Biofunctionalisation of Polypyrrole Nanowires Array with Sulfite Oxidase Coupled with the Integration of Platinum Nanoparticles for Ultrasensitive Amperometric Detection of Sulfite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus
2.3. Construction of Modified PPyNWA-PtNPs-SOx Electrode
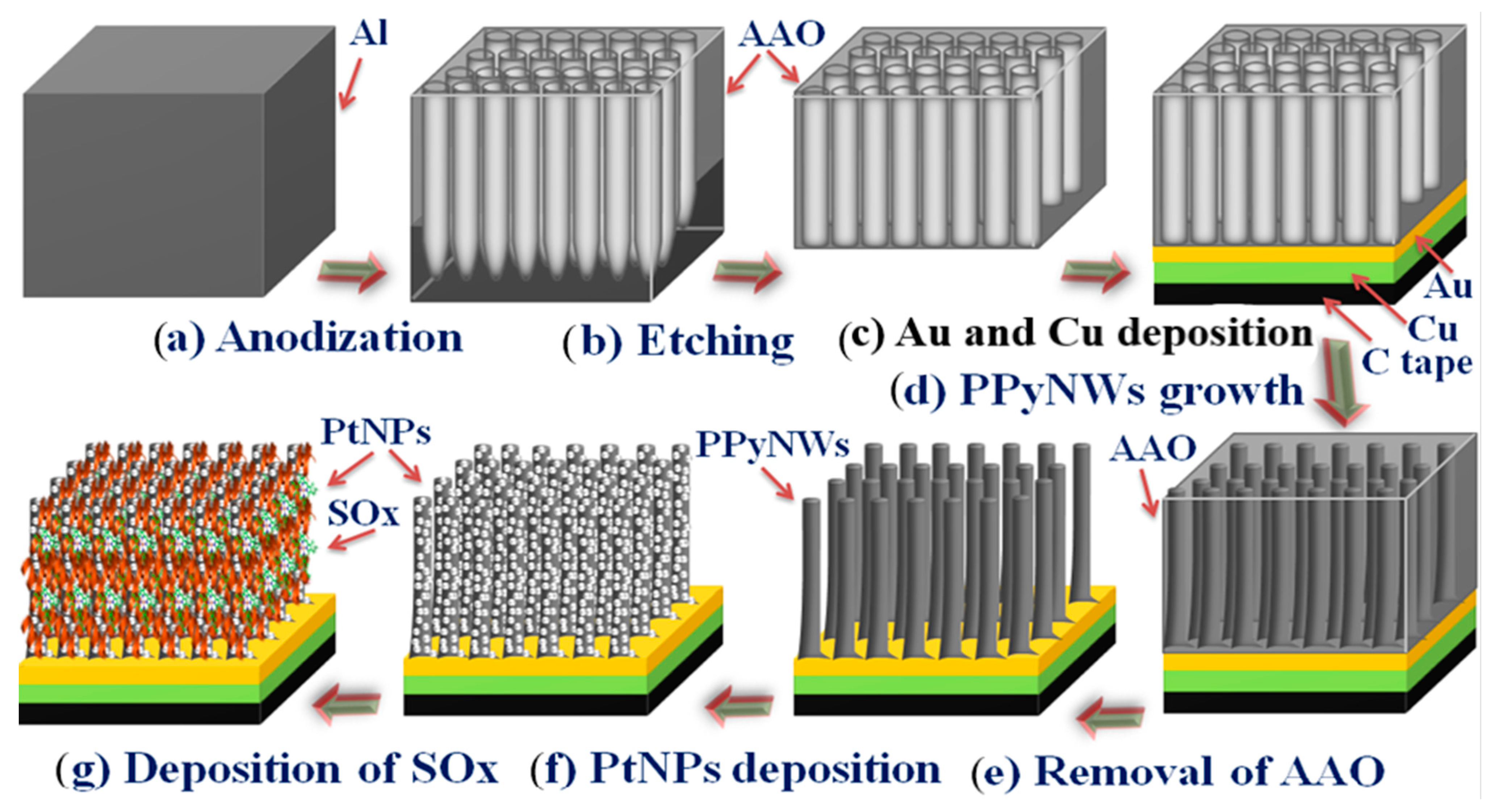
2.3.1. Fabrication of AAO Template
2.3.2. Growth of PPyNWA and Deposition of PtNPs
2.3.3. Adsorption of SOx
2.4. Amperometric Measurement with PPyNWA-PtNPs-SOx Biosensor
2.5. Optimisation of PPyNWA-PtNPs-SOx Biosensor
2.6. Detection of Sulfite
3. Results and Discussion
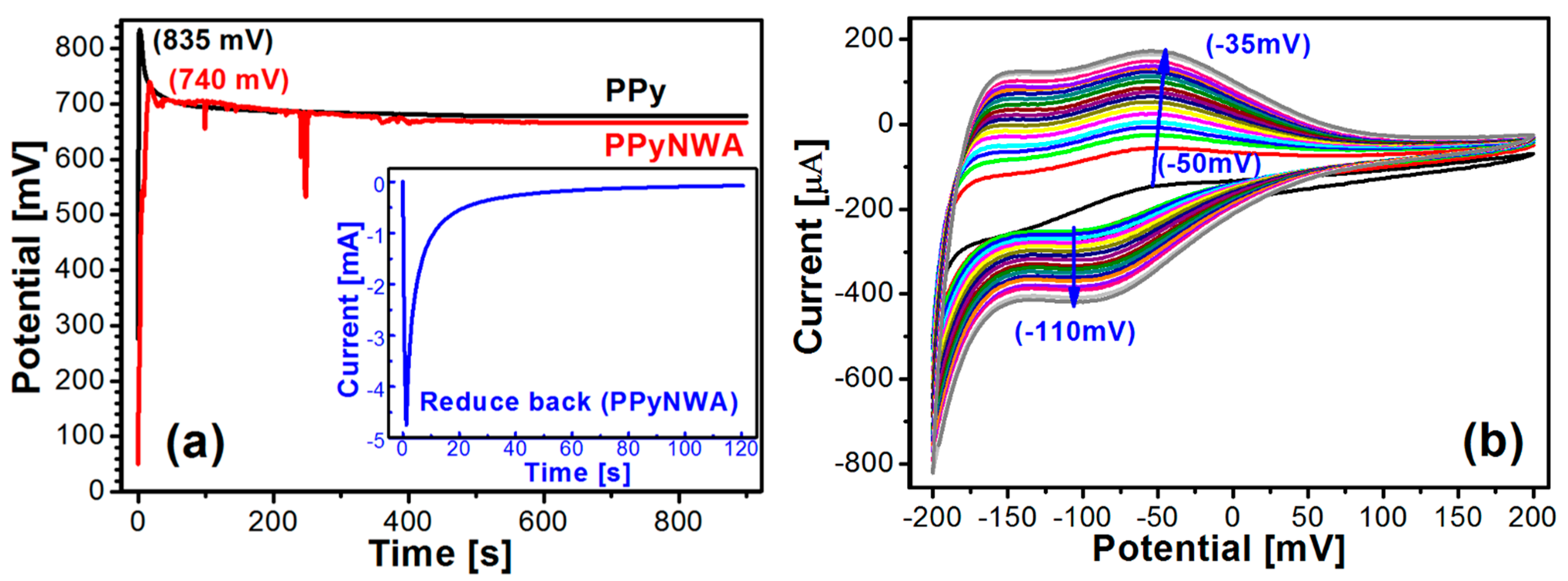
3.1. Formation and Biofunctionalisation of NWA
3.2. Morphological Study of PPyNWA-PtNPs by SEM
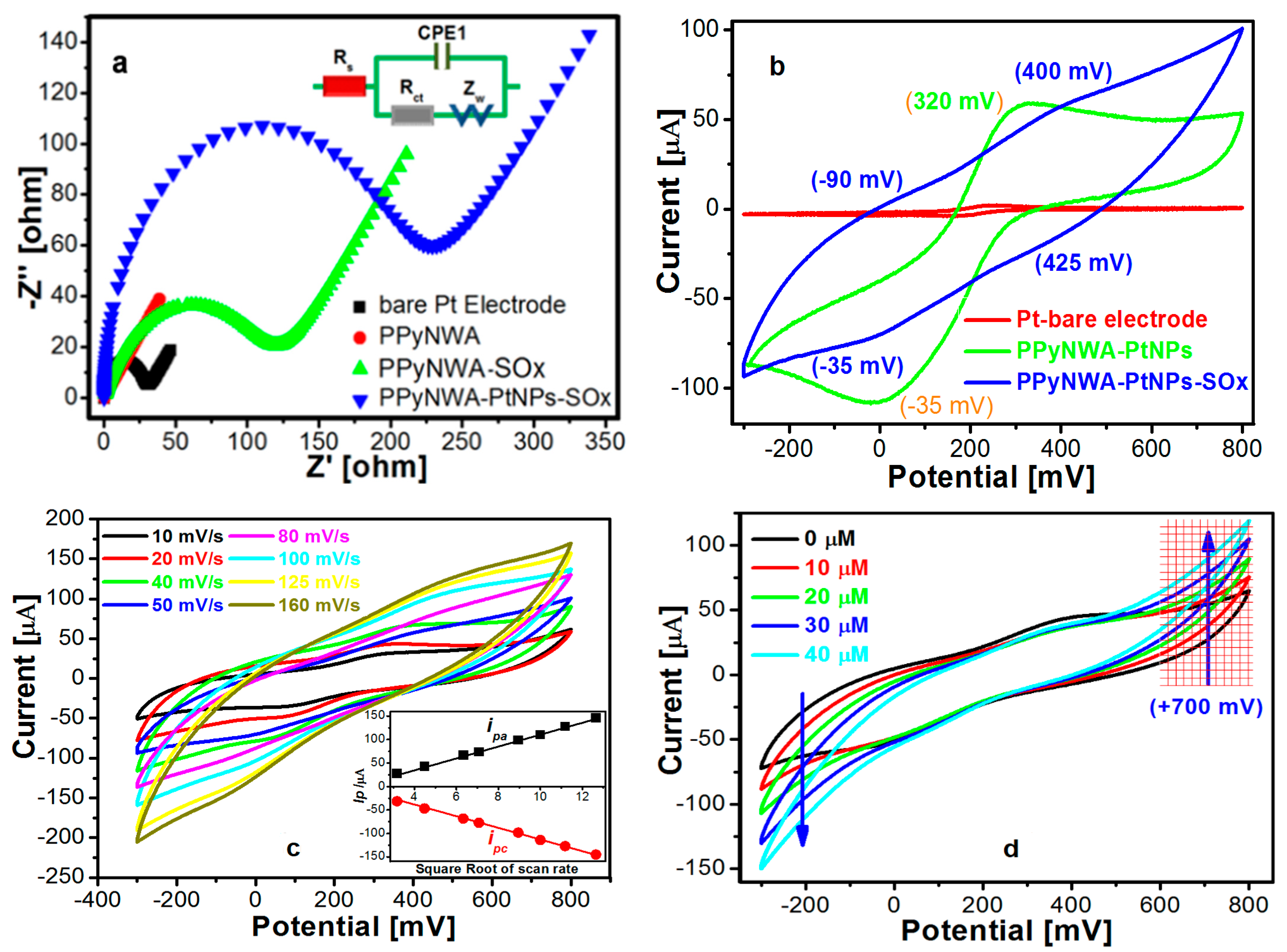
3.3. Electrochemical Impedance Analysis
3.4. Electrochemical Behaviour of PPyNWA-PtNPs-SOx
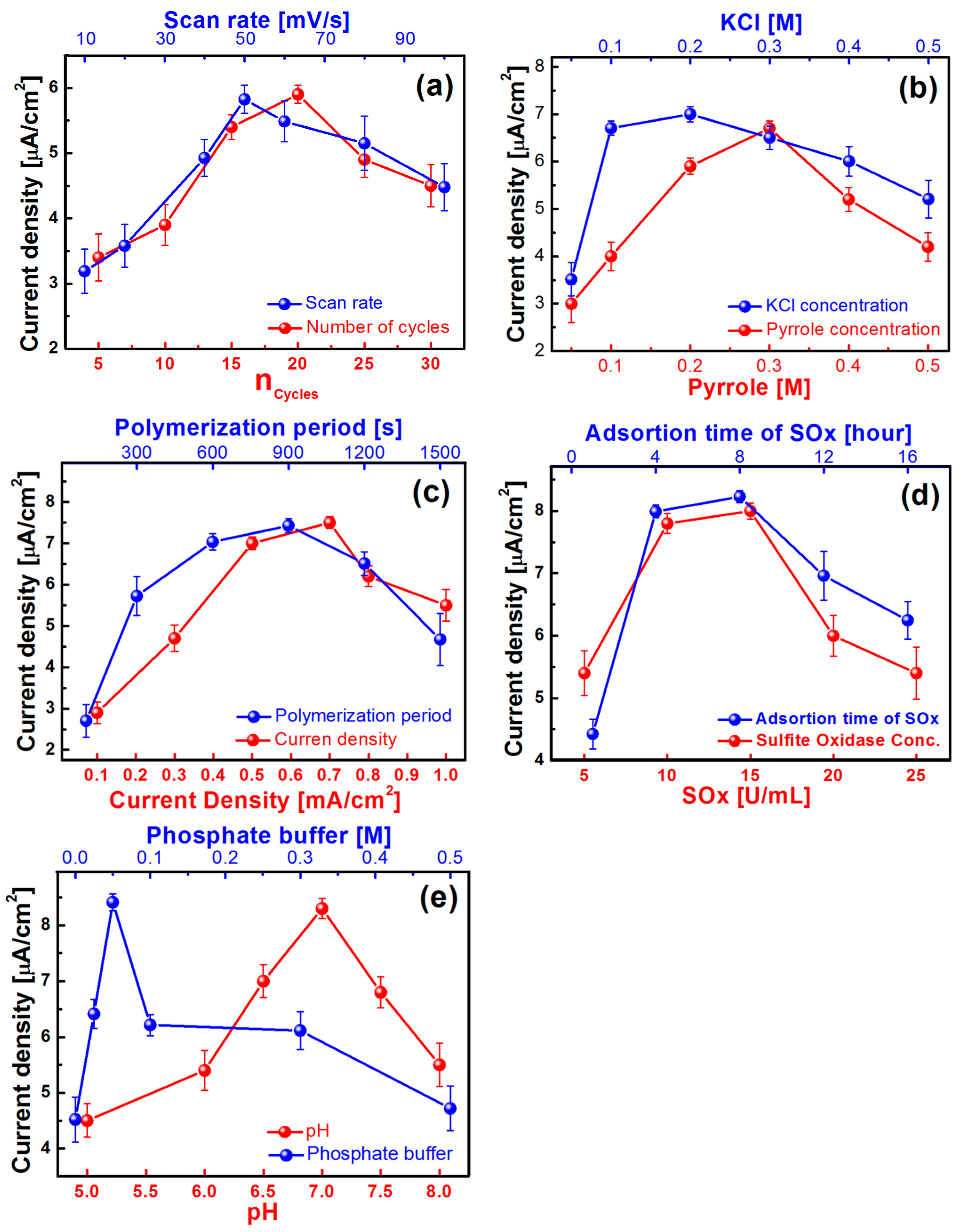
3.5. Optimisation of the Nanobiosensor
3.5.1. Deposition of PtNPs
3.5.2. Influence of Pyrrole and Potassium Chloride Concentration
3.5.3. Applied Current Density and Polymerisation Time
3.5.4. SOx Concentration and Adsorption Period
3.5.5. Influence of pH and Buffer Concentration
3.6. Analytical Performance and Applications
3.7. Interferences, Reproducibility, and Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petersen, S. Biofunctionalization. BioNanoMaterials 2017, 18, 20170007. [Google Scholar] [CrossRef]
- Hartmann, H.; Krastev, R. Biofunctionalization of surfaces using polyelectrolyte multilayers. BioNanoMaterials 2017, 18, 20160015. [Google Scholar] [CrossRef]
- Salehi, M. Bioconjugation of SERS nanotags and increasing the reproducibility of results. BioNanoMaterials 2017, 18, 20160011. [Google Scholar] [CrossRef]
- Conde, J.; Dias, J.T.; Grazú, V.; Moros, M.; Baptista, P.V.; de la Fuente, J.M. Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine. Front. Chem. 2014, 2, 48. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Ai, S.; Shi, W.; Zhu, L. A novel hydrogen peroxide biosensor based on horseradish peroxidase immobilized on gold nanoparticles–silk fibroin modified glassy carbon electrode and direct electrochemistry of horseradish peroxidase. Sens. Actuators B Chem. 2009, 137, 747–753. [Google Scholar] [CrossRef]
- Walter, J.G.; Urmann, K.; Modrejewski, J.; Scheper, T. Aptamer-modified nanomaterials: Principles and applications. BioNanoMaterials 2017, 18, 20160012. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Bordbar, A.K.; Zare, D.; Davoodi, D.; Noruzi, M.; Barkhi, M.; Tabatabaei, M. Immobilization of cellulase enzyme on superparamagnetic nanoparticles and determination of its activity and stability. Chem. Eng. J. 2011, 171, 669–673. [Google Scholar] [CrossRef]
- Abdul Rahim, M.Z.; Govender-Hondros, G.; Adeloju, S.B. A single step electrochemical integration of gold nanoparticles, cholesterol oxidase, cholesterol esterase and mediator with polypyrrole films for fabrication of free and total cholesterol nanobiosensors. Talanta 2018, 189, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Yogeswaran, U.; Chen, S.-M. A Review on the Electrochemical Sensors and Biosensors Composed of Nanowires as Sensing Material. Sensors 2008, 8, 290–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cronin, S.B.; Lin, Y.-M.; Rabin, O.; Black, M.R.; Dresselhaus, G.; Dresselhaus, M.S.; Gai, P.L. Bismuth nanowires for potential applications in nanoscale electronics technology. Microsc. Microanal. 2002, 8, 58–63. [Google Scholar] [CrossRef]
- Cui, J.; Adeloju, S.B.O.; Wu, Y. Integration of a highly ordered gold nanowires array with glucose oxidase for ultra-sensitive glucose detection. Anal. Chim. Acta 2014, 809, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Ogabiela, E.E.; Adeloju, S.B.O.; Cui, J.; Wu, Y.; Chen, W. A novel ultrasensitive phosphate amperometric nanobiosensor based on the integration of pyruvate oxidase with highly ordered gold nanowires array. Biosens. Bioelectron. 2015, 71, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Ogabiela, E.E.; Hui, J.; Wang, Y.; Zhang, Y.; Tong, L.; Zhang, J.; Adeloju, S.B.; Zhang, X.; Wu, Y. Electrochemical Biosensor based on Pt/Au Alloy Nanowire Array for Phosphate Detection. J. Electrochem. Soc. 2015, 162, B62. [Google Scholar] [CrossRef]
- Adeloju, S.B.; Shaw, S.J.; Wallace, G.G. Polypyrrole-based amperometric biosensor for sulfite determination. Electroanalysis 1994, 6, 865–870. [Google Scholar] [CrossRef]
- Adeloju, S.B.; Barisci, J.N.; Wallace, G.G. Electroimmobilisation of sulphite oxidase into a polypyrrole film and its utilisation for flow amperometric detection of sulphite. Anal. Chim. Acta 1996, 332, 145–153. [Google Scholar] [CrossRef]
- Adeloju, S.B.O.; Hussain, S. Potentiometric sulfite biosensor based on entrapment of sulfite oxidase in a polypyrrole film on a platinum electrode modified with platinum nanoparticles. Microchim. Acta 2016, 183, 1341–1350. [Google Scholar] [CrossRef]
- Babgaleh, T.; Kathleen, M.K.; Cecilia, W.; Karl, C.K. Sulfites—A Food and Drug Administration Review of Recalls and Reported Adverse Events. J. Food Prot. 2004, 67, 1806–1811. [Google Scholar]
- Zhi, L.; Gorelik, T.; Wu, J.; Kolb, U.; Müllen, K. Nanotubes Fabricated from Ni−Naphthalocyanine by a Template Method. J. Am. Chem. Soc. 2005, 127, 12792–12793. [Google Scholar] [CrossRef]
- Qu, F.; Yang, M.; Shen, G.; Yu, R. Electrochemical biosensing utilizing synergic action of carbon nanotubes and platinum nanowires prepared by template synthesis. Biosens. Bioelectron. 2007, 22, 1749–1755. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Xu, M.; Wang, Y.; Xia, H.; Zhang, S.; Guo, X.; Wu, S. Polypyrrole-Coated SnO2 Hollow Spheres and Their Application for Ammonia Sensor. J. Phys. Chem. C 2009, 113, 1662–1665. [Google Scholar] [CrossRef]
- Cui, J.; Wu, Y.; Wang, Y.; Zheng, H.; Xu, G.; Zhang, X. In-Situ Fabrication of AAO Template without Oxide Barrier Layer and Its Applications. J. Nanosc. Nanotech. 2012, 12, 3130–3134. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Adeloju, S.B.; Wu, Y.; Zhang, X. Modification of polypyrrole nanowires array with platinum nanoparticles and glucose oxidase for fabrication of a novel glucose biosensor. Anal. Chim. Acta 2012, 755, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Otero, T.; Martinez, J. Activation energy for polypyrrole oxidation: Film thickness influence. J. Solid State Electrochem. 2011, 15, 1169–1178. [Google Scholar] [CrossRef]
- Tkac, J.; Davis, J.J. An optimised electrode pre-treatment for SAM formation on polycrystalline gold. J. Electroanal. Chem. 2008, 621, 117–120. [Google Scholar] [CrossRef]
- Song, M.-J.; Kim, J.; Lee, S.; Lee, J.-H.; Lim, D.; Hwang, S.; Whang, D. Pt-polyaniline nanocomposite on boron-doped diamond electrode for amperometic biosensor with low detection limit. Microchim. Acta 2010, 171, 249–255. [Google Scholar] [CrossRef]
- Ameer, Q.; Adeloju, S.B. Galvanostatic Entrapment of Sulfite Oxidase into Ultrathin Polypyrrole Films for Improved Amperometric Biosensing of Sulfite. Electroanalysis 2008, 20, 2549–2556. [Google Scholar] [CrossRef]
- Santos, A.; Vojkuvka, L.; Pallarés, J.; Ferré-Borrull, J.; Marsal, L.F. In situ electrochemical dissolution of the oxide barrier layer of porous anodic alumina fabricated by hard anodization. J. Electroanal. Chem. 2009, 632, 139–142. [Google Scholar] [CrossRef]
- Wang, Z.; Brust, M. Fabrication of nanostructure via self-assembly of nanowires within the AAO template. Nanoscale Res. Lett. 2007, 2, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, D.; Ueda, M.; Ohtsuka, T. The effect of ultrasonic irradiation during electropolymerization of polypyrrole on corrosion prevention of the coated steel. Corros. Sci. 2008, 50, 286–291. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, D.; Bourgeois, L.; Wang, H.; Webley, P.A. Direct Electrodeposition of Porous Gold Nanowire Arrays for Biosensing Applications. ChemPhysChem 2009, 10, 436–441. [Google Scholar] [CrossRef]
- Xue, C.-H.; Zhou, R.-J.; Shi, M.-M.; Wu, G.; Zhang, X.-B.; Wang, M.; Chen, H.-Z. Electrochemistry of glucose oxidase immobilized on carbon nanotubes noncovalently functionalized by multihydroxyl and multicarboxyl groups. J. Electroanal. Chem. 2010, 642, 92–97. [Google Scholar] [CrossRef]
- Ameer, Q.; Adeloju, S.B. Development of a potentiometric catechol biosensor by entrapment of tyrosinase within polypyrrole film. Sens. Actuators B Chem. 2009, 140, 5–11. [Google Scholar] [CrossRef]
- Rawal, R.; Chawla, S.; Pundir, C.S. An electrochemical sulfite biosensor based on gold coated magnetic nanoparticles modified gold electrode. Biosens. Bioelectron. 2012, 31, 144–150. [Google Scholar] [CrossRef] [PubMed]



| Sample Code | Sample | Alcohol Content (% Alc/Vol) | Photometric Results (µM), n = 3 | Biosensor Results (µM), n = 3 |
|---|---|---|---|---|
| BE1 | Beer | 3.6 | 21.25 ± 0.70 | 21.95 ± 0.65 |
| BE2 | Beer | 5.4 | 17.50 ± 0.52 | 17.82 ± 0.60 |
| BE3 | Beer | 3.5 | 16.25 ± 0.65 | 16.47 ± 0.73 |
| WI1 | Yellow wine | 11.5 (contains preservative 220) | 43.75 ±0.40 | 44.11 ± 0.30 |
| WI2 | White wine | 14.5 | 6.25 ± 0.46 | 6.40 ± 0.52 |
| Parameter/Condition | This Work | PPy Sulfite Nanobiosensor [16] | PPy Biosensor [26] | PPy Flow Biosensor [15] |
|---|---|---|---|---|
| Electrode | ||||
| Working electrode material | Nanowires array | Pt—No nanowires or array | Pt—No nanowires or array | Pt—No nanowires or arrays |
| Immobilisation of SOx | ||||
| Mode of immobilisation | Adsorption for 8 h on top of PtNPs grown on top of PPyNWA (SOx adsorbed outside) | Entrapment within ultrathin PPy film grown on top of PtNPs (SOx trapped inside) | Entrapment within ultrathin PPy film grown on Pt electrode (SOx trapped inside) | Entrapment within PPy film grown on Pt electrode (SOx trapped inside) |
| SOx concentration | 10 U mL−1 | 5 U mL−1 | 5 U mL−1 | 10 U mL−1 |
| Location of PtNPs | On top of PPyNWA | Under PPy film | No PtNPs | No PtNPs |
| Location of SOx | On top of PtNPs | Inside PPy film | Inside PPy film | Inside PPy film |
| PPy formation conditions | ||||
| Polymerisation time | 900 s | 60 s | 120 s | 300 s |
| Py concentration | 0.3 M | 0.2 M | 0.1 M | 0.5 M |
| Applied current density | 0.7 mA cm−2 | 0.3 mA cm−2 | 0.2 mA cm−2 | 0.5 mA cm−2 |
| PPy formation | On NWA (without PtNPs) | On top of PtNPs | On top of Pt disc electrode | On top of Pt disc electrode |
| Measurement conditions | ||||
| Phosphate buffer solution | 50 mM | 25 mM | 50 mM and 10 mM KCl | 100 mM and 500 mM KCl |
| Applied potential | +700 mV | None | −700 mV | −900 mV |
| Detection mode | Amperometry | Potentiometry | Amperometry | Flow Amperometry |
| Performance | ||||
| Response time | 2 s | 3–5 s | 5–7 s | Flow-rate-dependent |
| Linear concentration range | 0.12 to 1200 μM | 0.75 to 65 μM | 0.9 to 400 μM | 0 to 200 mg/L |
| Minimum detectable concentration | 0.12 μM | 0.75 μM | 0.9 μM | N/A |
| Stability | 18 weeks | 12–13 weeks | 30 days | 21 days |
| Number of fabrication steps | 7 | 3 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Adeloju, S.B. Biofunctionalisation of Polypyrrole Nanowires Array with Sulfite Oxidase Coupled with the Integration of Platinum Nanoparticles for Ultrasensitive Amperometric Detection of Sulfite. Biosensors 2023, 13, 621. https://doi.org/10.3390/bios13060621
Hussain S, Adeloju SB. Biofunctionalisation of Polypyrrole Nanowires Array with Sulfite Oxidase Coupled with the Integration of Platinum Nanoparticles for Ultrasensitive Amperometric Detection of Sulfite. Biosensors. 2023; 13(6):621. https://doi.org/10.3390/bios13060621
Chicago/Turabian StyleHussain, Shahid, and Samuel B. Adeloju. 2023. "Biofunctionalisation of Polypyrrole Nanowires Array with Sulfite Oxidase Coupled with the Integration of Platinum Nanoparticles for Ultrasensitive Amperometric Detection of Sulfite" Biosensors 13, no. 6: 621. https://doi.org/10.3390/bios13060621




