide on volatile sulphur compounds in UHT milk. In Proceedings of the Fifth Euro-Global Summit and Expo on Food & Beverages, Alicante, Spain, 16–18 June 2015. [Google Scholar]
- Gerard, C.; Huguet, S.; Laurence, E.; Ferry, I.; Lafay, M.; Fouque, J.; Madar, O.; Rezai, K.; Giard, C. Permeability and Release of Decontaminating Agent Used in Cytotoxic Reconstitution Units: Diffusion of Hydrogen Peroxide in IV Bags. Pharm. Technol. Hosp. Pharm. 2017, 2, 17–21. [Google Scholar] [CrossRef]
- Arefin, S.; Sarker, M.A.H.; Islam, M.A.; Harun-ur-Rashid, M.; Islam, M.N. Use of Hydrogen Peroxide (H2O2) in raw cow’s milk preservation. J. Adv. Vet. Anim. Res. 2017, 4, 371. [Google Scholar] [CrossRef]
- Baharun, N.; Ling, O.P.; Ardani, M.R.; Ariffin, K.S.; Yaraghi, A.; Abdullah, N.S.; Putra, T.A.R.; Ismail, S. Effect of hydrogen peroxide and lead(II) nitrate on gold cyanide leaching of Malaysian mesothermal deposit gold ore. Physicochem. Probl. Miner. Process. 2020, 56, 905–918. [Google Scholar] [CrossRef]
- Heravi, M.; Ghalavand, N.; Hashemi, E. Hydrogen Peroxide as a Green Oxidant for the Selective Catalytic Oxidation of Benzylic and Heterocyclic Alcohols in Different Media: An Overview. Chemistry 2020, 2, 101–178. [Google Scholar] [CrossRef] [Green Version]
- Morais, A.; Salgado, J.R.; Sljukic, B.; Santos, D.; Sequeira, C. Electrochemical behaviour of carbon supported Pt electrocatalysts for H2O2 reduction. Int. J. Hydrog. Energy 2012, 37, 14143–14151. [Google Scholar] [CrossRef]
- Daniel, G.; Zhang, Y.; Lanzalaco, S.; Brombin, F.; Kosmala, T.; Granozzi, G.; Wang, A.; Brillas, E.; Sirés, I.; Durante, C. Chitosan-Derived Nitrogen-Doped Carbon Electrocatalyst for a Sustainable Upgrade of Oxygen Reduction to Hydrogen Peroxide in UV-Assisted Electro-Fenton Water Treatment. ACS Sustain. Chem. Eng. 2020, 8, 14425–14440. [Google Scholar] [CrossRef]
- Morais, A.L.; Rijo, P.; Batanero Hernán, M.B.; Nicolai, M. Biomolecules and Electrochemical Tools in Chronic Non-Communicable Disease Surveillance: A Systematic Review. Biosensors 2020, 10, 121. [Google Scholar] [CrossRef]
- Hernandez, K.; Berenguer-Murcia, A.; Rodrigues, R.C.; Fernandez-Lafuente, R. Hydrogen Peroxide in Biocatalysis. A Dangerous Liaison. Curr. Org. Chem. 2012, 16, 2652–2672. [Google Scholar] [CrossRef]
- Liang, F.; Hu, L.; Li, Y.; Majeed, S.; Li, H.; Cai, H.; Yang, X.; Xu, G. Low-potential determination of hydrogen peroxide, uric acid and uricase based on highly selective oxidation of p-hydroxyphenylboronic acid by hydrogen peroxide. Sens. Actuators B Chem. 2013, 178, 144–148. [Google Scholar] [CrossRef]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 2014, 1840, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Kehrer, J.P.; Koltz, L. Free radicals and related reactive species as mediators of tissue injury and disease: Implications for Health. Crit. Rev. Toxicol. 2015, 45, 765–798. [Google Scholar] [CrossRef] [PubMed]
- Molavian, H.; Tonekaboni, A.M.; Kohandel, M.; Sivaloganathan, S. The Synergetic Coupling among the Cellular Antioxidants Glutathione Peroxidase/Peroxiredoxin & Other Antioxidants and its Effect on the Concentration of H2O2. Sci. Rep. 2015, 5, 13620. [Google Scholar] [CrossRef]
- Lennicke, C.; Rahn, J.; Lichtenfels, R.; Wessjohann, L.A.; Seliger, B. Hydrogen peroxide–production, fate and role in redox signaling of tumor cells. Cell Commun. Signal 2015, 13, 39. [Google Scholar] [CrossRef] [Green Version]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [Green Version]
- Dimozi, A.; Mavrogonatou, E.; Sklirou, A.; Kletsas, D. Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur. Cells Mater. 2015, 30, 89–103. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Su, J.; Liu, W.; Altura, B.T.; Altura, B.M. Hydrogen peroxide induces apoptosis in cerebral vascular smooth muscle cells: Possible relation to neurodegenerative diseases and strokes. Brain Res. Bull. 2003, 62, 101–106. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [Green Version]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant Mechanisms in Renal Injury and Disease. Antioxid. Redox Signal. 2016, 25, 119–146. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signalling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Runchel, C.; Matsuzawa, A.; Ichijo, H. Mitogen-activated protein kinases in mammalian oxidative stress responses. Antioxid. Redox Signal 2011, 15, 205–218. [Google Scholar] [CrossRef]
- Tabner, B.J.; Turnbull, S.; Fullwood, N.J.; German, M.; Allsop, D. The production of hydrogen peroxide during early-stage protein aggregation: A common pathological mechanism in different neurodegenerative diseases? Biochem. Soc. Trans. 2005, 33, 548–550. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Yan, Y.; Zhu, L.; Li, X.; Li, G. An amperometric biosensor for the detection of hydrogen peroxide released from human breast cancer cells. Biosens. Bioelectron. 2013, 41, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Steinhorn, B.; Sorrentino, A.; Badole, S.; Bogdanova, Y.; Belousov, V.; Michel, T. Chemogenetic generation of hydrogen peroxide in the heart induces severe cardiac dysfunction. Nat. Commun. 2018, 9, 4044. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.-K.; Jeong, Y.S.; Kim, S.; Jang, J. Fluorescent polymer nanoparticle for selective sensing of intracellular hydrogen peroxide. ACS Nano 2012, 6, 8516–8524. [Google Scholar] [CrossRef]
- Magara, K.; Ikeda, T.; Sugimoto, T.; Hosoya, S. Quantitative Analysis of Hydrogen Peroxide by High Performance Liquid Chromatography. Japan Tappi J. 2007, 61, 1481–1493. [Google Scholar] [CrossRef] [Green Version]
- Tahirović, A.; Copra, A.; Omanović-Miklicanin, E.; Kalcher, K. A chemiluminescence sensor for the determination of hydrogen peroxide. Talanta 2007, 72, 1378–1385. [Google Scholar] [CrossRef]
- Gimeno, M.; Mayoral, M.; Andrés, J. A potentiometric titration for H2O2 determination in the presence of organic compounds. Anal. Methods 2013, 5, 1510–1514. [Google Scholar] [CrossRef]
- Dhara, K.; Mahapatra, D.R. Recent advances in electrochemical nonenzymatic hydrogen peroxide sensors based on nanomaterials: A review. J. Mater. Sci. 2019, 54, 12319–12357. [Google Scholar] [CrossRef]
- Ledo, A.; Fernandes, E.; Brett, C.; Barbosa, R. Enhanced Selectivity and Stability of Ruthenium Purple-Modified Carbon Fiber Microelectrodes for Detection of Hydrogen Peroxide in Brain Tissue. Sens. Actuators B Chem. 2020, 311, 127899. [Google Scholar] [CrossRef]
- Chaisuksant, R.; Chomsook, T.; Manthong, N.; Kalcher, K. Low Cost Hydrogen Peroxide Sensor from Manganese Oxides Modified Pencil Graphite Electrode. Procedia Chem. 2016, 20, 81–84. [Google Scholar] [CrossRef] [Green Version]
- Katsounaros, I.; Schneider, W.B.; Meier, J.; Benedikt, U.; Biedermann, P.U.; Auer, A.A.; Mayrhofer, K.J.J. Hydrogen peroxide electrochemistry on platinum: Towards understanding the oxygen reduction reaction mechanism. Phys. Chem. Chem. Phys. 2012, 14, 7384–7391. [Google Scholar] [CrossRef]
- Elewi, A.; AL-Shammaree, S.; Al-sammarraie, A. Hydrogen peroxide biosensor based on hemoglobin-modified gold nanoparticles–screen printed carbon electrode. Sens. Bio-Sens. Res. 2020, 28, 100340. [Google Scholar] [CrossRef]
- Chen, J.; Yu, Q.; Fu, W.; Chen, X.; Zhang, Q.; Dong, S.; Chen, H.; Zhang, S. A Highly Sensitive Amperometric Glutamate Oxidase Microbiosensor Based on a Reduced Graphene Oxide/Prussian Blue Nanocube/Gold Nanoparticle Composite Film-Modified Pt Electrode. Sensors 2020, 20, 2924. [Google Scholar] [CrossRef]
- Si, B.; Song, E. Recent Advances in the Detection of Neurotransmitters. Chemosensors 2018, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Sanford, A.L.; Morton, S.W.; Whitehouse, K.L.; Oara, H.M.; Lugo-Morales, L.Z.; Roberts, J.G.; Sombers, L.A. Voltammetric Detection of Hydrogen Peroxide at Carbon Fiber Microelectrodes. Anal. Chem. 2010, 82, 5205–5210. [Google Scholar] [CrossRef] [Green Version]
- Pullano, S.A.; Greco, M.; Bianco, M.G.; Foti, D.; Brunetti, A.; Fiorillo, A.S. Glucose biosensors in clinical practice: Principles, limits and perspectives of currently used devices. Theranostics 2022, 12, 493–511. Available online: https://www.thno.org/v12p0493.htm (accessed on 15 March 2022). [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [Green Version]
- Swamy, N.K.; Sandeep, S.; Santhosh, A.S. Enzyme Immobilization Methods and Role of Conductive Polymers in Fabrication of Enzymatic Biosensors. Indian J. Adv. Chem. Sci. 2017, S2, 1–5. [Google Scholar] [CrossRef]
- Sin, M.L.; Mach, K.E.; Wong, P.K.; Liao, J.C. Advances and challenges in biosensor-based diagnosis of infectious diseases. Expert Rev. Mol. Diagn. 2014, 14, 225–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Li, L.; Zhou, W.; Shao, Z.; Chen, X. Advances in Non-Enzymatic Glucose Biosensors Based on Metal Oxides. J. Mater. Chem. B 2016, 4, 7333–7349. [Google Scholar] [CrossRef] [PubMed]
- Defnet, P.A.; Han, C.; Zhang, B. Temporally-Resolved Ultrafast Hydrogen Adsorption and Evolution on Single Platinum Nanoparticles. Anal. Chem. 2019, 91, 4023–4030. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Chen, S.-M.; Thangaraj, B.; Elumalai, P.; Raja, P.; Chen, T.-W.; Kannan, R.; Kannaiyan, D.; George, G. A review of the advanced developments of electrochemical sensors for the detection of toxic and bioactive molecules. Inorg. Chem. Front. 2019, 6, 3418–3439. [Google Scholar] [CrossRef]
- Rai, M.; Gade, A.; Gaikwad, S.; Marcato, P.D.; Durán, N. Biomedical Applications of Nanobiosensors: The State-of-the-Art. J. Braz. Chem. Soc. 2012, 23, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Feng, B.; Li, H.; Li, H. Controlled Assembly of Hierarchical Metal Catalysts with Enhanced Performances. Cell 2019, 5, 805–837. [Google Scholar] [CrossRef] [Green Version]
- Prasai, B.; Wilson, A.; Wiley, B.J.; Ren, Y.; Petkov, V. On the road to metallic nanoparticles by rational design: Bridging the gap between atomic-level theoretical modeling and reality by total scattering experiments. Nanoscale 2015, 7, 17902–17922. [Google Scholar] [CrossRef]
- Gomez, C.G.; Silva, A.M.; Strumia, M.C.; Avalle, L.B.; Rojas, M.I. The origin of high electrocatalytic activity of hydrogen peroxide reduction reaction by a g-C3N4/HOPG sensor. Nanoscale 2017, 9, 11170–11179. [Google Scholar] [CrossRef]
- Welch, C.M.; Compton, R.G. The use of nanoparticles in electroanalysis: A review. Anal. Bioanal. Chem. 2006, 384, 601–619. [Google Scholar] [CrossRef]
- Yang, G.; Akhade, S.; Chen, X.; Liu, Y.; Lee, M.-S.; Glezakou, V.-A.; Rousseau, R.; Lercher, J. Nature of Hydrogen Adsorption on Platinum in the Aqueous Phase. Angew. Chem. Int. Ed. 2018, 58, 3527–3532. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Goodman, D.W. Dissociative adsorption and hydrogenolysis of ethane over clean and nickel-covered platinum (111). J. Phys. Chem. 1990, 94, 5342–5347. [Google Scholar] [CrossRef]
- Li, K.; Yan, S.; Chen, H.; Wang, B.; Li, G.; Shi, Y.; Xu, X. Facile Solvothermal Synthesis of Hybrid SnS2/Platinum Nanoparticles for Hydrogen Peroxide Biosensing. J. Bionanosci. 2015, 9, 335–340. [Google Scholar] [CrossRef]
- Chen, W.; Cao, J.; Fu, W.; Zhang, J.; Qian, G.; Yang, J.; Chen, D.; Zhou, X.; Yuan, W.; Duan, X. Molecular-Level Insights into the Notorious CO Poisoning of Platinum Catalyst. Angew. Chem. (Int. Ed. Engl.) 2022, 61, e202200190. [Google Scholar] [CrossRef] [PubMed]
- Molochas, C.; Tsiakaras, P. Carbon Monoxide Tolerant Pt-Based Electrocatalysts for H2-PEMFC Applications: Current Progress and Challenges. Catalysts 2021, 11, 1127. [Google Scholar] [CrossRef]
- Al-Akraa, I. A Simple and Effective Way to Overcome Carbon Monoxide Poisoning of Platinum Surfaces in Direct Formic Acid Fuel Cells. Int. J. Electrochem. Sci. 2019, 14, 8267–8275. [Google Scholar] [CrossRef]
- Valdés-López, V.; Mason, T.; Shearing, P.; Brett, D.J.L. Carbon monoxide poisoning and mitigation strategies for polymer electrolyte membrane fuel cells–A review. Prog. Energy Combust. Sci. 2020, 79, 100842. [Google Scholar] [CrossRef]
- Zhao, W.; Jin, J.; Wu, H.; Wang, S.; Fneg, C.; Yang, S.; Ding, Y. Electrochemical hydrogen peroxide sensor based on carbon supported Cu@Pt core-shell nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 185–190. [Google Scholar] [CrossRef]
- Yu, G.; Wu, W.; Pan, X.; Zhao, Q.; Wei, X.; Lu, Q. High Sensitive and Selective Sensing of Hydrogen Peroxide Released from Pheochromocytoma Cells Based on Pt-Au Bimetallic Nanoparticles Electrodeposited on Reduced Graphene Sheets. Sensors 2015, 15, 2709–2722. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, C.C. Eliminating the Interference of Oxygen for Sensing Hydrogen Peroxide with the Polyaniline Modified Electrode. Sensors 2008, 8, 8237–8247. [Google Scholar] [CrossRef]
- Mueller, J.E.; Krtil, P.; Kibler, L.A.; Jacob, T. Bimetallic alloys in action: Dynamic atomistic motifs for electrochemistry and catalysis. Phys. Chem. Chem. Phys. 2014, 16, 15029–15042. [Google Scholar] [CrossRef]
- Yang, Z.; Nakashima, N. A simple preparation of very high methanol tolerant cathode electrocatalyst for direct methanol fuel cell based on polymer-coated carbon nanotube/platinum. Sci. Rep. 2015, 5, 12236–12245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rick, J.; Tsai, M.-C.; Hwang, B.J. Biosensors Incorporating Bimetallic Nanoparticles. Nanomaterials 2016, 6, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, X.; Chen, C.; Zhao, H.; Chai, Y.; Lan, M. Novel snowflake-like Pt-Pd bimetallic clusters on screen-printed gold nanofilm electrode for H2O2 and glucose sensing. Biosens. Bioelectron. 2012, 36, 262–266. [Google Scholar] [CrossRef]
- Mei, H.; Wu, W.; Yu, B.; Wu, H.; Wang, S.; Xia, Q.-H. Nonenzymatic electrochemical sensor based on Fe@Pt core-shell nanoparticles for hydrogen peroxide, glucose and formaldehyde. Sens. Actuators B Chem. 2016, 223, 68–75. [Google Scholar] [CrossRef]
- Wang, T.; Pan, L.; Zhang, X.; Zou, J.-J. Insights into the Pt (111) Surface Aid in Predicting the Selective Hydrogenation Catalyst. Catalysts. 2020, 10, 1473. [Google Scholar] [CrossRef]
- Xin, H.; Linic, S. Communications: Exceptions to the d-band model of chemisorption on metal surfaces: The dominant role of repulsion between adsorbate states and metal d-states. J. Chem. Phys. 2010, 132, 221101. [Google Scholar] [CrossRef]
- Oliveira-Brett, A.M. Electron Transfer Reactions in Biological Systems. In Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–8. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Z.; Chen, C.; Wu, Y.; Zhu, Z.; Zhao, H.; Lan, M. A Novel Biomimetic Hydrogen Peroxide Biosensor Based on Pt Flowers-decorated Fe3O4/Graphene Nanocomposite. Electroanalysis 2017, 29, 1518–1523. [Google Scholar] [CrossRef]
- Tajabadi, M.T.; Sookhakian, M.; Zalnezhad, E.; Yoon, G.H.; Hamouda, A.M.S.; Azarang, M.; Basirun, W.J.; Alias, Y. Electrodeposition of flower-like platinum on electrophoretically grown nitrogen-doped graphene as a highly sensitive electrochemical non-enzymatic biosensor for hydrogen peroxide detection. Appl. Surf. Sci. 2016, 386, 418–426. [Google Scholar] [CrossRef]
- Detsi, E.; Tolbert, S.; Punzhin, S.; De Hosson, J.T.M. Metallic muscles and beyond: Nanofoams at work. J. Mater. Sci. 2015, 51, 615–634. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Li, J.; Ren, W.; Wen, D.; Dong, S.; Wang, E. Carbon Nanotube/Silica Coaxial Nanocable as a Three-Dimensional Support for Loading Diverse Ultra-High-Density Metal Nanostructures: Facile Preparation and Use as Enhanced Materials for Electrochemical Devices and SERS. Chem. Mater. 2009, 21, 2247–2257. [Google Scholar] [CrossRef]
- Thirumalraj, B.; Sakthivel, R.; Chen, S.-M.; Rajkumar, C.; Yu, L.-k.; Kubendhiran, S. A reliable electrochemical sensor for determination of H2O2 in biological samples using platinum nanoparticles supported graphite/gelatin hydrogel. Microchem. J. 2019, 146, 673–678. [Google Scholar] [CrossRef]
- Lakard, B. Electrochemical Biosensors Based on Conducting Polymers: A Review. Appl. Sci. 2020, 10, 6614. [Google Scholar] [CrossRef]
- Oliveira, R.C.P.; Milikić, J.; Daş, E.; Yurtcan, A.B.; Santos, D.M.F.; Šljukić, B. Platinum/polypyrrole-carbon electrocatalysts for direct borohydride-peroxide fuel cells. Appl. Catal. B Environ. 2018, 238, 454–464. [Google Scholar] [CrossRef]
- Xing, L.; Rong, Q.; Ma, Z. Non-enzymatic electrochemical sensing of hydrogen peroxide based on polypyrrole/platinum nanocomposites. Sen. Actuators B Chem. 2015, 221, 242–247. [Google Scholar] [CrossRef]
- Mei, H.; Wu, H.; Wu, W.; Wang, S.; Xia, Q. Ultrasensitive electrochemical assay of hydrogen peroxide and glucose based on PtNi alloy decorated MWCNTs. RSC Adv. 2015, 5, 102877–102884. [Google Scholar] [CrossRef]
- Gutierrez, F.A.; Giordana, I.S.; Fuertes, V.C.; Montemerlo, A.E.; Sieben, J.M.; Alvarez, A.E.; Rubianes, M.D.; Rivas, G.A. Analytical applications of Cu@PtPd/C nanoparticles for the quantification of hydrogen peroxide. Microchem. J. 2018, 141, 240–246. [Google Scholar] [CrossRef]
- Cardoso, D.S.P.; Santos, D.M.F.; Šljukić, B.; Sequeira, C.A.C.; Macciò, D.; Saccone, A. Platinum-rare earth cathodes for direct borohydride-peroxide fuel cells. J. Power Sources 2016, 307, 251–258. [Google Scholar] [CrossRef]
- Guan, H.; Zhao, Y.; Zhang, J.; Liu, Y.; Yuan, S.; Zhang, B. Uniformly dispersed PtNi alloy nanoparticles in porous N-doped carbon nanofibers with high selectivity and stability for hydrogen peroxide detection. Sens. Actuators B Chem. 2018, 261, 354–363. [Google Scholar] [CrossRef]
- Guan, H.; Zhao, Y.; Zhang, J.; Liu, Y.; Zhang, B. Rapid quantitative determination of hydrogen peroxide using an electrochemical sensor based on PtNi alloy/CeO2 plates embedded in N-doped carbon nanofibers. Electrochim. Acta 2018, 295, 997–1005. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Z.; Zhou, Q.; Xu, C.; Hou, J. Nanoporous platinum-copper flowers for non-enzymatic sensitive detection of hydrogen peroxide and glucose at near-neutral pH values. Mikrochim. Acta 2019, 186, 631. [Google Scholar] [CrossRef]
- Li, H.; Zhao, H.; He, H.; Shi, L.; Cai, X.; Lan, M. Pt-Pd bimetallic nanocoral modified carbon fiber microelectrode as a sensitive hydrogen peroxide sensor for cellular detection. Sens. Actuators B Chem. 2018, 260, 174–182. [Google Scholar] [CrossRef]
- Darby, M.T.; Sykes, E.C.H.; Michaelides, A.; Stamatakis, M. Carbon Monoxide Poisoning Resistance and Structural Stability of Single Atom Alloys. Top. Catal. 2018, 61, 428–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Statista Market and Consumption Website Data Homepage. Available online: https://www.statista.com/ (accessed on 23 March 2022).
- Zhang, Z.-P.; Zhang, Y.-W.; Jiang, H. Fundamentals and Structural Characterization of Shape-Controlled Bimetallic Nanostructures. In Bimetallic Nanostructures-Shape-Controlled Synthesis for Catalysis, Plasmonics, and Sensing Applications; Zhang, Y.-W., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 3–51. [Google Scholar]
- Katsounaros, I.; Schneider, W.B.; Meier, J.C.; Benedikt, U.; Biedermann, P.U.; Auer, A.A.; Cuesta, A.; Mayrhofer, K.J.J. The impact of spectator species on the interaction of H2O2 with platinum–implications for the oxygen reduction reaction pathways. Phys. Chem. Chem. Phys. 2013, 15, 8058–8068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castanheira, L.F.; Dubau, L.; Mermoux, M.; Berthomé, G.; Caque, N.; Rossinot, E.; Chatenet, M.; Maillard, F. Carbon Corrosion in Proton-Exchange Membrane Fuel Cells: From Model Experiments to Real-Life Operation in Membrane Electrode Assemblies. ACS Catal. 2014, 4, 2258–2267. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Hu, Y.; Wu, J.; Wei, H. Metal Oxide-Based Nanomaterials for Nanozymes. In Nanozymes: Next Wave of Artificial Enzymes; Wang, X., Guo, W., Hu, Y., Wu, J., Wei, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 57–91. [Google Scholar]
- Gao, L.Z.; Zhuang, J.; Nie, L.; Zhang, J.B.; Zhang, Y.; Gu, N.; Wang, T.H.; Feng, J.; Yang, D.L.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Zhou, Q.; Shi, G. Conducting Polymer-Based Catalysts. J. Am. Chem. Soc. 2016, 138, 2868–2876. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morais, A.; Rijo, P.; Batanero, B.; Nicolai, M. Low Platinum-Content Electrocatalysts for Highly Sensitive Detection of Endogenously Released H2O2. Biosensors 2022, 12, 672. https://doi.org/10.3390/bios12090672
Morais A, Rijo P, Batanero B, Nicolai M. Low Platinum-Content Electrocatalysts for Highly Sensitive Detection of Endogenously Released H2O2. Biosensors. 2022; 12(9):672. https://doi.org/10.3390/bios12090672
Chicago/Turabian StyleMorais, Ana, Patrícia Rijo, Belen Batanero, and Marisa Nicolai. 2022. "Low Platinum-Content Electrocatalysts for Highly Sensitive Detection of Endogenously Released H2O2" Biosensors 12, no. 9: 672. https://doi.org/10.3390/bios12090672







