Recent Progress in Nanomaterial-Based Biosensors and Theranostic Nanomedicine for Bladder Cancer
Abstract
:1. Introduction
2. Nanomaterial-Based Bladder Cancer Diagnosis
2.1. Urine Cancer Biomarker Test
2.1.1. Nanomaterial-Based Electrochemical Sensors
Electrochemical ELISA
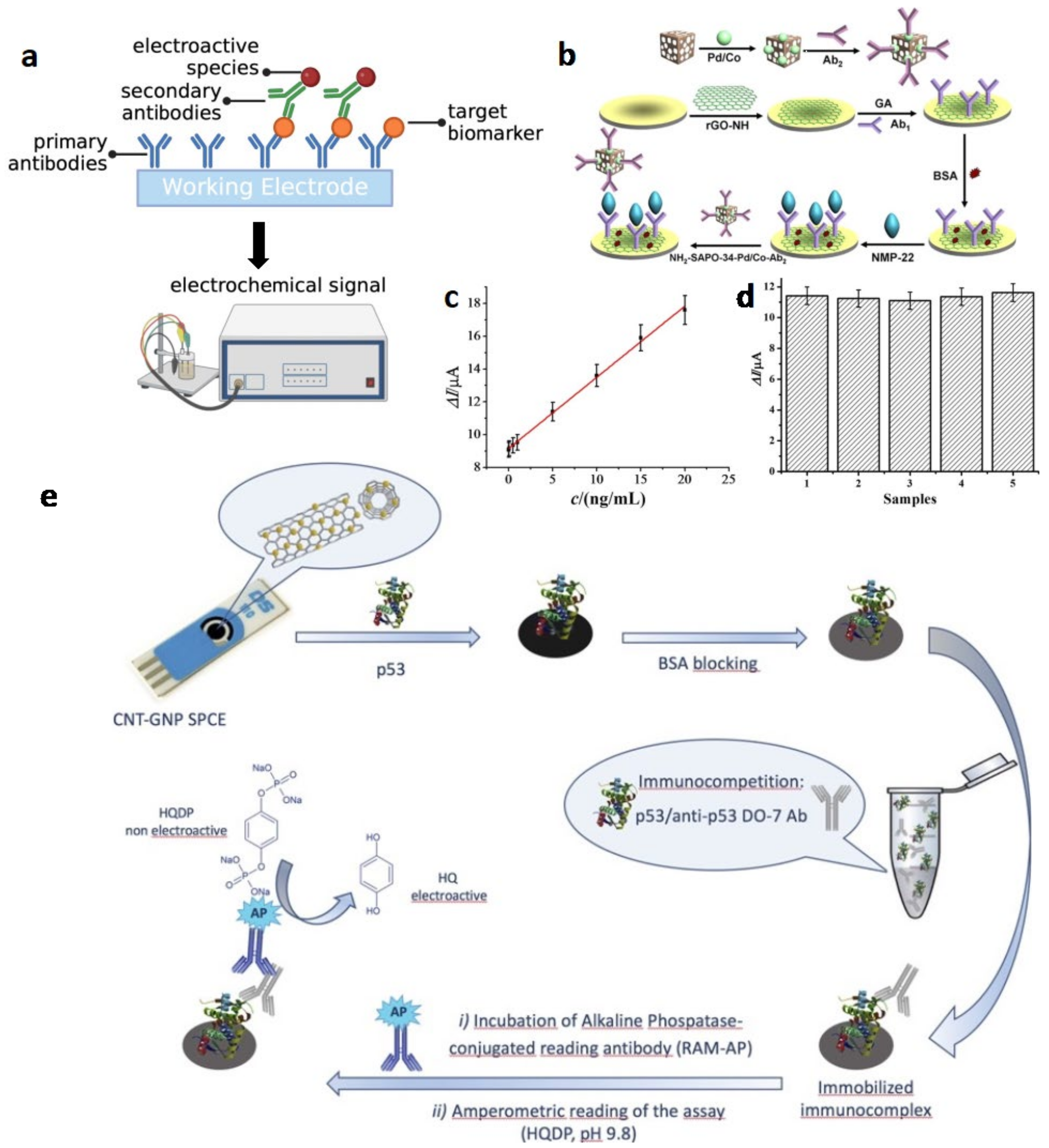
Label-Free Electrochemical Sensor
| No. | Technique | Biomarker | Working Electrode Modification | Electroactive Species | LOD | Linear Range | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | eELISA (sandwich) | NMP-22 | Ab1/NH2-rGO/GCE | Ab2-Pd/Co NPs | 0.33 pg/mL | 0.001–20 ng/mL | Wu et al. (2016) [33] |
| 2 | eELISA (sandwich) | MMP-9 | Ab1-MPs/ITO | Ab2-ALP | 1.9 pM (standard solution); BRREAK 2.3 pM (standard urine) | 10 pM–10 nM | Song et al. (2020) [34] |
| 3 | eELISA (sandwich) | NUMA1, CFHR1 | Ab1/FNAB/gold electrode | Ab2-ALP | 1.13 ng/mL, BRREAK 0.97 ng/mL | 1–100 ng/mL | Arya and Estrela (2018) [41] |
| 4 | eELISA (sandwich) | ApoA1 | Ab1-biotin/avidin/ITO | Ab2-ALP | 1 pM | 1 pM–100 nM | Kim et al. (2019) [42] |
| 5 | eELISA (competitive) | p53 | CNT/GNP-SPCEs | Anti-p53 Ab/RAM-ALP | 14 pM | 20 pM–10 nM | Giannetto et al. (2017) [35] |
| 6 | Label-free electrochemical sensor | NMP-22 | Au@Ab/Pd/Ag/NH2-GS/GCE | Au@Pd/Ag NPs | 3.3 pg/mL | 0.01–18 ng/mL | Li et al. (2014) [38] |
| 7 | Label-free electrochemical sensor | NMP-22 | MIPs/ZnO nanorods | 1.0 pg/mL | 128–588 ng/mL | Lee et al. (2016) [39] | |
| 8 | Label-free electrochemical sensor | NMP-22 | Ab/AuPdPt NPs | GCE/rGO-TEPA | 0.01 U/mL | 0.040–20 U/mL | Ma et al. (2015) [43] |
| 9 | Label-free electrochemical sensor | MMP-7 | Au electrode/CNT/GNPs- designed peptide substrate | CNT/GNPs | 6 pg/mL | 1 × 10−2 to 1 × 103 ng/mL | Palomar et al. (2020) [40] |
Field-Effect Transistor (FET)
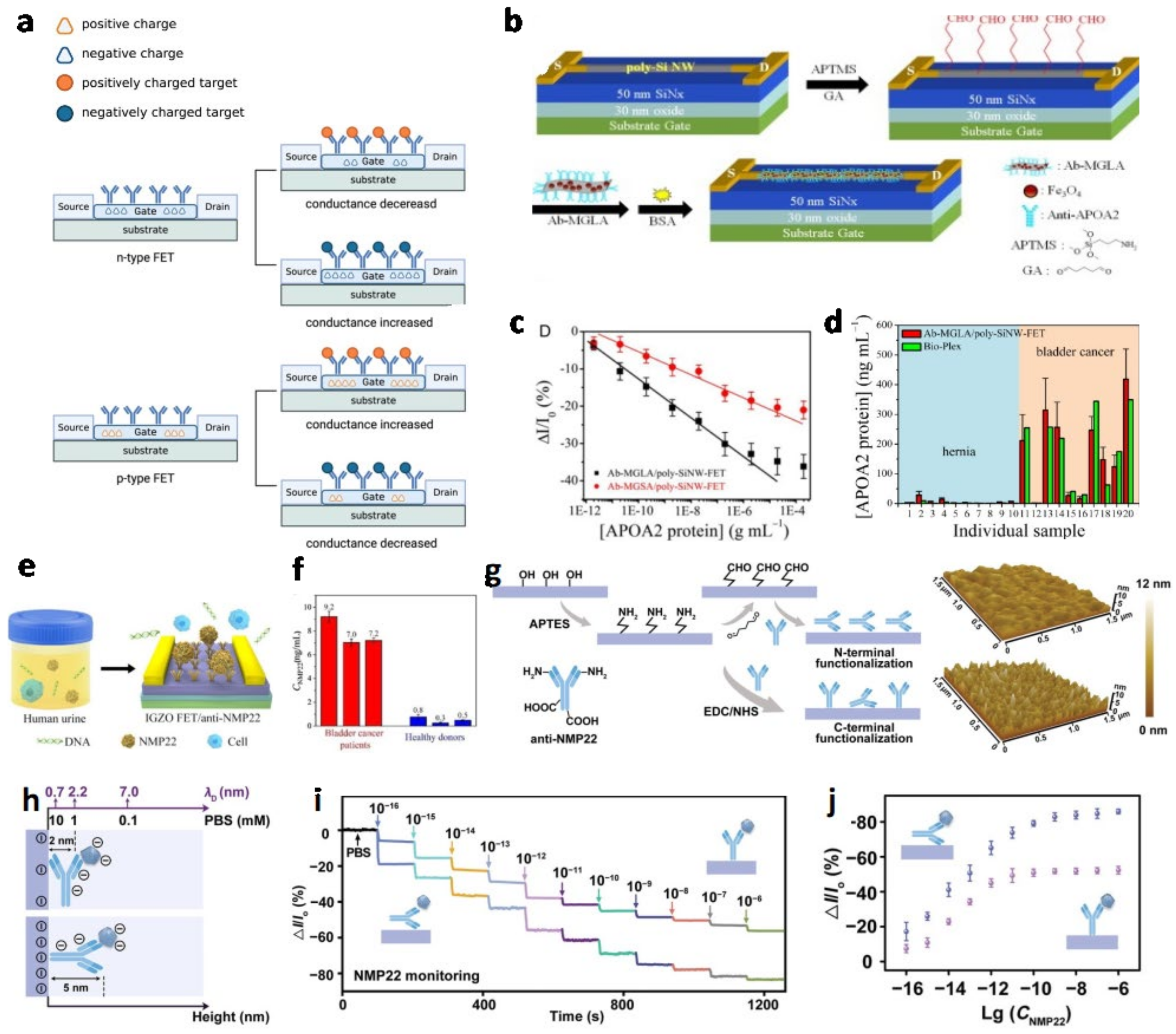
| No. | Biomarker | Gate | LOD | Linear Range | Ref. |
|---|---|---|---|---|---|
| 1 | APOA2 | Ab-MGLA/poly-SiNW | 6.7 pg/mL | 9.5 pg/mL and 1.95 µg/mL | Chen et al. (2015) [22] |
| 2 | NMP22, CK8 | Ab-MoS2 | 0.027 aM, 0.019 aM | 10−6–10−1 pg/mL | Yang et al. (2020) [46] |
| 3 | microRNA-21 | ssDNA-IGZO | 19.8 amol/L | --- | Guo et al. (2021) [47] |
| 4 | NMP22 | Ab-IGZO | 2.7 amol/L | 0.0001–0.1 pg/mL | Li et al. (2020) [48] |
| 6 | NMP22 | N-terminal Ab-IGZO | 3.2 × 10−17 g/mL | 10−16–10−12 g mL−1 | Yang et al. (2022) [49] |
| 5 | APOA1 | Ab-SiNW | 1 ng/mL | 0.2 ng/mL-10 μg/mL | Lin et al. (2017) [50] |
2.1.2. Optical Methods
Fluorescence Assays
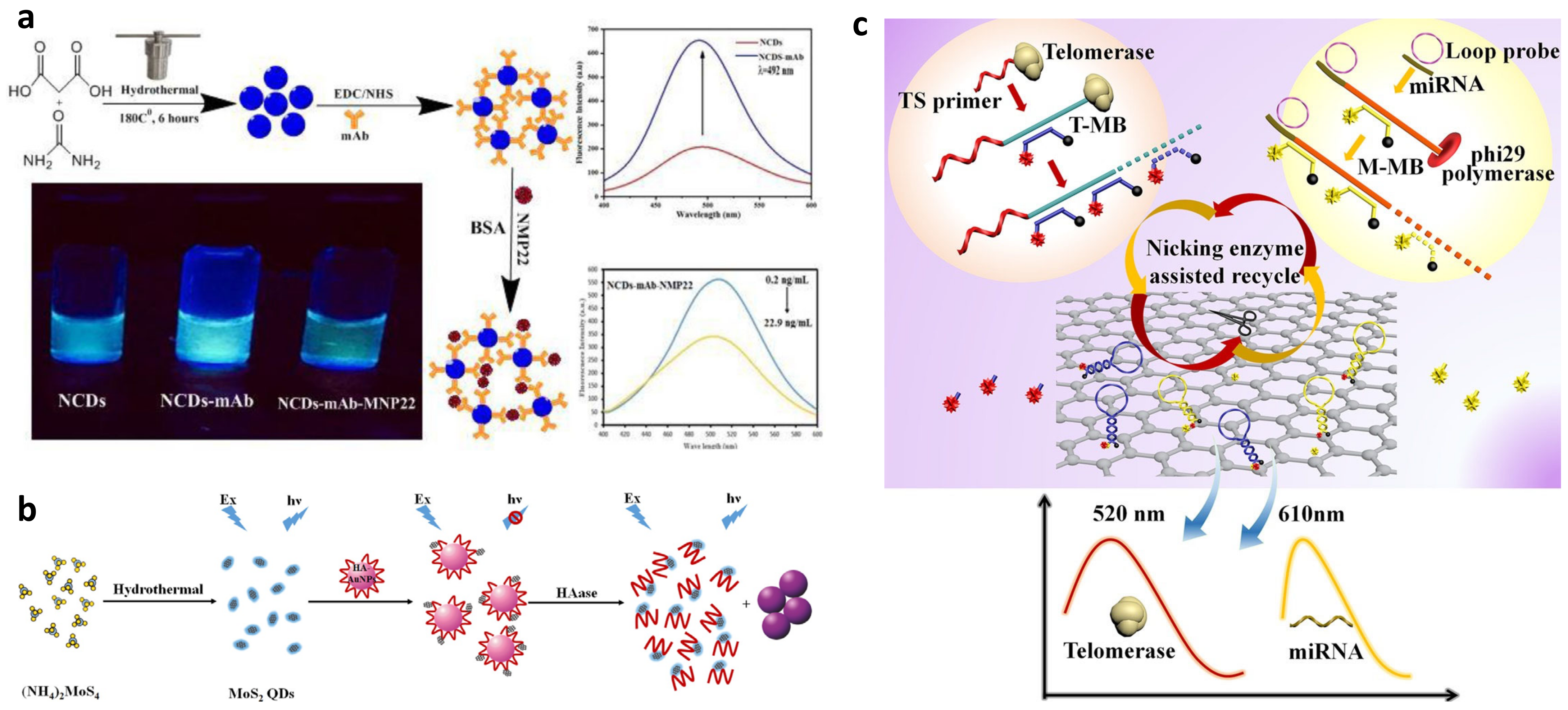
Dynamic Light Scattering
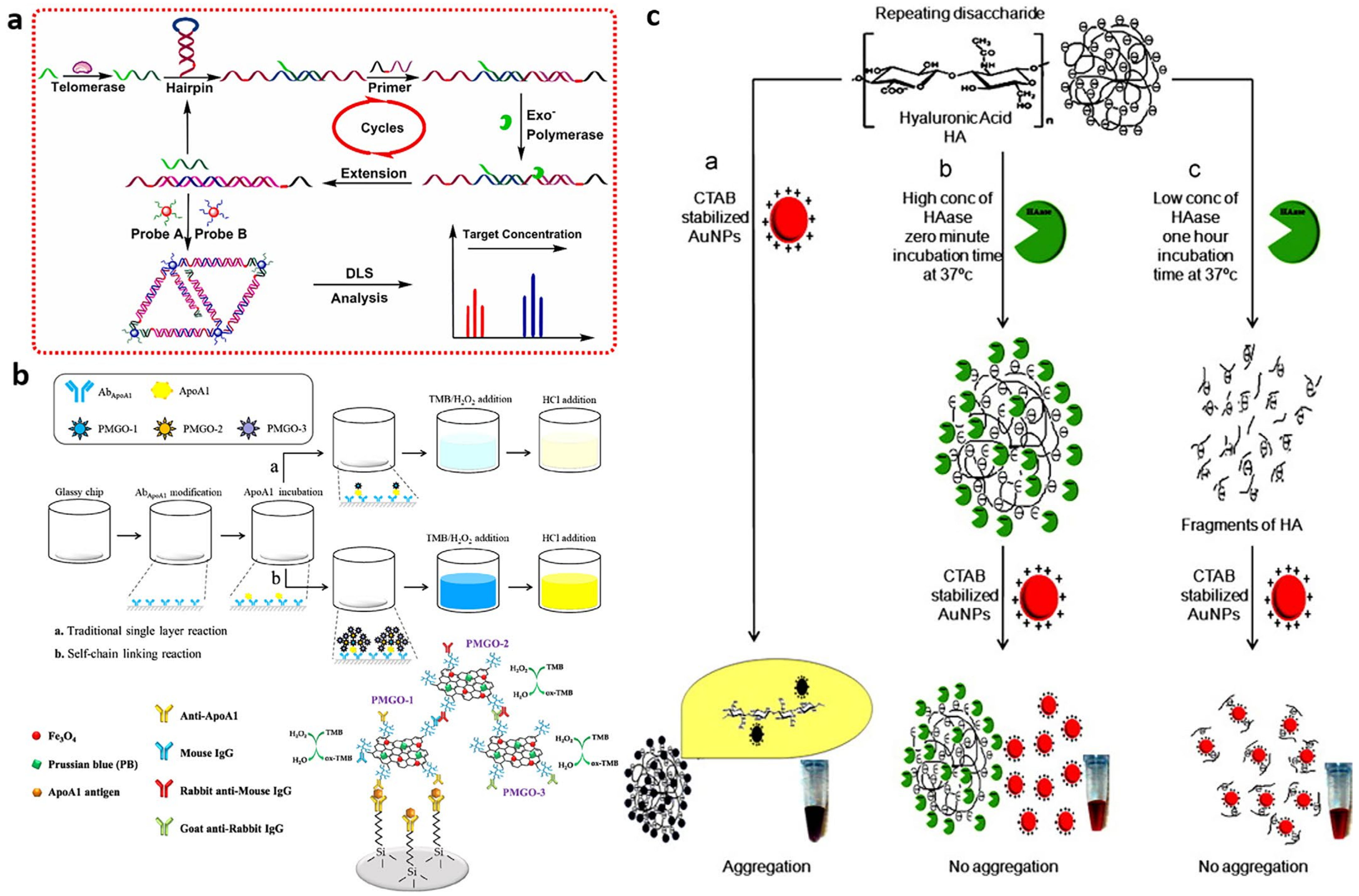
Colorimetric Assay
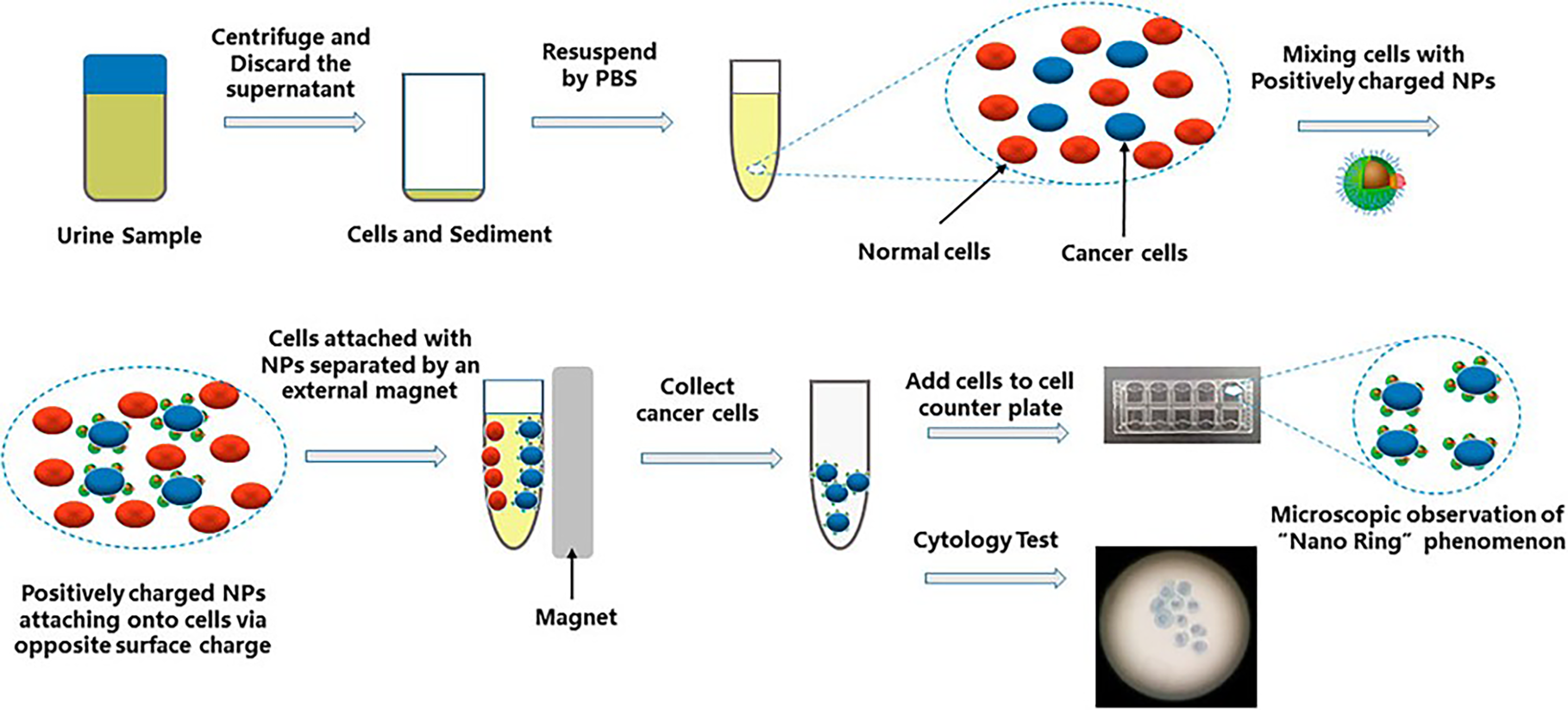
2.2. Urinary Nano-Cytology
2.3. Nanomedicine Advanced Imaging Techniques in the Bladder Cancer Diagnosis
2.3.1. Optical Imaging in Bladder Cancer Diagnosis
In Vivo Imaging in Bladder Cancer Diagnosis
Biopsy in Bladder Cancer Diagnosis
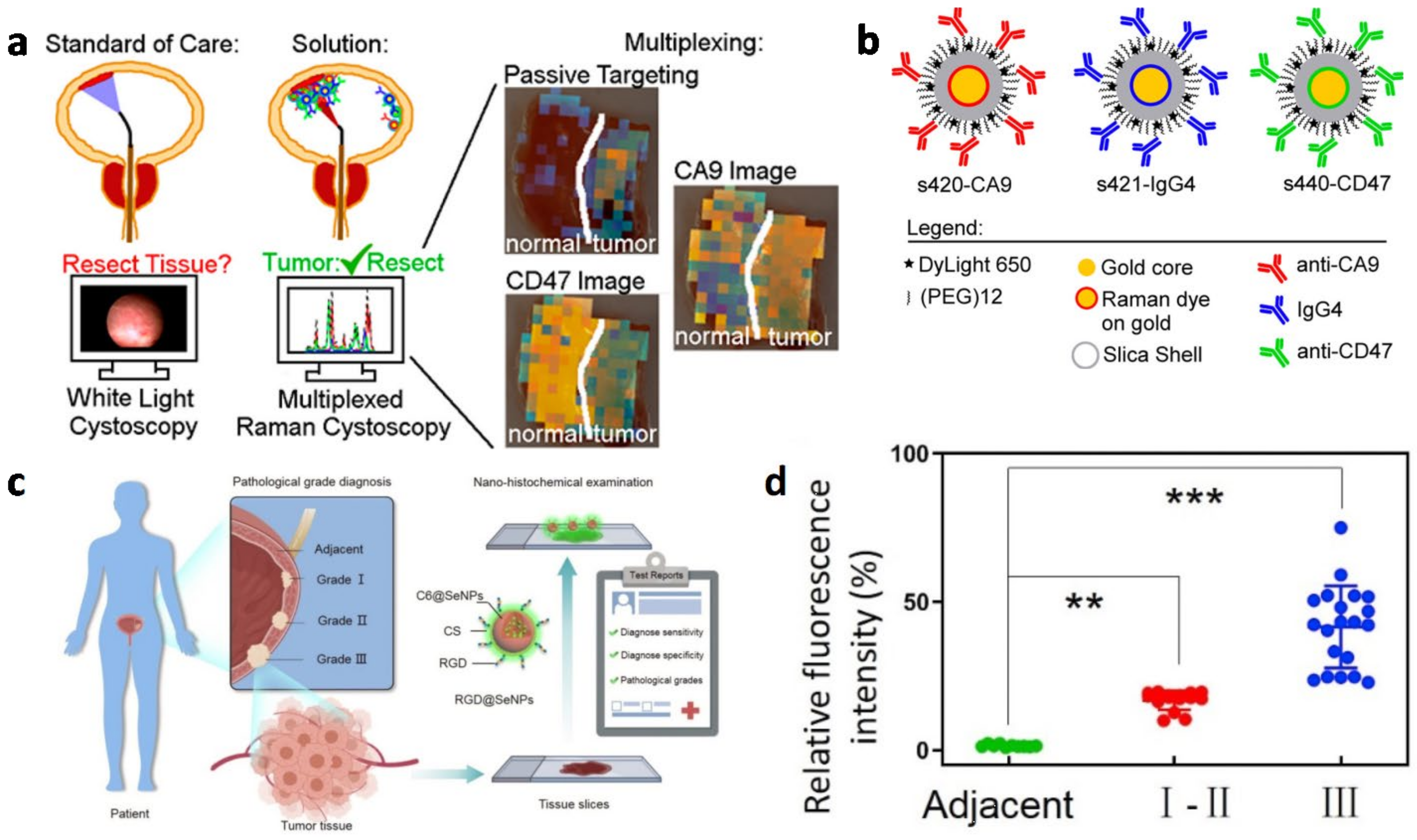
2.3.2. Magnetic Resonance Imaging in Bladder Cancer Diagnosis
2.3.3. Multimodal Imaging
3. Nanomedicine-Based Bladder Cancer Therapy
3.1. Nanocarriers-Based Intravesical Delivery for Chemotherapy of Bladder Cancer
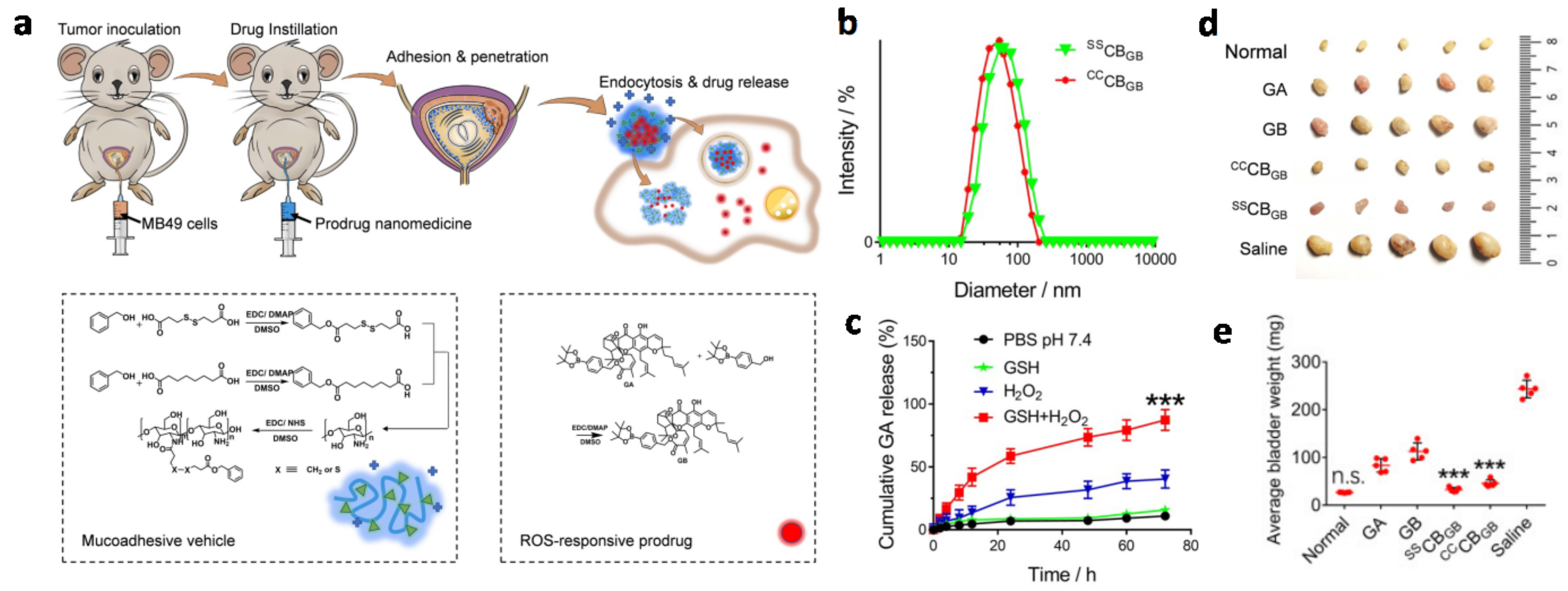
3.1.1. Mucoadhesive Nanocarriers for Intravesical Drug Delivery
Synthetic Polymeric Mucoadhesive Nanocarriers
Chitosan-Based Mucoadhesive Nanocarriers
3.1.2. Other Polymeric Nanoparticles for Intravesical Drug Delivery

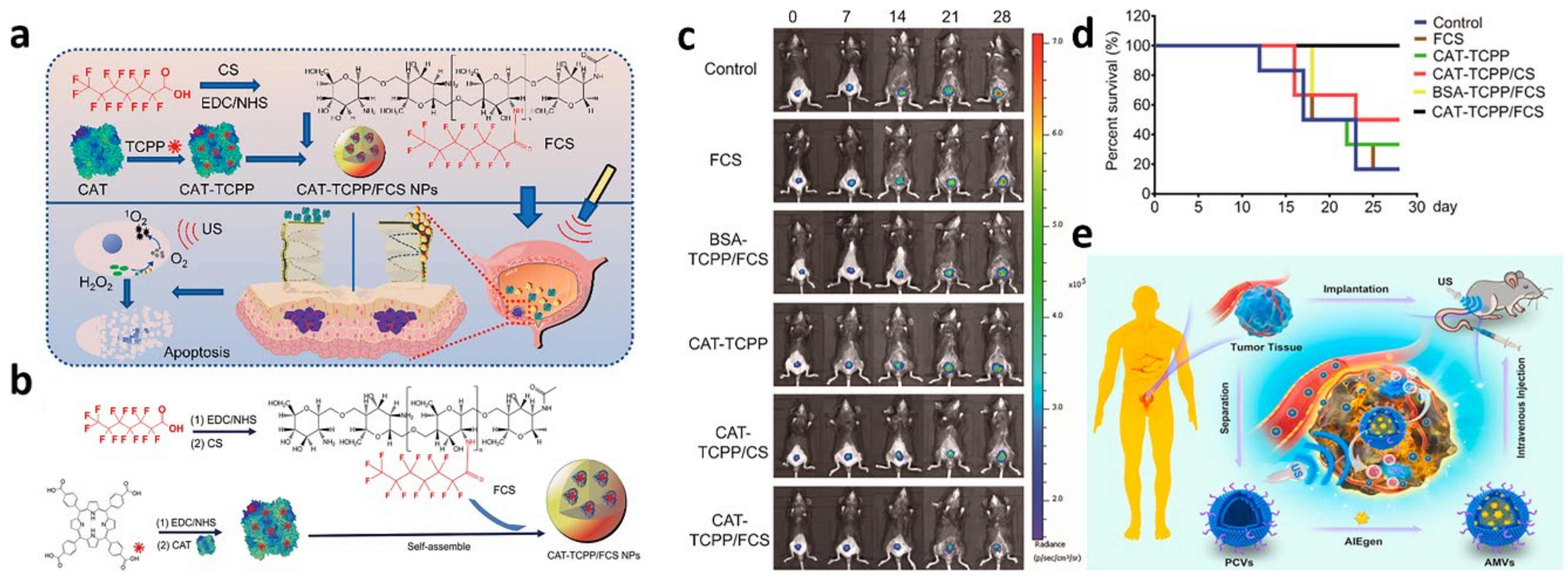
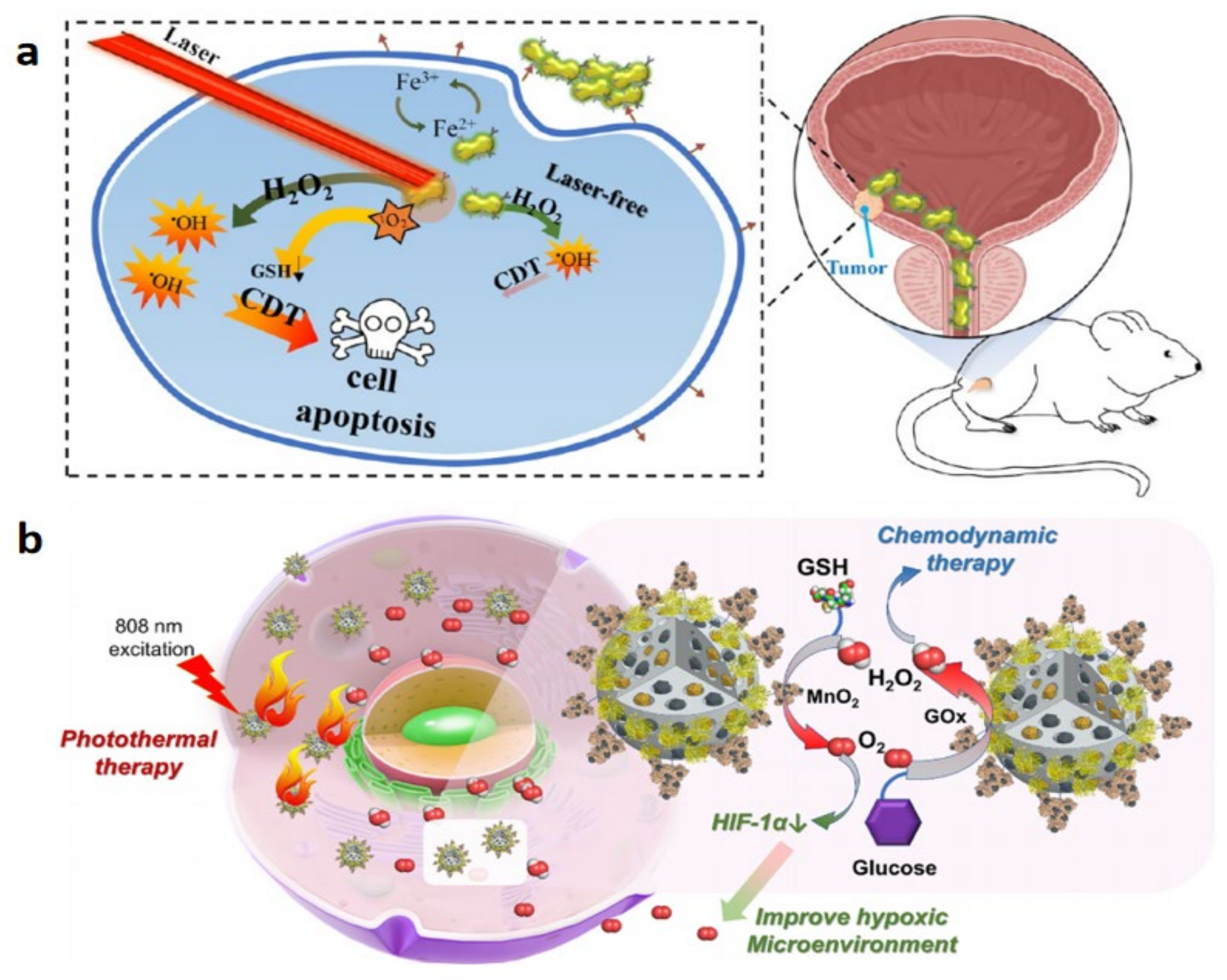
3.2. Photothermal Therapy for Bladder Cancer
| No | Technique | Material System | Route of Administration | Advantage | Biosafety | Ref. |
|---|---|---|---|---|---|---|
| 1 | PTT | FePPy-NH2 NPs | intravenous injection |
| good biosafety | Liu et al. (2022) [121] |
| 2 | PTT | NanoDOX and SWCNTs | intravenous injection |
| low adverse effect | Gao et al. (2017) [122] |
| 3 | PTT | anti-EGFR conjugated GNPs | intravesical instillation |
| reduced damage to surrounding cells | Chen et al. (2016) [123] |
| 4 | PTT | 2MDTT-BBTD | intravesical instillation |
| reduced damage to surrounding cells | Zeng et al. (2021) [124] |
| 5 | PTT | GOX@MBSA-PPy-MnO2+ | intravenous injection |
| not clear | Chen et al. (2021) [125] |
| 6 | PTT | smart SI gel system | intravenous injection |
| good biosafety | Guo et al. (2018) [126] |
| 7 | PDT | PLZ4-nanoporphyrin | intravesical instillation |
| fewer toxicity issues | Lin et al. (2016) [78] |
| 8 | PDT | HAS-MnO2-Ce6 NPs | intravenous injection |
| not clear | Lin et al. (2018) [127] |
| 9 | SDT | CAT-TCPP/FCS NPs | intravesical instillation |
| no obvious side effect | Li et al. (2020) [128] |
| 10 | SDT | MVs/AIEgen hybrid system | intravenous injection |
| tiny damage to internal organs | Duo et al. (2021) [129] |
| 11 | CDT+PDT | Au@Chl/Fe-CPBA | intravesical instillation |
| reduced systemic toxicity | Liao et al. (2022) [130] |
| 12 | CDT+PTT | GOx@MBSA-PPy-MnO2 NPs | tumor injection |
| not clear or little | Chen et al. (2021) [125] |

3.3. Photodynamic Therapy of Bladder Cancer
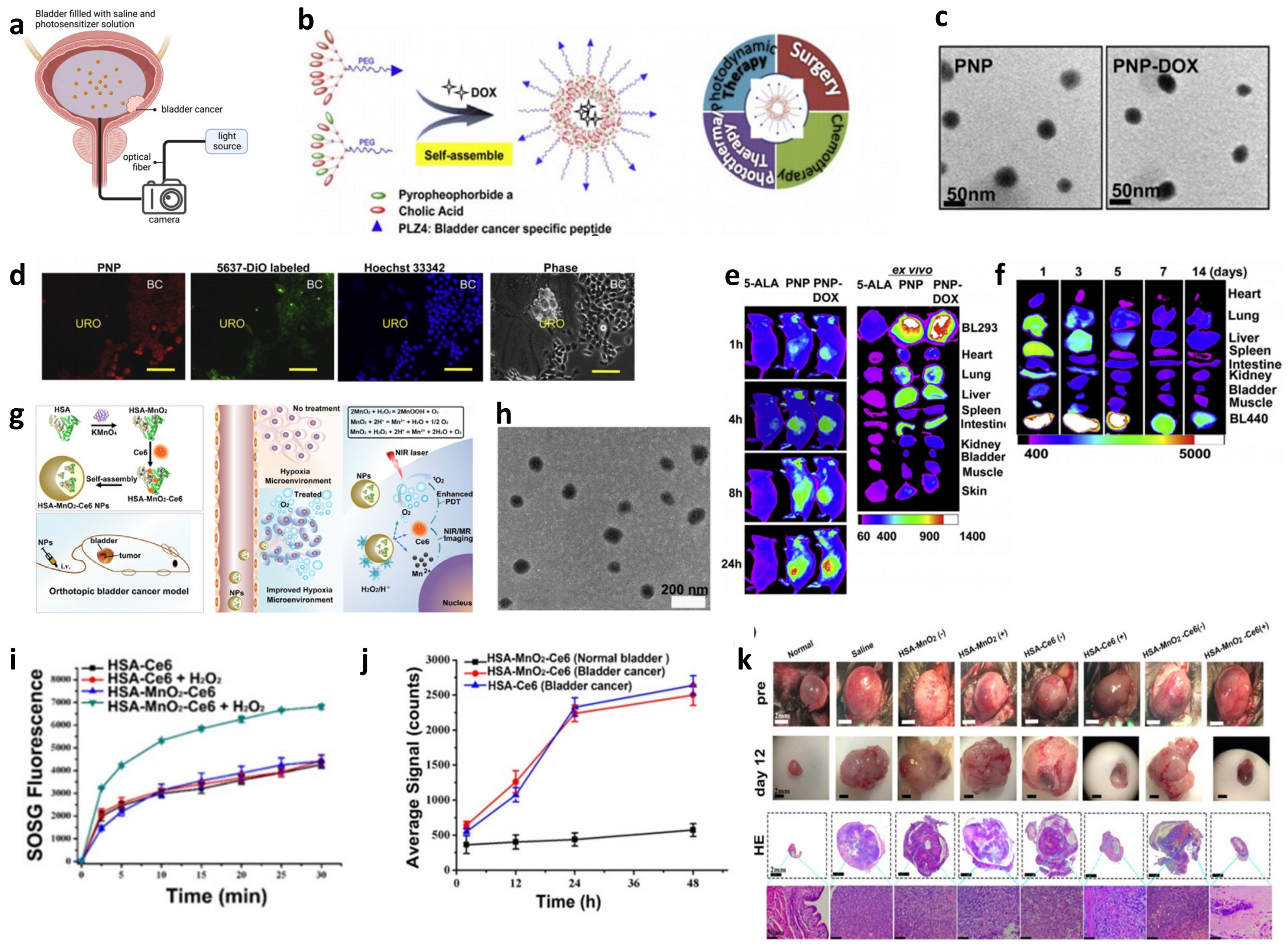
3.4. Sonodynamic Therapy of Bladder Cancer
3.5. Chemodynamic Therapy of Bladder Cancer
3.6. Other Novel Achievements in Nanomedicine for Bladder Cancer Therapy
3.7. Biosafety for Nanomaterials-Based Bladder Cancer Therapy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Kamat, A.M.; Hahn, N.M.; Efstathiou, J.A.; Lerner, S.P.; Malmström, P.-U.; Choi, W.; Guo, C.C.; Lotan, Y.; Kassouf, W. Bladder cancer. Lancet 2016, 388, 2796–2810. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.; Xiao, J.-F.; Agarwal, N.; Duex, J.E.; Theodorescu, D. Advances in bladder cancer biology and therapy. Nat. Rev. Cancer 2021, 21, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Bellmunt, J.; Comperat, E.; De Santis, M.; Huddart, R.; Loriot, Y.; Necchi, A.; Valderrama, B.P.; Ravaud, A.; Shariat, S.F.; et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, G.; Wu, S. Advances in Diagnosis and Therapy for Bladder Cancer. Cancers 2022, 14, 3181. [Google Scholar] [CrossRef]
- Jones, R.; Latinovic, R.; Charlton, J.; Gulliford, M.C. Alarm symptoms in early diagnosis of cancer in primary care: Cohort study using General Practice Research Database. BMJ 2007, 334, 1040. [Google Scholar] [CrossRef] [Green Version]
- Alanee, S.; Alvarado-Cabrero, I.; Murugan, P.; Kumar, R.; Nepple, K.G.; Paner, G.P.; Patel, M.I.; Raspollini, M.R.; Lopez-Beltran, A.; Konety, B.R. Update of the International Consultation on Urological Diseases on bladder cancer 2018: Non-urothelial cancers of the urinary bladder. World J. Urol. 2019, 37, 107–114. [Google Scholar] [CrossRef]
- Barani, M.; Hosseinikhah, S.M.; Rahdar, A.; Farhoudi, L.; Arshad, R.; Cucchiarini, M.; Pandey, S. Nanotechnology in Bladder Cancer: Diagnosis and Treatment. Cancers 2021, 13, 2214. [Google Scholar] [CrossRef]
- Crabb, S.J.; Douglas, J. The latest treatment options for bladder cancer. Br. Med. Bull. 2018, 128, 85–95. [Google Scholar] [CrossRef]
- Jordan, B.; Meeks, J.J. T1 bladder cancer: Current considerations for diagnosis and management. Nat. Rev. Urol. 2019, 16, 23–34. [Google Scholar] [CrossRef]
- GuhaSarkar, S.; Banerjee, R. Intravesical drug delivery: Challenges, current status, opportunities and novel strategies. J. Control. Release Off. J. Control. Release Soc. 2010, 148, 147–159. [Google Scholar] [CrossRef]
- Booth, C.M.; Karim, S.; Brennan, K.; Siemens, D.R.; Peng, Y.; Mackillop, W.J. Perioperative chemotherapy for bladder cancer in the general population: Are practice patterns finally changing? Urol. Oncol. 2018, 36, 89.e13–89.e20. [Google Scholar] [CrossRef]
- Pettenati, C.; Ingersoll, M.A. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat. Rev. Urol. 2018, 15, 615–625. [Google Scholar] [CrossRef]
- Wołącewicz, M.; Hrynkiewicz, R.; Grywalska, E.; Suchojad, T.; Leksowski, T.; Roliński, J.; Niedźwiedzka-Rystwej, P. Immunotherapy in Bladder Cancer: Current Methods and Future Perspectives. Cancers 2020, 12, 1181. [Google Scholar] [CrossRef]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; Mshs, M.D. Bladder Cancer: A Review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef]
- Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef] [Green Version]
- Onoue, S.; Yamada, S.; Chan, H.K. Nanodrugs: Pharmacokinetics and safety. Int. J. Nanomed. 2014, 9, 1025–1037. [Google Scholar] [CrossRef] [Green Version]
- Gradishar, W.J.; Krasnojon, D.; Cheporov, S.; Makhson, A.N.; Manikhas, G.M.; Clawson, A.; Bhar, P. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J. Clin. Oncol. 2009, 27, 3611–3619. [Google Scholar] [CrossRef]
- Gennari, A.; Sun, Z.; Hasler-Strub, U.; Colleoni, M.; Kennedy, M.J.; Von Moos, R.; Cortés, J.; Vidal, M.J.; Hennessy, B.; Walshe, J.; et al. A randomized phase II study evaluating different maintenance schedules of nab-paclitaxel in the first-line treatment of metastatic breast cancer: Final results of the IBCSG 42-12/BIG 2-12 SNAP trial. Ann. Oncol. 2018, 29, 661–668. [Google Scholar] [CrossRef] [Green Version]
- Ko, Y.-J.; Canil, C.M.; Mukherjee, S.D.; Winquist, E.; Elser, C.; Eisen, A.; Reaume, M.N.; Zhang, L.; Sridhar, S.S. Nanoparticle albumin-bound paclitaxel for second-line treatment of metastatic urothelial carcinoma: A single group, multicentre, phase 2 study. Lancet Oncol. 2013, 14, 769–776. [Google Scholar] [CrossRef]
- Grivas, P.D.; Hussain, M.; Hafez, K.; Daignault-Newton, S.; Wood, D.; Lee, C.T.; Weizer, A.; Montie, J.E.; Hollenbeck, B.; Montgomery, J.S.; et al. A phase II trial of neoadjuvant nab-paclitaxel, carboplatin, and gemcitabine (ACaG) in patients with locally advanced carcinoma of the bladder. Urology 2013, 82, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Chen, Y.T.; Tsai, R.Y.; Chen, M.C.; Chen, S.L.; Xiao, M.C.; Chen, C.L.; Hua, M.Y. A sensitive and selective magnetic graphene composite-modified polycrystalline-silicon nanowire field-effect transistor for bladder cancer diagnosis. Biosens. Bioelectron. 2015, 66, 198–207. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, C.; Wang, J.; Chen, L.; Chen, J.; Chen, T.; Zeng, Q. Application of nanotechnology in the diagnosis and treatment of bladder cancer. J. Nanobiotechnol. 2021, 19, 393. [Google Scholar] [CrossRef] [PubMed]
- Batista, R.; Vinagre, N.; Meireles, S.; Vinagre, J.; Prazeres, H.; Leao, R.; Maximo, V.; Soares, P. Biomarkers for Bladder Cancer Diagnosis and Surveillance: A Comprehensive Review. Diagnostics 2020, 10, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharm. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Lokeshwar, V.B.; Selzer, M.G. Urinary bladder tumor markers. Urol. Oncol. 2006, 24, 528–537. [Google Scholar] [CrossRef]
- Tagit, O.; Hildebrandt, N. Fluorescence Sensing of Circulating Diagnostic Biomarkers Using Molecular Probes and Nanoparticles. ACS Sens. 2017, 2, 31–45. [Google Scholar] [CrossRef]
- Findlay, J.W.; Smith, W.C.; Lee, J.W.; Nordblom, G.D.; Das, I.; DeSilva, B.S.; Khan, M.N.; Bowsher, R.R. Validation of immunoassays for bioanalysis: A pharmaceutical industry perspective. J. Pharm. Biomed. Anal. 2000, 21, 1249–1273. [Google Scholar] [CrossRef]
- Chikkaveeraiah, B.V.; Bhirde, A.A.; Morgan, N.Y.; Eden, H.S.; Chen, X. Electrochemical immunosensors for detection of cancer protein biomarkers. ACS Nano 2012, 6, 6546–6561. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.E.; Kwak, J.W.; Park, J.W. Nanotechnology for early cancer detection. Sensors 2010, 10, 428–455. [Google Scholar] [CrossRef]
- Arya, S.K.; Estrela, P. Recent Advances in Enhancement Strategies for Electrochemical ELISA-Based Immunoassays for Cancer Biomarker Detection. Sensors 2018, 18, 10. [Google Scholar] [CrossRef] [Green Version]
- Kokkinos, C.; Economou, A.; Prodromidis, M.I. Electrochemical immunosensors: Critical survey of different architectures and transduction strategies. TrAC Trends Anal. Chem. 2016, 79, 88–105. [Google Scholar] [CrossRef]
- Wu, D.; Wang, Y.; Zhang, Y.; Ma, H.; Yan, T.; Du, B.; Wei, Q. Sensitive Electrochemical Immunosensor for Detection of Nuclear Matrix Protein-22 based on NH2-SAPO-34 Supported Pd/Co Nanoparticles. Sci. Rep. 2016, 6, 24551. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Kim, Y.J.; Kang, H.-L.; Yoon, S.; Hong, D.-K.; Kim, W.-H.; Shin, I.-S.; Seong, W.K.; Lee, K.-N. Sensitivity Improvement in Electrochemical Immunoassays Using Antibody Immobilized Magnetic Nanoparticles with a Clean ITO Working Electrode. BioChip J. 2020, 14, 308–316. [Google Scholar] [CrossRef]
- Giannetto, M.; Bianchi, M.V.; Mattarozzi, M.; Careri, M. Competitive amperometric immunosensor for determination of p53 protein in urine with carbon nanotubes/gold nanoparticles screen-printed electrodes: A potential rapid and noninvasive screening tool for early diagnosis of urinary tract carcinoma. Anal. Chim. Acta 2017, 991, 133–141. [Google Scholar] [CrossRef]
- Joung, C.K.; Kim, H.N.; Lim, M.C.; Jeon, T.J.; Kim, H.Y.; Kim, Y.R. A nanoporous membrane-based impedimetric immunosensor for label-free detection of pathogenic bacteria in whole milk. Biosens. Bioelectron. 2013, 44, 210–215. [Google Scholar] [CrossRef]
- Lin, J.; Wei, Z.; Chu, P. A label-free immunosensor by controlled fabrication of monoclonal antibodies and gold nanoparticles inside the mesopores. Anal. Biochem. 2012, 421, 97–102. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Li, Y.; Cao, W.; Ma, H.; Wu, D.; Du, B.; Wei, Q. A label-free electrochemical immunosensor based on Au@Pd/Ag yolk-bimetallic shell nanoparticles and amination graphene for detection of nuclear matrix protein 22. Sens. Actuators B Chem. 2014, 202, 67–73. [Google Scholar] [CrossRef]
- Lee, M.H.; Thomas, J.L.; Chang, Y.C.; Tsai, Y.S.; Liu, B.D.; Lin, H.Y. Electrochemical sensing of nuclear matrix protein 22 in urine with molecularly imprinted poly(ethylene-co-vinyl alcohol) coated zinc oxide nanorod arrays for clinical studies of bladder cancer diagnosis. Biosens. Bioelectron. 2016, 79, 789–795. [Google Scholar] [CrossRef]
- Palomar, Q.; Xu, X.; Selegård, R.; Aili, D.; Zhang, Z. Peptide decorated gold nanoparticle/carbon nanotube electrochemical sensor for ultrasensitive detection of matrix metalloproteinase-7. Sens. Actuators B Chem. 2020, 325, 128789. [Google Scholar] [CrossRef]
- Arya, S.K.; Estrela, P. Electrochemical ELISA-based platform for bladder cancer protein biomarker detection in urine. Biosens. Bioelectron. 2018, 117, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-E.; Kim, Y.J.; Song, S.; Lee, K.-N.; Seong, W.K. A simple electrochemical immunosensor platform for detection of Apolipoprotein A1 (Apo-A1) as a bladder cancer biomarker in urine. Sens. Actuators B Chem. 2019, 278, 103–109. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, X.; Li, X.; Li, R.; Du, B.; Wei, Q. Electrochemical immunosensor for detecting typical bladder cancer biomarker based on reduced graphene oxide-tetraethylene pentamine and trimetallic AuPdPt nanoparticles. Talanta 2015, 143, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Vu, C.A.; Chen, W.Y. Field-Effect Transistor Biosensors for Biomedical Applications: Recent Advances and Future Prospects. Sensors 2019, 19, 4214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, A.; Lieber, C.M. Nano-Bioelectronics. Chem. Rev. 2016, 116, 215–257. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zeng, B.; Li, Y.; Liang, H.; Yang, Y.; Yuan, Q. Construction of MoS2 field effect transistor sensor array for the detection of bladder cancer biomarkers. Sci. China Chem. 2020, 63, 997–1003. [Google Scholar] [CrossRef]
- Guo, J.; Shen, R.; Shen, X.; Zeng, B.; Yang, N.; Liang, H.; Yang, Y.; Yuan, Q. Construction of high stability indium gallium zinc oxide transistor biosensors for reliable detection of bladder cancer-associated microRNA. Chin. Chem. Lett. 2021, 33, 979–982. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, B.; Yang, Y.; Liang, H.; Yang, Y.; Yuan, Q. Design of high stability thin-film transistor biosensor for the diagnosis of bladder cancer. Chin. Chem. Lett. 2020, 31, 1387–1391. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Huang, W.; Wan, G.; Xia, M.; Chen, D.; Zhang, Y.; Wang, Y.; Guo, F.; Tan, J.; et al. Integrated Urinalysis Devices Based on Interface-Engineered Field-Effect Transistor Biosensors Incorporated With Electronic Circuits. Adv. Mater. 2022, 34, e2203224. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Lin, W.-S.; Wong, J.-C.; Hsu, W.-C.; Peng, Y.-S.; Chen, C.-L. Bottom-up assembly of silicon nanowire conductometric sensors for the detection of apolipoprotein A1, a biomarker for bladder cancer. Microchim. Acta 2017, 184, 2419–2428. [Google Scholar] [CrossRef]
- Wu, L.; Qu, X. Cancer biomarker detection: Recent achievements and challenges. Chem. Soc. Rev. 2015, 44, 2963–2997. [Google Scholar] [CrossRef]
- Chan, W.C.; Nie, S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998, 281, 2016–2018. [Google Scholar] [CrossRef] [Green Version]
- Bruchez, M., Jr.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281, 2013–2016. [Google Scholar] [CrossRef] [Green Version]
- Wegner, K.D.; Hildebrandt, N. Quantum dots: Bright and versatile in vitro and in vivo fluorescence imaging biosensors. Chem. Soc. Rev. 2015, 44, 4792–4834. [Google Scholar] [CrossRef] [Green Version]
- Othman, H.O.; Salehnia, F.; Hosseini, M.; Hassan, R.; Faizullah, A.; Ganjali, M.R. Fluorescence immunoassay based on nitrogen doped carbon dots for the detection of human nuclear matrix protein NMP22 as biomarker for early stage diagnosis of bladder cancer. Microchem. J. 2020, 157, 104966. [Google Scholar] [CrossRef]
- Gu, W.; Yan, Y.; Zhang, C.; Ding, C.; Xian, Y. One-Step Synthesis of Water-Soluble MoS2 Quantum Dots via a Hydrothermal Method as a Fluorescent Probe for Hyaluronidase Detection. ACS Appl. Mater. Interfaces 2016, 8, 11272–11279. [Google Scholar] [CrossRef]
- Xu, J.; Wei, X.; Zhang, X.; Cai, Z.; Wei, Y.; Liu, W.; Yang, J.; Li, Y.; Cai, X.; Bai, T.; et al. Multiplexed detection of bladder cancer microRNAs based on core-shell-shell magnetic quantum dot microbeads and cascade signal amplification. Sens. Actuators B Chem. 2021, 349, 130824. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Li, T.; Shen, R.; Li, G.; Ling, L. Strand displacement amplification-coupled dynamic light scattering method to detect urinary telomerase for non-invasive detection of bladder cancer. Biosens. Bioelectron. 2019, 131, 143–148. [Google Scholar] [CrossRef]
- Duan, R.; Zhang, Z.; Zheng, F.; Wang, L.; Guo, J.; Zhang, T.; Dai, X.; Zhang, S.; Yang, D.; Kuang, R.; et al. Combining Protein and miRNA Quantification for Bladder Cancer Analysis. ACS Appl. Mater. Interfaces 2017, 9, 23420–23427. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, M.; Ge, Q.; Qu, X.; Guo, Q.; Hu, X.; Sun, Q. Ratiometric Quantum Dot-Ligand System Made by Phase Transfer for Visual Detection of Double-Stranded DNA and Single-Nucleotide Polymorphism. Anal. Chem. 2016, 88, 1768–1774. [Google Scholar] [CrossRef]
- Raj, P.; Lee, S.Y.; Lee, T.Y. Carbon Dot/Naphthalimide Based Ratiometric Fluorescence Biosensor for Hyaluronidase Detection. Materials 2021, 14, 1313. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Mao, G.; Zhong, Y.; Wu, G.; Wu, W.; Zhan, Y.; He, Z.; Huang, W. Highly sensitive ratiometric fluorescent paper sensor for the urine assay of cancer. Talanta 2019, 194, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; Lin, Q.; Wei, Y.; Wang, J.; Li, Y.; Yang, R.; Yuan, Q. Highly Sensitive Detection of Bladder Cancer-Related miRNA in Urine Using Time-Gated Luminescent Biochip. ACS Sens. 2019, 4, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zou, L.; Zhang, J.; Li, G.; Ling, L. Non-invasive diagnosis of bladder cancer by detecting telomerase activity in human urine using hybridization chain reaction and dynamic light scattering. Anal. Chim. Acta 2019, 1065, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Li, X.; Zhang, J.; Ling, L. A Highly Sensitive Catalytic Hairpin Assembly-Based Dynamic Light-Scattering Biosensors for Telomerase Detection in Bladder Cancer Diagnosis. Anal. Chem. 2020, 92, 12656–12662. [Google Scholar] [CrossRef]
- Peng, C.; Hua, M.Y.; Li, N.S.; Hsu, Y.P.; Chen, Y.T.; Chuang, C.K.; Pang, S.T.; Yang, H.W. A colorimetric immunosensor based on self-linkable dual-nanozyme for ultrasensitive bladder cancer diagnosis and prognosis monitoring. Biosens. Bioelectron. 2019, 126, 581–589. [Google Scholar] [CrossRef]
- Nossier, A.I.; Eissa, S.; Ismail, M.F.; Hamdy, M.A.; Azzazy, H.M. Direct detection of hyaluronidase in urine using cationic gold nanoparticles: A potential diagnostic test for bladder cancer. Biosens. Bioelectron. 2014, 54, 7–14. [Google Scholar] [CrossRef]
- Nossier, A.I.; Mohammed, O.S.; Fakhr, E.-D.R.R.; Zaghloul, A.S.; Eissa, S. Gelatin-modified gold nanoparticles for direct detection of urinary total gelatinase activity: Diagnostic value in bladder cancer. Talanta 2016, 161, 511–519. [Google Scholar] [CrossRef]
- Acharya, A.P.; Theisen, K.M.; Correa, A.; Meyyappan, T.; Apfel, A.; Sun, T.; Tarin, T.V.; Little, S.R. An Inexpensive, Point-of-Care Urine Test for Bladder Cancer in Patients Undergoing Hematuria Evaluation. Adv. Healthc. Mater. 2017, 6, 1700808. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Comperat, E.M.; Cowan, N.C.; Gakis, G.; Hernandez, V.; Linares Espinos, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef]
- Xu, J.; Zeng, S.; Li, J.; Gao, L.; Le, W.; Huang, X.; Wang, G.; Chen, B.; Zhang, Z.; Xu, C. Novel Non-Invasive Diagnosis of Bladder Cancer in Urine Based on Multifunctional Nanoparticles. Front. Cell Dev. Biol. 2021, 9, 813420. [Google Scholar] [CrossRef]
- Pan, Y.; Volkmer, J.P.; Mach, K.E.; Rouse, R.V.; Liu, J.J.; Sahoo, D.; Chang, T.C.; Metzner, T.J.; Kang, L.; van de Rijn, M.; et al. Endoscopic molecular imaging of human bladder cancer using a CD47 antibody. Sci. Transl. Med. 2014, 6, 260ra148. [Google Scholar] [CrossRef]
- Davis, R.M.; Kiss, B.; Trivedi, D.R.; Metzner, T.J.; Liao, J.C.; Gambhir, S.S. Surface-Enhanced Raman Scattering Nanoparticles for Multiplexed Imaging of Bladder Cancer Tissue Permeability and Molecular Phenotype. ACS Nano 2018, 12, 9669–9679. [Google Scholar] [CrossRef]
- Witjes, J.A.; Douglass, J. The role of hexaminolevulinate fluorescence cystoscopy in bladder cancer. Nat. Clin. Pract. Urol. 2007, 4, 542–549. [Google Scholar] [CrossRef]
- Mark, J.R.; Gelpi-Hammerschmidt, F.; Trabulsi, E.J.; Gomella, L.G. Blue light cystoscopy for detection and treatment of non-muscle invasive bladder cancer. Can. J. Urol. 2012, 19, 6227–6231. [Google Scholar]
- He, M.H.; Chen, L.; Zheng, T.; Tu, Y.; He, Q.; Fu, H.L.; Lin, J.C.; Zhang, W.; Shu, G.; He, L.; et al. Potential Applications of Nanotechnology in Urological Cancer. Front. Pharm. 2018, 9, 745. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.Y.; Li, Y.; Liu, Q.; Chen, J.L.; Zhang, H.; Lac, D.; Zhang, H.; Ferrara, K.W.; Wachsmann-Hogiu, S.; Li, T.; et al. Novel theranostic nanoporphyrins for photodynamic diagnosis and trimodal therapy for bladder cancer. Biomaterials 2016, 104, 339–351. [Google Scholar] [CrossRef] [Green Version]
- Zhong, L.; Cai, S.; Huang, Y.; Yin, L.; Yang, Y.; Lu, C.; Yang, H. DNA Octahedron-Based Fluorescence Nanoprobe for Dual Tumor-Related mRNAs Detection and Imaging. Anal. Chem. 2018, 90, 12059–12066. [Google Scholar] [CrossRef]
- Xue, J.; Shan, L.; Chen, H.; Li, Y.; Zhu, H.; Deng, D.; Qian, Z.; Achilefu, S.; Gu, Y. Visual detection of STAT5B gene expression in living cell using the hairpin DNA modified gold nanoparticle beacon. Biosens. Bioelectron. 2013, 41, 71–77. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Q.; Wu, Y.; Zhang, B.; Peng, W.; Piao, J.; Zhou, Y.; Gao, W.; Gong, X.; Chang, J. The construction of a novel nucleic acids detection microplatform based on the NSET for one-step detecting TK1-DNA and microRNA-21. Biosens. Bioelectron. 2017, 97, 26–33. [Google Scholar] [CrossRef]
- Choi, C.H.J.; Hao, L.; Narayan, S.P.; Auyeung, E.; Mirkin, C.A. Mechanism for the endocytosis of spherical nucleic acid nanoparticle conjugates. Proc. Natl. Acad. Sci. USA 2013, 110, 7625–7630. [Google Scholar] [CrossRef]
- Ding, F.; Mou, Q.; Ma, Y.; Pan, G.; Guo, Y.; Tong, G.; Choi, C.H.J.; Zhu, X.; Zhang, C. A Crosslinked Nucleic Acid Nanogel for Effective siRNA Delivery and Antitumor Therapy. Angew. Chem. 2018, 57, 3064–3068. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhang, P.; Liang, X.; Cui, D.; Shao, Q.; Zhang, H.; Zhang, M.; Yang, T.; Wang, L.; Zhang, N.; et al. R11 peptides can promote the molecular imaging of spherical nucleic acids for bladder cancer margin identification. Nano Res. 2022, 15, 2278–2287. [Google Scholar] [CrossRef]
- Parikh, A.R.; Leshchiner, I.; Elagina, L.; Goyal, L.; Levovitz, C.; Siravegna, G.; Livitz, D.; Rhrissorrakrai, K.; Martin, E.E.; Van Seventer, E.E.; et al. Liquid versus tissue biopsy for detecting acquired resistance and tumor heterogeneity in gastrointestinal cancers. Nat. Med. 2019, 25, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, S.; Krajewska, M.; Shabaik, A.; Wang, H.G.; Irie, S.; Fong, L.; Reed, J.C. Immunohistochemical analysis of in vivo patterns of Bcl-X expression. Cancer Res. 1994, 54, 5501–5507. [Google Scholar] [PubMed]
- Liu, H.; Mei, C.; Deng, X.; Lin, W.; He, L.; Chen, T. Rapid visualizing and pathological grading of bladder tumor tissues by simple nanodiagnostics. Biomaterials 2021, 264, 120434. [Google Scholar] [CrossRef]
- Kunjachan, S.; Ehling, J.; Storm, G.; Kiessling, F.; Lammers, T. Noninvasive Imaging of Nanomedicines and Nanotheranostics: Principles, Progress, and Prospects. Chem. Rev. 2015, 115, 10907–10937. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, S.K.; Luo, Y.; O’Donnell, M.A.; Assouline, J.G. Peptide-Mediated Targeting Mesoporous Silica Nanoparticles: A Novel Tool for Fighting Bladder Cancer. J. Biomed. Nanotechnol. 2017, 13, 232–242. [Google Scholar] [CrossRef]
- Hoosein, M.M.; Rajesh, A. MR imaging of the urinary bladder. Magn. Reson. Imaging Clin. N. Am. 2014, 22, 129–134. [Google Scholar] [CrossRef]
- Key, J.; Dhawan, D.; Cooper, C.L.; Knapp, D.W.; Kim, K.; Kwon, I.C.; Choi, K.; Park, K.; Decuzzi, P.; Leary, J.F. Multicomponent, peptide-targeted glycol chitosan nanoparticles containing ferrimagnetic iron oxide nanocubes for bladder cancer multimodal imaging. Int. J. Nanomed. 2016, 11, 4141–4155. [Google Scholar] [CrossRef] [Green Version]
- Naito, S.; Algaba, F.; Babjuk, M.; Bryan, R.T.; Sun, Y.-H.; Valiquette, L.; de la Rosette, J. The Clinical Research Office of the Endourological Society (CROES) Multicentre Randomised Trial of Narrow Band Imaging-Assisted Transurethral Resection of Bladder Tumour (TURBT) Versus Conventional White Light Imaging-Assisted TURBT in Primary Non-Muscle-invasive Bladder Cancer Patients: Trial Protocol and 1-year Results. Eur. Urol. 2016, 70, 506–515. [Google Scholar] [CrossRef]
- Tong, T.; Guan, Y.; Gao, Y.; Xing, C.; Zhang, S.; Jiang, D.; Yang, X.; Kang, Y.; Pang, J. Smart nanocarriers as therapeutic platforms for bladder cancer. Nano Res. 2021, 15, 2157–2176. [Google Scholar] [CrossRef]
- Tyagi, P.; Wu, P.-C.; Chancellor, M.; Yoshimura, N.; Huang, L. Recent advances in intravesical drug/gene delivery. Mol. Pharm. 2006, 3, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Lewis, S.A. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am. J. Physiol. Ren. Physiol. 2000, 278, F867–F874. [Google Scholar] [CrossRef]
- Apfelthaler, C.; Anzengruber, M.; Gabor, F.; Wirth, M. Poly—(L)—Glutamic acid drug delivery system for the intravesical therapy of bladder cancer using WGA as targeting moiety. Eur. J. Pharm. Biopharm. 2017, 115, 131–139. [Google Scholar] [CrossRef]
- Leonard, F.; Kulkarni, R.K.; Brandes, G.; Nelson, J.; Cameron, J.J. Synthesis and degradation of poly (alkyl α-cyanoacrylates). J. Appl. Polym. Sci. 1966, 10, 259–272. [Google Scholar] [CrossRef]
- Chang, L.C.; Wu, S.C.; Tsai, J.W.; Yu, T.J.; Tsai, T.R. Optimization of epirubicin nanoparticles using experimental design for enhanced intravesical drug delivery. Int. J. Pharm. 2009, 376, 195–203. [Google Scholar] [CrossRef]
- Kainthan, R.K.; Janzen, J.; Kizhakkedathu, J.N.; Devine, D.V.; Brooks, D.E. Hydrophobically derivatized hyperbranched polyglycerol as a human serum albumin substitute. Biomaterials 2008, 29, 1693–1704. [Google Scholar] [CrossRef]
- Kainthan, R.K.; Mugabe, C.; Burt, H.M.; Brooks, D.E. Unimolecular micelles based on hydrophobically derivatized hyperbranched polyglycerols: Ligand binding properties. Biomacromolecules 2008, 9, 886–895. [Google Scholar] [CrossRef]
- Mugabe, C.; Matsui, Y.; So, A.I.; Gleave, M.E.; Heller, M.; Zeisser-Labouebe, M.; Heller, L.; Chafeeva, I.; Brooks, D.E.; Burt, H.M. In vitro and in vivo evaluation of intravesical docetaxel loaded hydrophobically derivatized hyperbranched polyglycerols in an orthotopic model of bladder cancer. Biomacromolecules 2011, 12, 949–960. [Google Scholar] [CrossRef]
- Mugabe, C.; Raven, P.A.; Fazli, L.; Baker, J.H.; Jackson, J.K.; Liggins, R.T.; So, A.I.; Gleave, M.E.; Minchinton, A.I.; Brooks, D.E.; et al. Tissue uptake of docetaxel loaded hydrophobically derivatized hyperbranched polyglycerols and their effects on the morphology of the bladder urothelium. Biomaterials 2012, 33, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Lai, S.K.; Suk, J.S.; Pace, A.; Cone, R.; Hanes, J. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that “slip” through the human mucus barrier. Angew. Chem. Int. Ed. Engl. 2008, 47, 9726–9729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.; Li, F.; Qiu, H.; Xu, W.; Li, P.; Hou, Y.; Ding, J.; Chen, X. Synergistically Enhanced Mucoadhesive and Penetrable Polypeptide Nanogel for Efficient Drug Delivery to Orthotopic Bladder Cancer. Research 2020, 2020, 8970135. [Google Scholar] [CrossRef] [PubMed]
- Eroğlu, M.; Irmak, S.; Acar, A.; Denkbaş, E.B. Design and evaluation of a mucoadhesive therapeutic agent delivery system for postoperative chemotherapy in superficial bladder cancer. Int. J. Pharm. 2002, 235, 51–59. [Google Scholar] [CrossRef]
- Bilensoy, E.; Sarisozen, C.; Esendagli, G.; Dogan, A.L.; Aktas, Y.; Sen, M.; Mungan, N.A. Intravesical cationic nanoparticles of chitosan and polycaprolactone for the delivery of Mitomycin C to bladder tumors. Int. J. Pharm. 2009, 371, 170–176. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, Y.-S.; Park, K.; Lee, S.; Nam, H.Y.; Min, K.H.; Jo, H.G.; Park, J.H.; Choi, K.; Jeong, S.Y.; et al. Antitumor efficacy of cisplatin-loaded glycol chitosan nanoparticles in tumor-bearing mice. J. Control. Release 2008, 127, 41–49. [Google Scholar] [CrossRef]
- Saravanakumar, G.; Min, K.H.; Min, D.S.; Kim, A.Y.; Lee, C.-M.; Cho, Y.W.; Lee, S.C.; Kim, K.; Jeong, S.Y.; Park, K.; et al. Hydrotropic oligomer-conjugated glycol chitosan as a carrier of paclitaxel: Synthesis, characterization, and in vivo biodistribution. J. Control. Release 2009, 140, 210–217. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Hou, J.; Sun, B.; Zhu, B.; Qiao, Z.; Su, Y.; Zhu, X. Paclitaxel/Chitosan Nanosupensions Provide Enhanced Intravesical Bladder Cancer Therapy with Sustained and Prolonged Delivery of Paclitaxel. ACS Appl. Bio Mater. 2018, 1, 1992–2001. [Google Scholar] [CrossRef]
- Xu, X.; Liu, K.; Jiao, B.; Luo, K.; Ren, J.; Zhang, G.; Yu, Q.; Gan, Z. Mucoadhesive nanoparticles based on ROS activated gambogic acid prodrug for safe and efficient intravesical instillation chemotherapy of bladder cancer. J. Control. Release 2020, 324, 493–504. [Google Scholar] [CrossRef]
- Endres, T.K.; Beck-Broichsitter, M.; Samsonova, O.; Renette, T.; Kissel, T.H. Self-assembled biodegradable amphiphilic PEG-PCL-lPEI triblock copolymers at the borderline between micelles and nanoparticles designed for drug and gene delivery. Biomaterials 2011, 32, 7721–7731. [Google Scholar] [CrossRef]
- Sun, P.; Zhou, D.; Gan, Z. Novel reduction-sensitive micelles for triggered intracellular drug release. J. Control. Release 2011, 155, 96–103. [Google Scholar] [CrossRef]
- Greish, K.; Ray, A.; Bauer, H.; Larson, N.; Malugin, A.; Pike, D.; Haider, M.; Ghandehari, H. Anticancer and antiangiogenic activity of HPMA copolymer-aminohexylgeldanamycin-RGDfK conjugates for prostate cancer therapy. J. Control. Release 2011, 151, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Mitra, A.; Coleman, T.; Borgman, M.; Nan, A.; Ghandehari, H.; Line, B.R. Polymeric conjugates of mono- and bi-cyclic alphaVbeta3 binding peptides for tumor targeting. J. Control. Release 2006, 114, 175–183. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, G.; Gan, Z. c(RGDfK) decorated micellar drug delivery system for intravesical instilled chemotherapy of superficial bladder cancer. J. Control. Release 2013, 169, 204–210. [Google Scholar] [CrossRef]
- De Souza, B.M.; da Silva, A.V.R.; Resende, V.M.F.; Arcuri, H.A.; Dos Santos Cabrera, M.P.; Ruggiero Neto, J.; Palma, M.S. Characterization of two novel polyfunctional mastoparan peptides from the venom of the social wasp Polybia paulista. Peptides 2009, 30, 1387–1395. [Google Scholar] [CrossRef]
- Wang, K.-r.; Zhang, B.-z.; Zhang, W.; Yan, J.-x.; Li, J.; Wang, R. Antitumor effects, cell selectivity and structure-activity relationship of a novel antimicrobial peptide polybia-MPI. Peptides 2008, 29, 963–968. [Google Scholar] [CrossRef]
- Oršolić, N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2012, 31, 173–194. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Lei, Q.; Wang, F.; Deng, D.; Wang, S.; Tian, L.; Shen, W.; Cheng, Y.; Liu, Z.; Wu, S. Fluorinated Polymer Mediated Transmucosal Peptide Delivery for Intravesical Instillation Therapy of Bladder Cancer. Small 2019, 15, e1900936. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, M.; Jin, H.; Tao, K.; Tang, C.; Fan, Y.; Liu, S.; Liu, Y.; Hou, Y.; Zhang, H. Fe(III)-Doped Polyaminopyrrole Nanoparticle for Imaging-Guided Photothermal Therapy of Bladder Cancer. ACS Biomater. Sci. Eng. 2022, 8, 502–511. [Google Scholar] [CrossRef]
- Gao, H.; Bi, Y.; Wang, X.; Wang, M.; Zhou, M.; Lu, H.; Gao, J.; Chen, J.; Hu, Y. Near-Infrared Guided Thermal-Responsive Nanomedicine against Orthotopic Superficial Bladder Cancer. ACS Biomater. Sci. Eng. 2017, 3, 3628–3634. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Wu, Y.-J.; Chen, J.-J. Photo-thermal therapy of bladder cancer with Anti-EGFR antibody conjugated gold nanoparticles. Front. Biosci. 2016, 21, 1211–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, S.; Gao, H.; Li, C.; Xing, S.; Xu, Z.; Liu, Q.; Feng, G.; Ding, D. Boosting Photothermal Theranostics via TICT and Molecular Motions for Photohyperthermia Therapy of Muscle-Invasive Bladder Cancer. Adv. Healthc. Mater. 2021, 10, e2101063. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Yu, K.-J.; Jhou, J.-W.; Pang, H.-H.; Weng, W.-H.; Lin, W.-S.; Yang, H.-W. Glucose/Glutathione Co-triggered Tumor Hypoxia Relief and Chemodynamic Therapy to Enhance Photothermal Therapy in Bladder Cancer. ACS Appl. Bio Mater. 2021, 4, 7485–7496. [Google Scholar] [CrossRef]
- Guo, P.; Shang, W.; Peng, L.; Wang, L.; Wang, H.; Han, Z.; Jiang, H.; Tian, J.; Wang, K.; Xu, W. A gel system for single instillation of non-muscle-invasive bladder Cancer: A “divide-and-rule” strategy. J. Control. Release 2018, 285, 46–55. [Google Scholar] [CrossRef]
- Lin, T.; Zhao, X.; Zhao, S.; Yu, H.; Cao, W.; Chen, W.; Wei, H.; Guo, H. O-generating MnO nanoparticles for enhanced photodynamic therapy of bladder cancer by ameliorating hypoxia. Theranostics 2018, 8, 990–1004. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Deng, D.; Xiao, Z.; Dong, Z.; Wang, Z.; Lei, Q.; Gao, S.; Huang, G.; Zhang, E.; et al. Fluorinated Chitosan To Enhance Transmucosal Delivery of Sonosensitizer-Conjugated Catalase for Sonodynamic Bladder Cancer Treatment Post-intravesical Instillation. ACS Nano 2020, 14, 1586–1599. [Google Scholar] [CrossRef]
- Duo, Y.; Zhu, D.; Sun, X.; Suo, M.; Zheng, Z.; Jiang, W.; Tang, B.Z. Patient-derived microvesicles/AIE luminogen hybrid system for personalized sonodynamic cancer therapy in patient-derived xenograft models. Biomaterials 2021, 272, 120755. [Google Scholar] [CrossRef]
- Liao, M.-Y.; Huang, T.-C.; Chin, Y.-C.; Cheng, T.-Y.; Lin, G.-M. Surfactant-Free Green Synthesis of Au@ Chlorophyll Nanorods for NIR PDT-Elicited CDT in Bladder Cancer Therapy. ACS Appl. Bio Mater. 2022, 5, 2819–2833. [Google Scholar] [CrossRef]
- Felsher, D.W. Cancer revoked: Oncogenes as therapeutic targets. Nat. Rev. Cancer 2003, 3, 375–380. [Google Scholar] [CrossRef]
- Marijnissen, J.P.; Star, W.M.; in ’t Zandt, H.J.; D’Hallewin, M.A.; Baert, L. In situ light dosimetry during whole bladder wall photodynamic therapy: Clinical results and experimental verification. Phys. Med. Biol. 1993, 38, 567–582. [Google Scholar] [CrossRef]
- Yavari, N.; Andersson-Engels, S.; Segersten, U.; Malmstrom, P.-U. An overview on preclinical and clinical experiences with photodynamic therapy for bladder cancer. Can. J. Urol. 2011, 18, 5778–5786. [Google Scholar]
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The history of photodetection and photodynamic therapy. Photochem. Photobiol. 2001, 74, 656–669. [Google Scholar] [CrossRef]
- Kelly, J.F.; Snell, M.E. Hematoporphyrin derivative: A possible aid in the diagnosis and therapy of carcinoma of the bladder. J. Urol. 1976, 115, 150–151. [Google Scholar] [CrossRef]
- Berger, A.P.; Steiner, H.; Stenzl, A.; Akkad, T.; Bartsch, G.; Holtl, L. Photodynamic therapy with intravesical instillation of 5-aminolevulinic acid for patients with recurrent superficial bladder cancer: A single-center study. Urology 2003, 61, 338–341. [Google Scholar] [CrossRef]
- Shackley, D.C.; Briggs, C.; Gilhooley, A.; Whitehurst, C.; O’Flynn, K.J.; Betts, C.D.; Moore, J.V.; Clarke, N.W. Photodynamic therapy for superficial bladder cancer under local anaesthetic. BJU Int. 2002, 89, 665–670. [Google Scholar] [CrossRef] [Green Version]
- Ballut, S.; Makky, A.; Loock, B.; Michel, J.-P.; Maillard, P.; Rosilio, V. New strategy for targeting of photosensitizers. Synthesis of glycodendrimeric phenylporphyrins, incorporation into a liposome membrane and interaction with a specific lectin. Chem. Commun. 2009, 224–226. [Google Scholar] [CrossRef]
- Sano, K.; Nakajima, T.; Choyke, P.L.; Kobayashi, H. Markedly enhanced permeability and retention effects induced by photo-immunotherapy of tumors. ACS Nano 2013, 7, 717–724. [Google Scholar] [CrossRef] [Green Version]
- Black, P.C.; Shetty, A.; Brown, G.A.; Esparza-Coss, E.; Metwalli, A.R.; Agarwal, P.K.; McConkey, D.J.; Hazle, J.D.; Dinney, C.P.N. Validating bladder cancer xenograft bioluminescence with magnetic resonance imaging: The significance of hypoxia and necrosis. BJU Int. 2010, 106, 1799–1804. [Google Scholar] [CrossRef]
- Li, G.; Yuan, S.; Deng, D.; Ou, T.; Li, Y.; Sun, R.; Lei, Q.; Wang, X.; Shen, W.; Cheng, Y.; et al. Fluorinated Polyethylenimine to Enable Transmucosal Delivery of Photosensitizer-Conjugated Catalase for Photodynamic Therapy of Orthotopic Bladder Tumors Postintravesical Instillation. Adv. Funct. Mater. 2019, 29, 1901932. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, X.; Gong, F.; Chao, Y.; Cheng, L. Newly developed strategies for improving sonodynamic therapy. Mater. Horiz. 2020, 7, 2028–2046. [Google Scholar] [CrossRef]
- Qian, X.; Zheng, Y.; Chen, Y. Micro/Nanoparticle-Augmented Sonodynamic Therapy (SDT): Breaking the Depth Shallow of Photoactivation. Adv. Mater. 2016, 28, 8097–8129. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Chen, Y.; Shi, J. Nanoenzyme-Augmented Cancer Sonodynamic Therapy by Catalytic Tumor Oxygenation. ACS Nano 2018, 12, 3780–3795. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Hou, B.; Xie, S. Application of nanosonosensitizer materials in cancer sono-dynamic therapy. RSC Adv. 2022, 12, 22722–22747. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhong, X.; Liu, Z.; Cheng, L. Recent progress of chemodynamic therapy-induced combination cancer therapy. Nano Today 2020, 35, 100946. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, Y.; He, M.; Bu, W. Chemodynamic therapy: Tumour microenvironment-mediated Fenton and Fenton-like reactions. Angew. Chem. Int. Ed. 2019, 58, 946–956. [Google Scholar] [CrossRef]
- Zhang, C.; Bu, W.; Ni, D.; Zhang, S.; Li, Q.; Yao, Z.; Zhang, J.; Yao, H.; Wang, Z.; Shi, J. Synthesis of Iron Nanometallic Glasses and Their Application in Cancer Therapy by a Localized Fenton Reaction. Angew. Chem. 2016, 128, 2141–2146. [Google Scholar] [CrossRef]
- Mei, L.; Ma, D.; Gao, Q.; Zhang, X.; Fu, W.; Dong, X.; Xing, G.; Yin, W.; Gu, Z.; Zhao, Y. Glucose-responsive cascaded nanocatalytic reactor with self-modulation of the tumor microenvironment for enhanced chemo-catalytic therapy. Mater. Horiz. 2020, 7, 1834–1844. [Google Scholar] [CrossRef]
- Lin, L.S.; Song, J.; Song, L.; Ke, K.; Liu, Y.; Zhou, Z.; Shen, Z.; Li, J.; Yang, Z.; Tang, W. Simultaneous Fenton-like ion delivery and glutathione depletion by MnO2-based nanoagent to enhance chemodynamic therapy. Angew. Chem. 2018, 130, 4996–5000. [Google Scholar] [CrossRef]
- Miao, Z.; Chen, S.; Xu, C.Y.; Ma, Y.; Qian, H.; Xu, Y.; Chen, H.; Wang, X.; He, G.; Lu, Y.; et al. PEGylated rhenium nanoclusters: A degradable metal photothermal nanoagent for cancer therapy. Chem. Sci. 2019, 10, 5435–5443. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhong, X.; Zha, Z.; He, G.; Miao, Z.; Lei, H.; Luo, Q.; Zhang, R.; Liu, Z.; Cheng, L. Biodegradable CoS2 nanoclusters for photothermal-enhanced chemodynamic therapy. Appl. Mater. Today 2020, 18, 100464. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Peng, F.; Tu, Y.; Adhikari, A.; Hintzen, J.C.J.; Löwik, D.W.P.M.; Wilson, D.A. A peptide functionalized nanomotor as an efficient cell penetrating tool. Chem. Commun. 2017, 53, 1088–1091. [Google Scholar] [CrossRef]
- Wang, J.; Gao, W. Nano/Microscale motors: Biomedical opportunities and challenges. ACS Nano 2012, 6, 5745–5751. [Google Scholar] [CrossRef]
- Liu, L.; Mo, H.; Wei, S.; Raftery, D. Quantitative analysis of urea in human urine and serum by 1H nuclear magnetic resonance. Analyst 2012, 137, 595–600. [Google Scholar] [CrossRef] [Green Version]
- Hortelão, A.C.; Carrascosa, R.; Murillo-Cremaes, N.; Patiño, T.; Sánchez, S. Targeting 3D Bladder Cancer Spheroids with Urease-Powered Nanomotors. ACS Nano 2019, 13, 429–439. [Google Scholar] [CrossRef]
- Bao, Q.; Hu, P.; Ren, W.; Guo, Y.; Shi, J. Tumor Cell Dissociation Removes Malignant Bladder Tumors. Chem 2020, 6, 2283–2299. [Google Scholar] [CrossRef]
- Chin, Y.C.; Yang, L.X.; Hsu, F.T.; Hsu, C.W.; Chang, T.W.; Chen, H.Y.; Chen, L.Y.; Chia, Z.C.; Hung, C.H.; Su, W.C.; et al. Iron oxide@chlorophyll clustered nanoparticles eliminate bladder cancer by photodynamic immunotherapy-initiated ferroptosis and immunostimulation. J. Nanobiotechnol. 2022, 20, 373. [Google Scholar] [CrossRef]
- Lamm, D.L. Efficacy and safety of bacille Calmette-Guerin immunotherapy in superficial bladder cancer. Clin. Infect. Dis. 2000, 31 (Suppl. S3), S86–S90. [Google Scholar] [CrossRef]
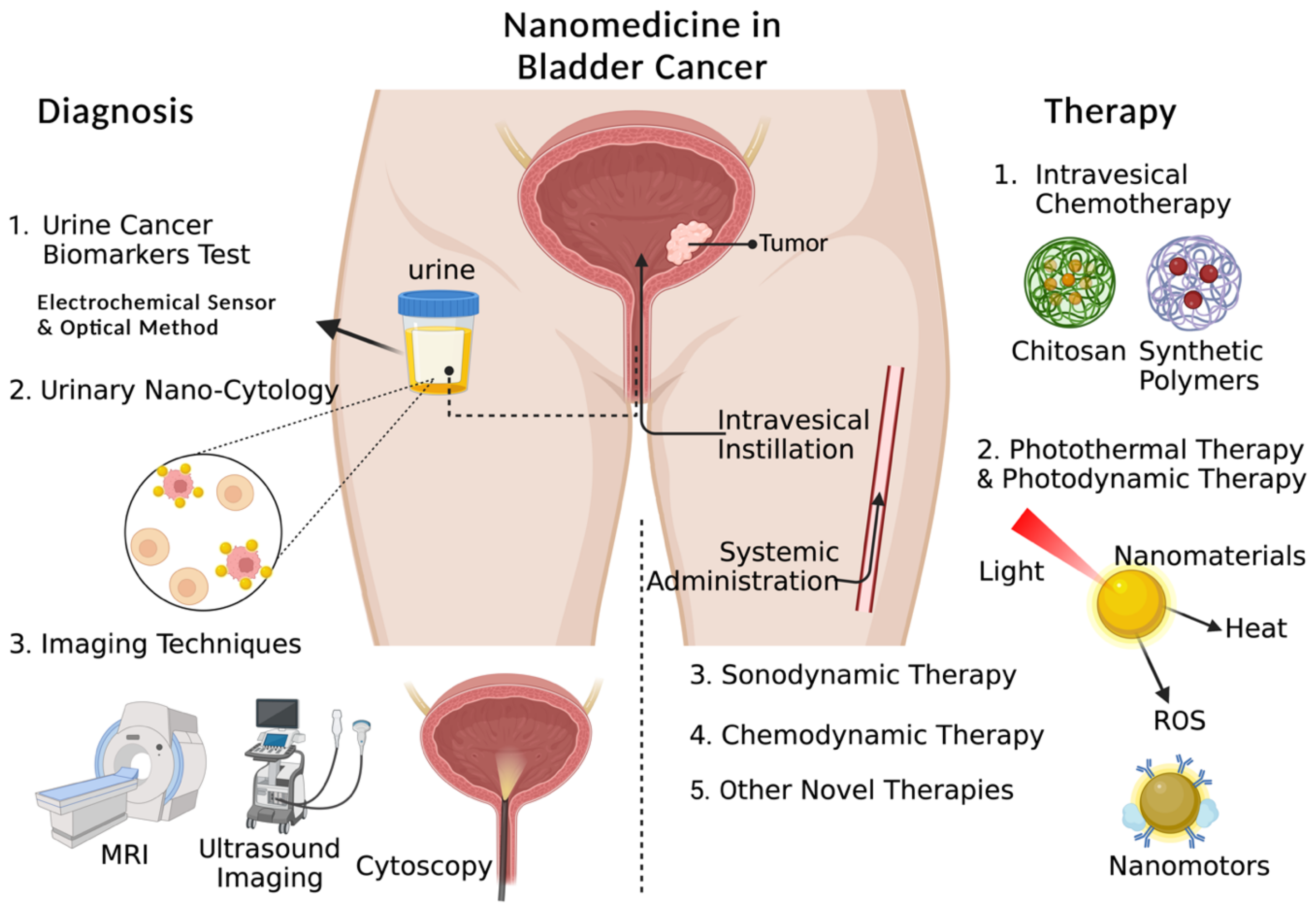


| NCT Number | Nanoparticles | Drug | Condition | States | Population | Sponsor/ Collaborators | Study Start | Status |
|---|---|---|---|---|---|---|---|---|
| NCT05519241 | PLZ4-coated paclitaxel-loaded micelles (PPM) | PTX | Non-muscle-invasive Bladder Cancer | Phase 1 | 18 years and older |
| October 2022 | Recruiting |
| NCT00585689 | Paclitaxel albumin-stabilized nanoparticle (Nab-paclitaxel) | PTX | Bladder Cancer | Phase 2 | 18 years and older |
| December 2007 | Completed |
| NCT02718742 | Paclitaxel Albumin-Stabilized Nanoparticle Formulation | PTX |
| Phase 2 | 18 years and older |
| June 2016 | Withdrawn |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, F.-X.; Xu, X.; Ding, H.; Yu, L.; Huang, H.; Hao, J.; Wu, C.; Liang, R.; Zhang, S. Recent Progress in Nanomaterial-Based Biosensors and Theranostic Nanomedicine for Bladder Cancer. Biosensors 2023, 13, 106. https://doi.org/10.3390/bios13010106
Song F-X, Xu X, Ding H, Yu L, Huang H, Hao J, Wu C, Liang R, Zhang S. Recent Progress in Nanomaterial-Based Biosensors and Theranostic Nanomedicine for Bladder Cancer. Biosensors. 2023; 13(1):106. https://doi.org/10.3390/bios13010106
Chicago/Turabian StyleSong, Fan-Xin, Xiaojian Xu, Hengze Ding, Le Yu, Haochen Huang, Jinting Hao, Chenghao Wu, Rui Liang, and Shaohua Zhang. 2023. "Recent Progress in Nanomaterial-Based Biosensors and Theranostic Nanomedicine for Bladder Cancer" Biosensors 13, no. 1: 106. https://doi.org/10.3390/bios13010106





