High-Performance FET-Based Dopamine-Sensitive Biosensor Platform Based on SOI Substrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
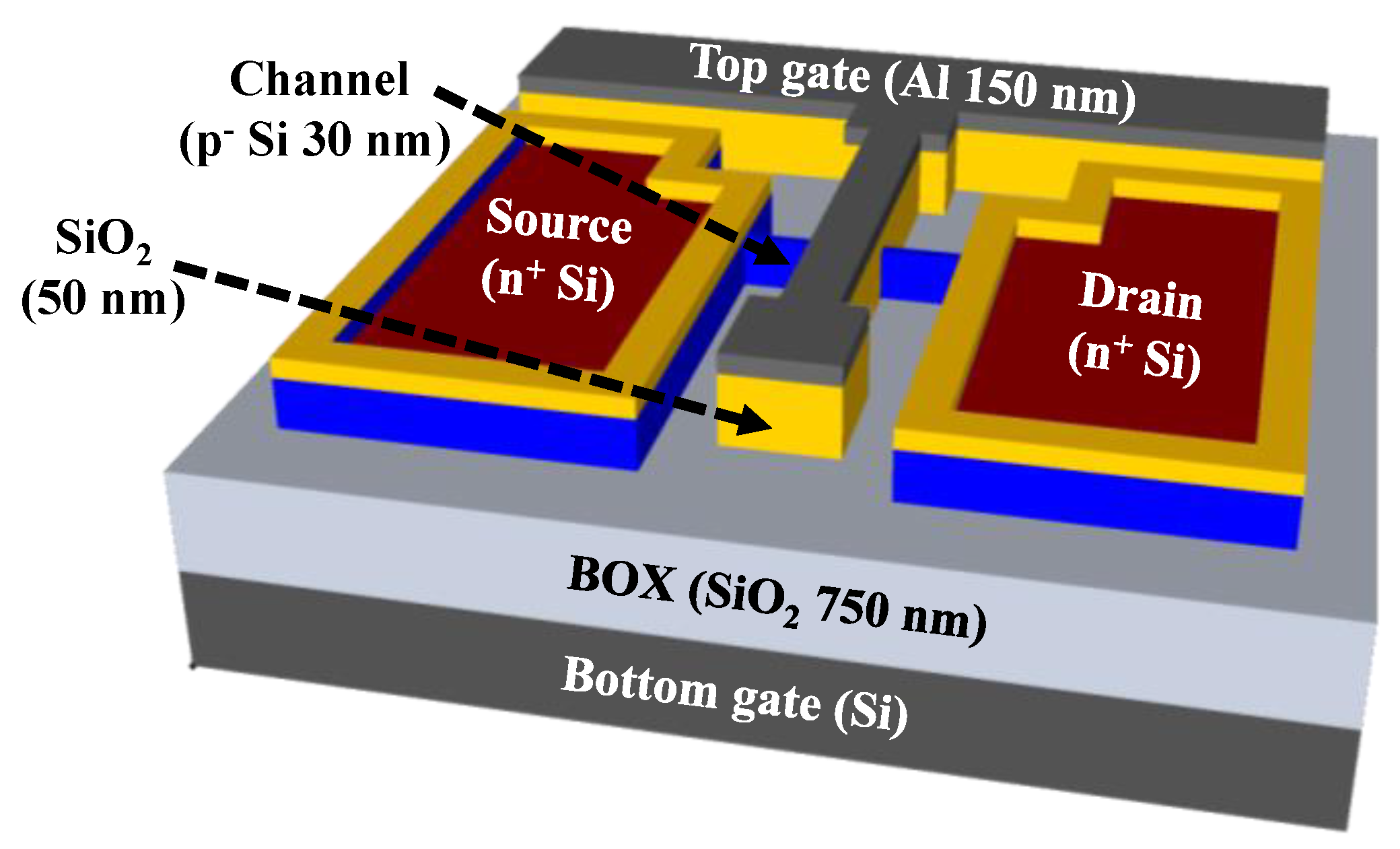
2.2. Fabrication of SOI DG FET Transducer Unit
2.3. Fabrication of DA-Sensitive EG Sensing Unit
2.4. Device Characterization
3. Results
3.1. Electrical Characteristics of SOI DG FET Transducer Unit
3.2. Self-Amplification through Capacitive Coupling of SOI DG FET Transducer Unit
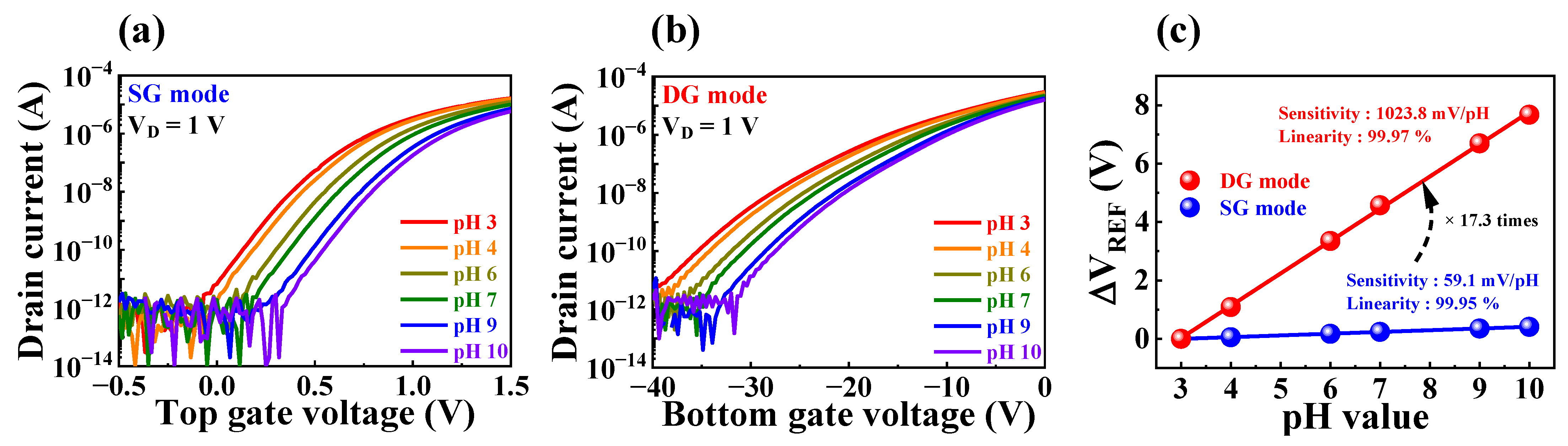
3.3. pH Sensing Characteristics of the Fabricated FET-Type Biosensor Platform
3.4. Non-Ideal Effects of the Fabricated FET-Type Biosensor Platform
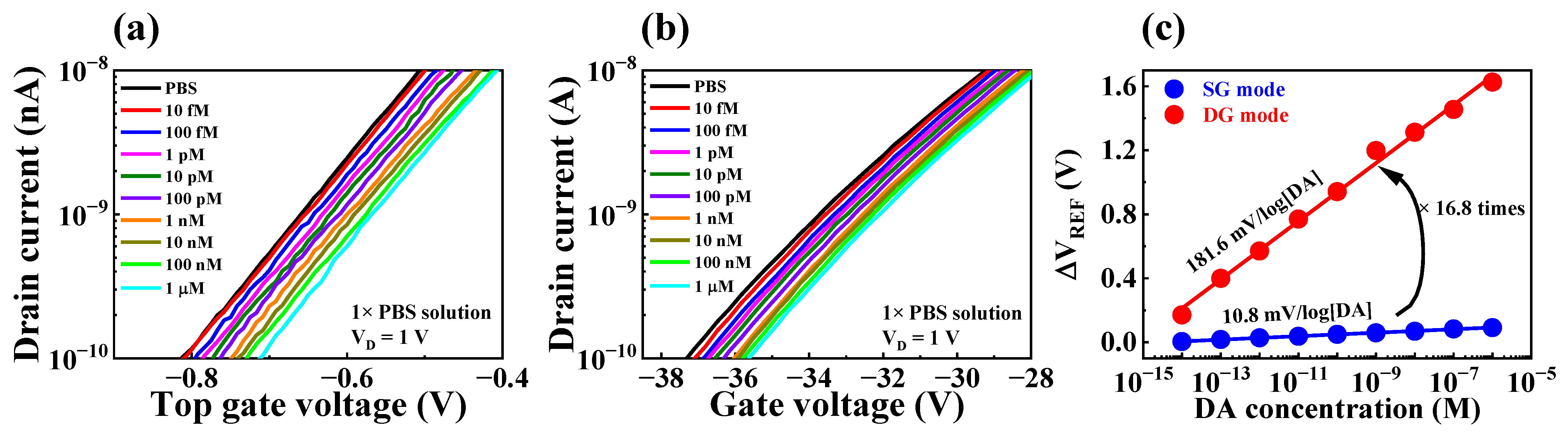
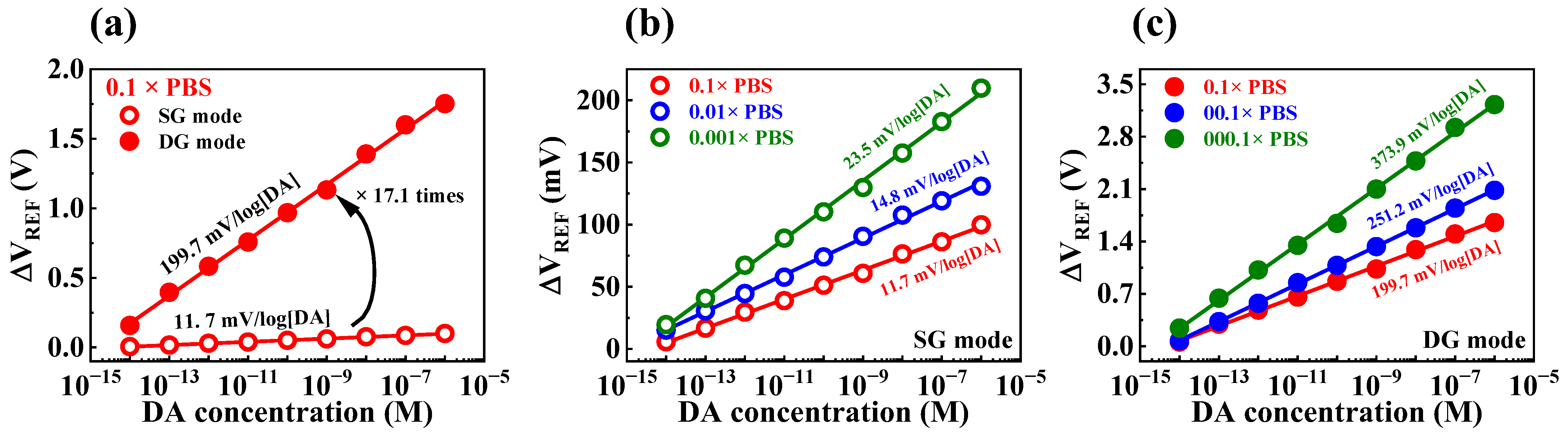
3.5. DA Sensing Characteristics of the Fabricated DA-Sensitive FET-Type Biosensor
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franco, R.; Reyes-Resina, I.; Navarro, G. Dopamine in health and disease: Much more than a neurotransmitter. Biomedicines 2021, 9, 109. [Google Scholar] [CrossRef]
- Basu, S.; Dasgupta, P.S. Dopamine, a neurotransmitter, influences the immune system. J. Neuroimmunol. 2000, 102, 113–124. [Google Scholar] [CrossRef]
- Lin, C.-H.; Hsiao, C.-Y.; Hung, C.-H.; Lo, Y.-R.; Lee, C.-C.; Su, C.-J.; Lin, H.-C.; Ko, F.-H.; Huang, T.-Y.; Yang, Y.-S. Ultrasensitive detection of dopamine using a polysilicon nanowire field-effect transistor. Chem. Commun. 2008, 44, 5749–5751. [Google Scholar] [CrossRef]
- Nieoullon, A.; Coquerel, A. Dopamine: A key regulator to adapt action, emotion, motivation and cognition. Curr. Opin. Neurol. 2003, 16, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Palit, S.; Singh, K.; Lou, B.-S.; Her, J.-L.; Pang, S.-T.; Pan, T.-M. Ultrasensitive dopamine detection of indium-zinc oxide on pet flexible based extended-gate field-effect transistor. Sens. Actuator B Chem. 2020, 310, 127850. [Google Scholar] [CrossRef]
- Elsworth, J.D.; Roth, R.H. Dopamine synthesis, uptake, metabolism, and receptors: Relevance to gene therapy of Parkinson’s disease. Exp. Neurol. 1997, 144, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Kaminga, A.C.; Wen, S.W.; Wu, X.; Acheampong, K.; Liu, A. Dopamine and dopamine receptors in Alzheimer’s disease: A systematic review and network meta-analysis. Front. Aging Neurosci. 2019, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, S.W.S.; Nyberg, L.; Bäckman, L. Intra-individual variability in behavior: Links to brain structure, neurotransmission and neuronal activity. Trends Neurosci. 2006, 29, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Justice, J.B. Quantitative microdialysis of neurotransmitters. J. Neurosci. Meth. 1993, 48, 263–276. [Google Scholar] [CrossRef]
- Smith, A.D.; Olson, R.J.; Justice, J.B. Quantitative microdialysis of dopamine in the striatum: Effect of circadian variation. J. Neurosci. Methods 1992, 44, 33–41. [Google Scholar] [CrossRef]
- O’Neill, R.D. Microvoltammetric techniques and sensors for monitoring neurochemical dynamics in vivo: A review. Analyst 1994, 119, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-S.; Kim, S.; Kim, M. Ion-sensitive field-effect transistor for biological sensing. Sensors 2009, 9, 7111–7131. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshani, K.N.; Singh, S.; Mohammed, M.K.A. Dielectric/charge density modulated junctionless fet based label-free biosensor. Inorg. Chem. Commun. 2023, 148, 110350. [Google Scholar] [CrossRef]
- Hosseini, S.N.; Das, P.S.; Lazarjan, V.K.; Gagnon-Turcotte, G.; Bouzid, K.; Gosselin, B. Recent advances in CMOS electrochemical biosensor design for microbial monitoring: Review and design methodology. IEEE Trans. Biomed. Circuits Syst. 2023, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Bomer, J.G.; Carlen, E.T.; van den Berg, A. Al2O3/silicon nanoISFET with near ideal Nernstian response. Nano Lett. 2011, 11, 2334–2341. [Google Scholar] [CrossRef]
- Bergveld, P. Thirty years of ISFETOLOGY what happened in the past 30 years and what may happen in the next 30 years. Sen. Actuator B Chem. 2003, 88, 1–20. [Google Scholar] [CrossRef]
- van der spiegel, J.; Lauks, I.; Chan, P.; Babic, D. The extended gate chemically sensitive field effect transistor as multi-species microprobe. Sens. Actuator. 1983, 4, 291–298. [Google Scholar] [CrossRef]
- Alvarez-Serna, B.E.; Ramírez-Chavarría, R.G.; Castillo-Villanueva, E.; Carrillo-Reyes, J.; Ramírez-Zamora, R.M.; Buitrón, G.; Alvarez-Icaza, L. Label-free and portable field-effect sensor for monitoring rt-lamp products to detect SARS-CoV-2 in wastewater. Talanta 2023, 253, 124060. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Huang, C.-H.; Lin, J.-H.; Yu, L.-S.; Huang, I.-Y. Development of an EGFET microsensor with 3D structure for high-specificity cardiac troponin I detection. J. Micromech. Microeng. 2023, 33, 045001. [Google Scholar] [CrossRef]
- Cho, S.-K.; Cho, W.-J. Highly sensitive and transparent urea-EnFET based point-of-care diagnostic test sensor with a triple-gate a-IGZO TFT. Sensors 2021, 21, 4748. [Google Scholar] [CrossRef]
- Chou, H.-Y.; Chiang, J.-L.; Yu, C.-T.R.; Chen, J.-M.M.; Wuu, D.-S. Sensing property of Ga2O3-based extended-gate field-effect transistors for a living cell viability sensor. Sens. Actuator A Phys. 2023, 349, 114071. [Google Scholar] [CrossRef]
- Spijkman, M.J.; Brondijk, J.J.; Geuns, T.C.; Smits, E.C.; Cramer, T.; Zerbetto, F.; Stoliar, P.; Biscarini, F.; Blom, P.W.; de Leeuw, D.M. Dual-gate organic field-effect transistors as potentiometric sensors in aqueous solution. Adv. Funct. Mater. 2010, 20, 898–905. [Google Scholar] [CrossRef]
- Jang, H.-J.; Cho, W.-J. Fabrication of high-performance fully depleted silicon-on-insulator based dual-gate ion-sensitive field-effect transistor beyond the Nernstian limit. Appl. Phys. Lett. 2012, 100, 073701. [Google Scholar] [CrossRef]
- Liu, N.; Hui Liu, Y.; Feng, P.; Qiang Zhu, L.; Shi, Y.; Wan, Q. Enhancing the pH sensitivity by laterally synergic modulation in dual-gate electric-double-layer transistors. Appl. Phys. Lett. 2015, 106, 073507. [Google Scholar] [CrossRef]
- Cho, S.-K.; Cho, W.-J. Ultra-high sensitivity pH-sensors using silicon nanowire channel dual-gate field-effect transistors fabricated by electrospun polyvinylpyrrolidone nanofibers pattern template transfer. Sens. Actuator B Chem. 2021, 326, 128835. [Google Scholar] [CrossRef]
- Cambre, J.N.; Sumerlin, B.S. Biomedical applications of boronic acid polymers. Polymer 2011, 52, 4631–4643. [Google Scholar] [CrossRef]
- Gu, L.; Jiang, X.; Liang, Y.; Zhou, T.; Shi, G. Double recognition of dopamine based on a boronic acid functionalized poly(aniline-co-anthranilic acid)–molecularly imprinted polymer composite. Analyst 2013, 138, 5461–5469. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Lee, L.Y.S.; So, M.-H.; Wong, K.-Y. A dopamine electrochemical sensor based on molecularly imprinted poly(acrylamidophenylboronic acid) film. Electroanalysis 2013, 25, 1085–1094. [Google Scholar] [CrossRef]
- Bartczak, D.; Kanaras, A.G. Preparation of peptide-functionalized gold nanoparticles using one pot EDC/sulfo-NHS coupling. Langmuir 2011, 27, 10119–10123. [Google Scholar] [CrossRef]
- Yan, Q.; Zheng, H.-N.; Jiang, C.; Li, K.; Xiao, S.-J. EDC/NHS activation mechanism of polymethacrylic acid: Anhydride versus NHS-ester. RSC Adv. 2015, 5, 69939–69947. [Google Scholar] [CrossRef]
- Bousse, L.; De Rooij, N.F.; Bergveld, P. Operation of chemically sensitive field-effect sensors as a function of the insulator-electrolyte interface. IEEE Trans. Electron. Devices 1983, 30, 1263–1270. [Google Scholar] [CrossRef]
- Healy, T.W. Site-binding model of the electrical double layer at the oxide/water interface. J. Chem. Soc.-Perkin Trans. 1974, 70, 1807–1818. [Google Scholar]
- Healy, T.W.; White, L.R. Ionizable surface group models of aqueous interfaces. Adv. Colloid Interface Sci. 1978, 9, 303–345. [Google Scholar] [CrossRef]
- Landheer, D.; Aers, G.; McKinnon, W.R.; Deen, M.J.; Ranuarez, J.C. Model for the field effect from layers of biological macromolecules on the gates of metal-oxide-semiconductor transistors. J. Appl. Phys. 2005, 98, 044701. [Google Scholar] [CrossRef]
- Majeed, L.; Amin, S.I.; Rasool, Z.; Bashir, I.; Kumar, N.; Anand, S. TCAD device modeling and simulation study of organic field effect transistor-based pH sensor with tunable sensitivity for surpassing Nernst limit. Electronics 2023, 12, 536. [Google Scholar] [CrossRef]
- Tsai, C.-N.; Chou, J.-C.; Sun, T.-P.; Hsiung, S.-K. Study on the sensing characteristics and hysteresis effect of the tin oxide pH electrode. Sens. Actuator B Chem. 2005, 108, 877–882. [Google Scholar] [CrossRef]
- Fung, C.D.; Cheung, P.W.; Ko, W.H. A generalized theory of an electrolyte-insulator-semiconductor field-effect transistor. IEEE Trans. Electron. Devices 1986, 33, 8–18. [Google Scholar] [CrossRef]
- Jamasb, S.; Collins, S.; Smith, R.L. A physical model for drift in pH ISFETs. Sens. Actuator B Chem. 1998, 49, 146–155. [Google Scholar] [CrossRef]
- Bousse, L.; Bergveld, P. The role of buried OH sites in the response mechanism of inorganic-gate pH-sensitive ISFETs. Sens. Actuator 1984, 6, 65–78. [Google Scholar] [CrossRef]
- Shoute, L.C.T.; Abdelrasoul, G.N.; Ma, Y.; Duarte, P.A.; Edwards, C.; Zhuo, R.; Zeng, J.; Feng, Y.; Charlton, C.L.; Kanji, J.N.; et al. Label-free impedimetric immunosensor for point-of-care detection of COVID-19 antibodies. Microsyst. Nanoeng. 2023, 9, 3. [Google Scholar] [CrossRef]
- Lai, P.-H.; Tseng, L.-S.; Yang, C.-M.; Lu, M.S.-C. Design and characterization of a 16 × 16 CMOS capacitive DNA sensor array. IEEE Sens. J. 2023, 23, 8120–8127. [Google Scholar] [CrossRef]

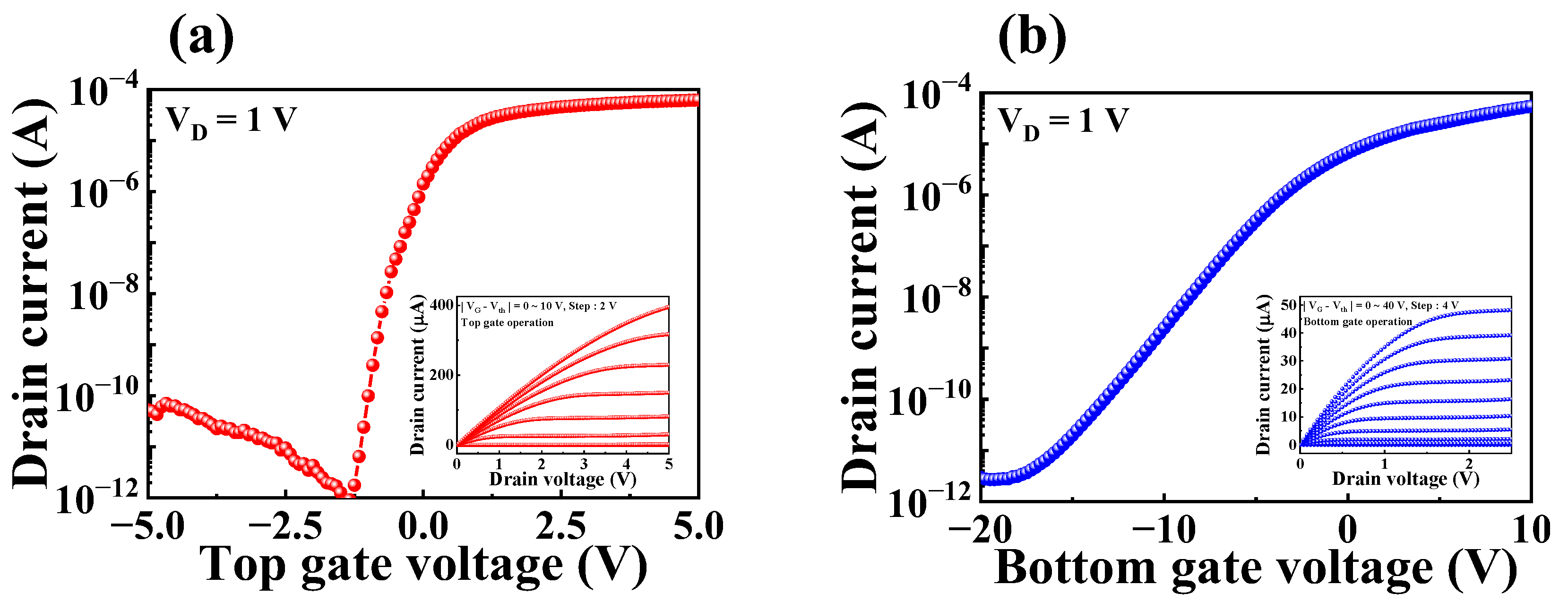


| Operation Mode | VTH (V) | ION/OFF | μFE (cm2/V·S) | SS (mV/dec) |
|---|---|---|---|---|
| Top-gate | −1.1 | 7.7 × 107 | 398.3 | 135.4 |
| Bottom-gate | −16.2 | 1.8 × 107 | 98.8 | 2224.9 |
| Operation Mode | Sensitivity (mV/pH) | VH (mV) | RD (mV/h) | VH to Sensitivity | RD to Sensitivity |
|---|---|---|---|---|---|
| SG | 59.1 | 3.7 | 6.8 | 6.2% | 11% |
| DG | 1023.9 | 36.2 | 48.7 | 3.5% | 4.7% |
| PBS Concentration | DA Sensitivity (mV/log[DA]) | Amplification Ratio | |
|---|---|---|---|
| SG Mode | DG Mode | ||
| 1× | 10.8 | 181.6 | 16.8 |
| 0.1× | 11.7 | 199.7 | 17.1 |
| 0.01× | 14.8 | 251.2 | 16.9 |
| 0.001× | 23.5 | 373.9 | 15.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyun, T.-H.; Cho, W.-J. High-Performance FET-Based Dopamine-Sensitive Biosensor Platform Based on SOI Substrate. Biosensors 2023, 13, 516. https://doi.org/10.3390/bios13050516
Hyun T-H, Cho W-J. High-Performance FET-Based Dopamine-Sensitive Biosensor Platform Based on SOI Substrate. Biosensors. 2023; 13(5):516. https://doi.org/10.3390/bios13050516
Chicago/Turabian StyleHyun, Tae-Hwan, and Won-Ju Cho. 2023. "High-Performance FET-Based Dopamine-Sensitive Biosensor Platform Based on SOI Substrate" Biosensors 13, no. 5: 516. https://doi.org/10.3390/bios13050516





