Discovery of Novel Stimulators of Pax7 and/or MyoD: Enhancing the Efficacy of Cultured Meat Production through Culture Media Enrichment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Differentiation
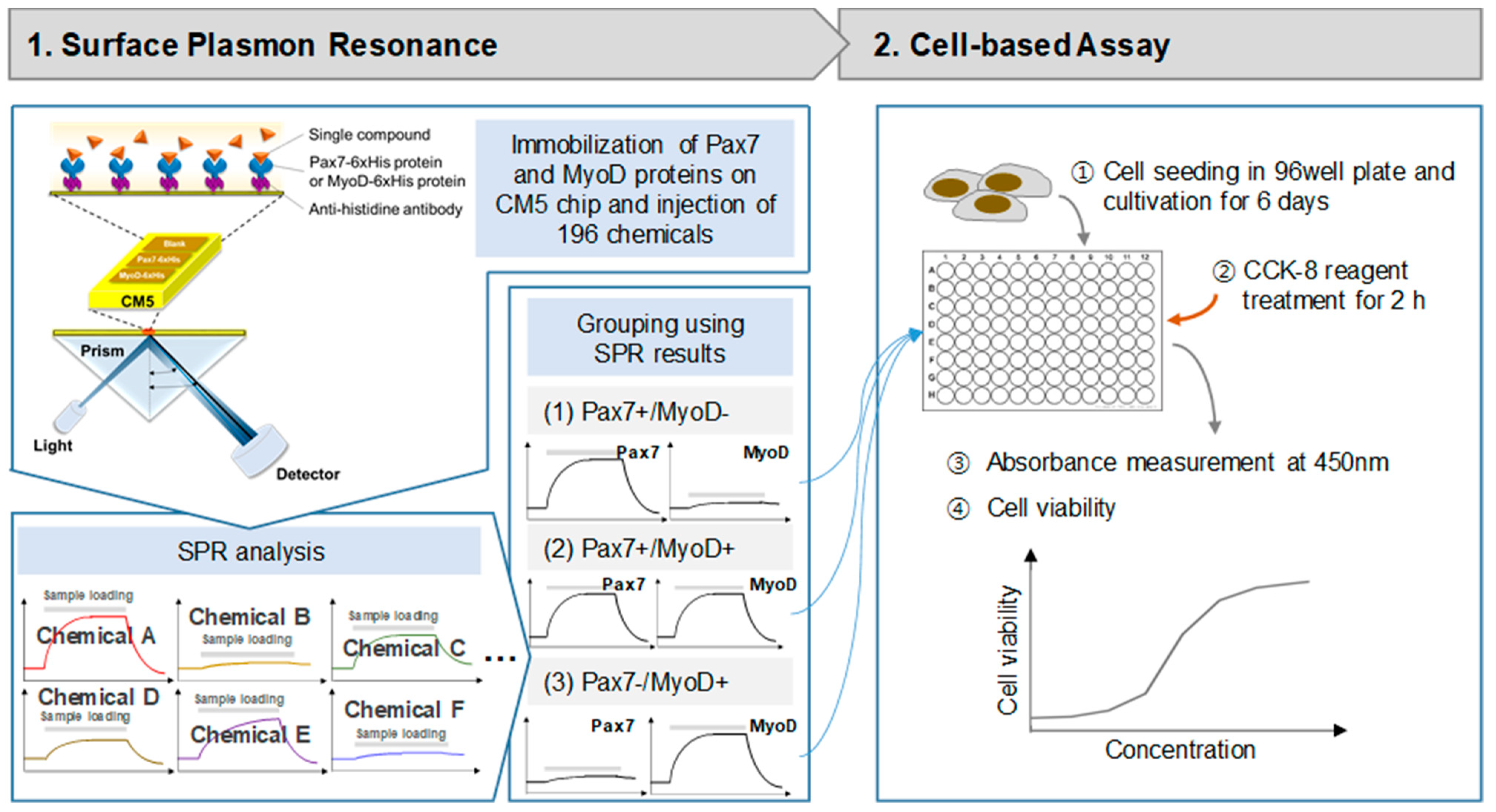
2.2. Surface Plasmon Resonance (SPR)
2.3. Cell Proliferation and Cytotoxicity
2.4. Immunofluorescence
2.5. Statistical Analysis
3. Results
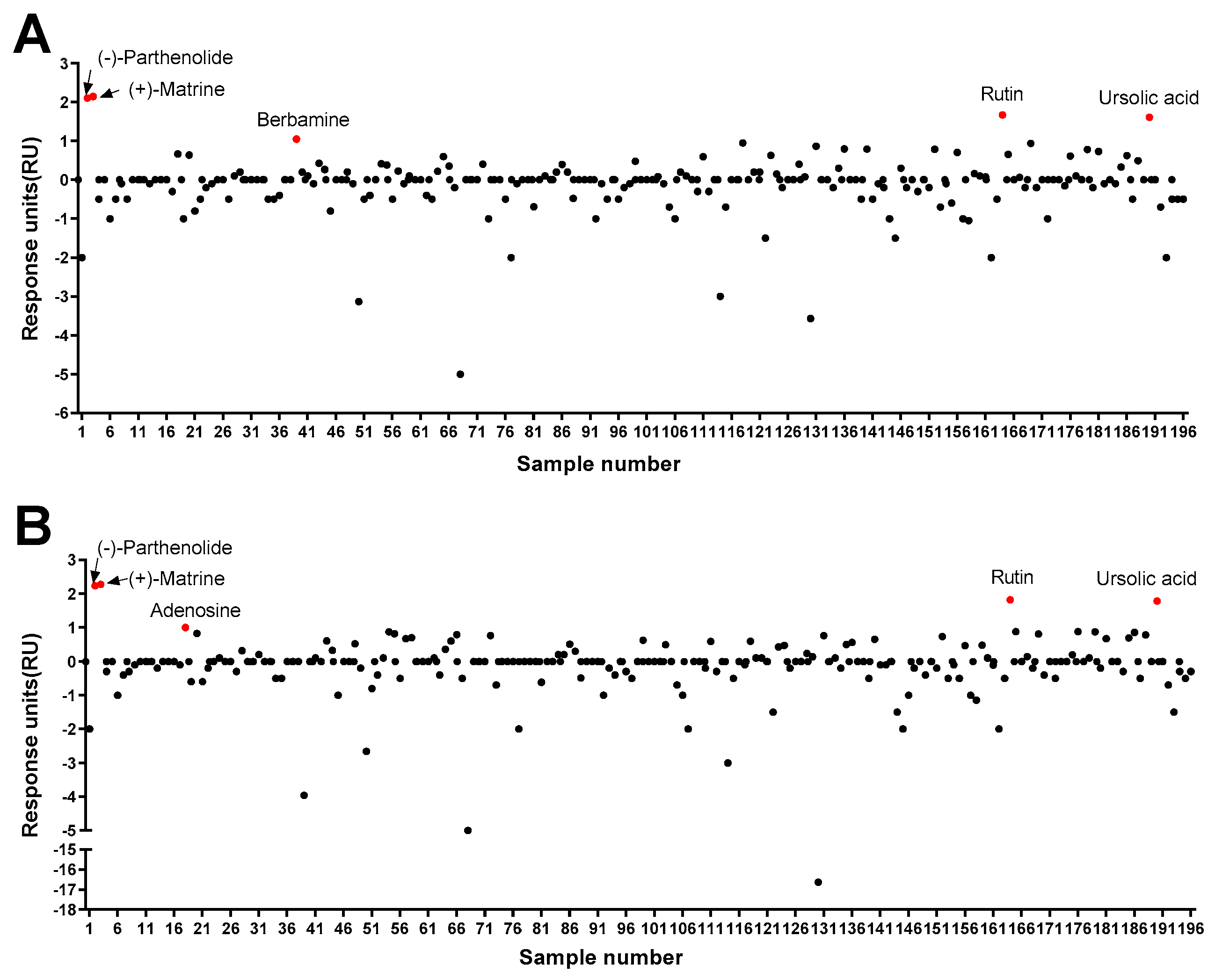
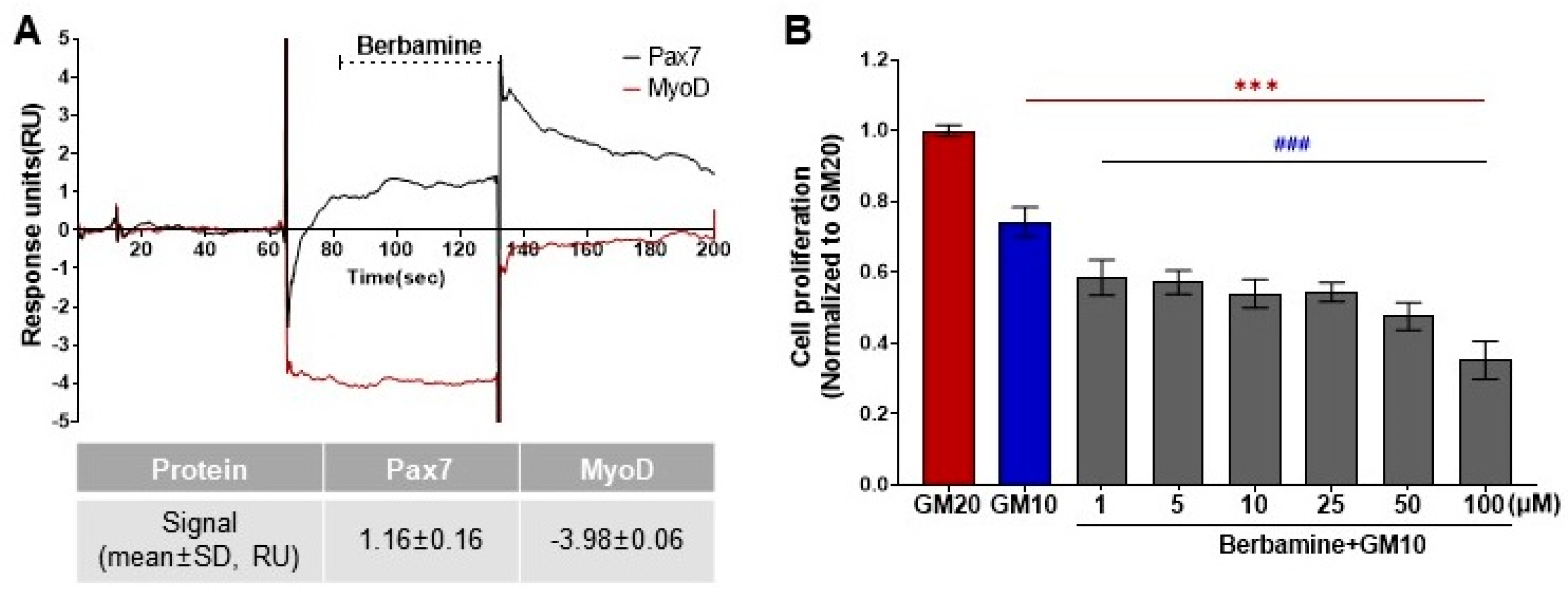
3.1. Characterization of a Chemical That Specifically Binds to Pax7
3.2. Characterization of Pax7+/MyoD+ Chemicals
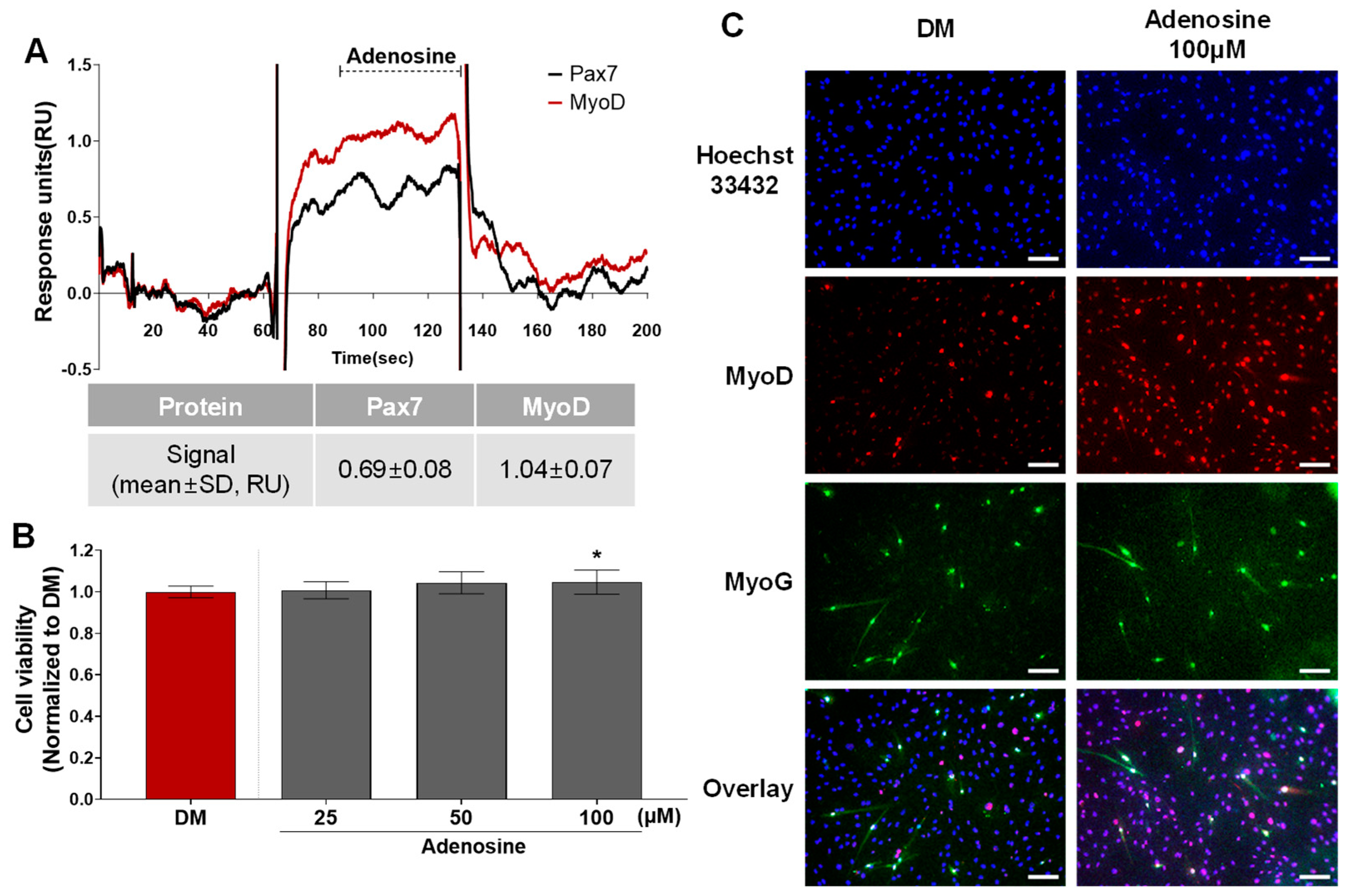
3.3. Effects of Pax7−/MyoD+ Chemicals on HWSC Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, D.Y.; Lee, S.Y.; Yun, S.H.; Jeong, J.W.; Kim, J.H.; Kim, H.W.; Choi, J.S.; Kim, G.-D.; Joo, S.T.; Choi, I.; et al. Review of the current research on fetal bovine serum and the development of cultured meat. Food Sci. Anim. Resour. 2022, 42, 775–799. [Google Scholar] [CrossRef] [PubMed]
- Post, M.J. An alternative animal protein source: Cultured beef. Ann. N. Y. Acad. Sci. 2014, 1328, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.R.; Shim, J.; Park, J.-H.; Kim, Y.-S.; Kim, M.J. Discovery of orphan olfactory receptor 6M1 as a new anticancer target in MCF-7 cells by a combination of surface plasmon resonance-based and cell-based systems. Sensors 2021, 21, 3468. [Google Scholar] [CrossRef] [PubMed]
- Maynard, J.A.; Lindquist, N.C.; Sutherland, J.N.; Lesuffleur, A.; Warrington, A.E.; Rodriguez, M.; Oh, S.H. Surface plasmon resonance for high-throughput ligand screening of membrane-bound proteins. Biotechnol. J. 2009, 4, 1542–1558. [Google Scholar] [CrossRef] [PubMed]
- Situ, C.; Mooney, M.H.; Elliott, C.T.; Buijs, J. Advances in surface plasmon resonance biosensor technology towards high-throughput, food-safety analysis. TrAC Trends Anal. Chem. 2010, 29, 1305–1315. [Google Scholar] [CrossRef]
- Xu, C.-P.; Qi, Y.; Cui, Z.; Yang, Y.-J.; Wang, J.; Hu, Y.-J.; Yu, B.; Wang, F.-Z.; Yang, Q.-P.; Sun, H.-T. Discovery of novel elongator protein 2 inhibitors by compound library screening using surface plasmon resonance. RSC Adv. 2019, 9, 1696–1704. [Google Scholar] [CrossRef]
- Jeanplong, F.; Falconer, S.J.; Oldham, J.M.; Thomas, M.; Gray, T.S.; Hennebry, A.; Matthews, K.G.; Kemp, F.C.; Patel, K.; Berry, C.; et al. Discovery of a mammalian splice variant of myostatin that stimulates myogenesis. PLoS ONE 2013, 8, e81713. [Google Scholar] [CrossRef]
- Keller, A.; Peltzer, J.; Carpentier, G.; Horváth, I.; Oláh, J.; Duchesnay, A.; Orosz, F.; Ovádi, J. Interactions of enolase isoforms with tubulin and microtubules during myogenesis. Biochim. Biophys. Acta 2007, 1770, 919–926. [Google Scholar] [CrossRef]
- Díaz-Ramos, À.; Roig-Borrellas, A.; García-Melero, A.; López-Alemany, R. α-Enolase, a multifunctional protein: Its role on pathophysiological situations. J. Biomed. Biotechnol. 2012, 2012, 156795. [Google Scholar] [CrossRef]
- Williams, C. Biotechnology match making: Screening orphan ligands and receptors. Curr. Opin. Biotechnol. 2000, 11, 42–46. [Google Scholar] [CrossRef]
- Motohashi, N.; Asakura, A. Muscle satellite cell heterogeneity and self-renewal. Front. Cell Dev. Biol. 2014, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Günther, S.; Kim, J.; Kostin, S.; Lepper, C.; Fan, C.M.; Braun, T. Myf5-positive satellite cells contribute to pax7-dependent long-term maintenance of adult muscle stem cells. Cell Stem Cell 2013, 13, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Von Maltzahn, J.; Jones, A.E.; Parks, R.J.; Rudnicki, M.A. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc. Natl. Acad. Sci. USA 2013, 110, 16474–16479. [Google Scholar] [CrossRef]
- Kitzmann, M.; Fernandez, A. Crosstalk between cell cycle regulators and the myogenic factor MyoD in skeletal myoblasts. Cell Mol. Life Sci. 2001, 58, 571–579. [Google Scholar] [CrossRef]
- Sabourin, L.A.; Girgis-Gabardo, A.; Seale, P.; Asakura, A.; Rudnicki, M.A. Reduced differentiation potential of primary myoD−/− myogenic cells derived from adult skeletal muscle. J. Cell Biol. 1999, 144, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Schüler, S.C.; Hüttner, S.S.; von Eyss, B.; von Maltzahn, J. Adult stem cells at work: Regenerating skeletal muscle. Cell. Mol. Life Sci. 2019, 76, 2559–2570. [Google Scholar] [CrossRef]
- Sousa-Victor, P.; García-Prat, L.; Muñoz-Cánoves, P. Control of satellite cell function in muscle regeneration and its disruption in ageing. Nat. Rev. Mol. Cell Biol. 2022, 23, 204–226. [Google Scholar] [CrossRef]
- Choi, K.-H.; Yoon, J.W.; Kim, M.; Lee, H.J.; Jeong, J.; Ryu, M.; Jo, C.; Lee, C.-K. Muscle stem cell isolation and in vitro culture for meat production: A methodological review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 429–457. [Google Scholar] [CrossRef]
- Yu, I.; Choi, J.; Kim, M.K.; Kim, M.J. The Comparison of Commercial Serum-Free Media for Hanwoo Satellite Cell Proliferation and the Role of Fibroblast Growth Factor 2. Food Sci. Anim. Resour. 2023, 43, 1017–1030. [Google Scholar] [CrossRef]
- Zhou, J.; Qi, Q.; Wang, C.; Qian, Y.; Liu, G.; Wang, Y.; Fu, L. Surface plasmon resonance (SPR) biosensors for food allergen detection in food matrices. Biosens. Bioelectron. 2019, 142, 111449. [Google Scholar] [CrossRef]
- Lan, Y.; Wang, S.; Yin, Y.; Hoffmann, W.C.; Zheng, X. Using a surface plasmon resonance biosensor for rapid detection of Salmonella Typhimurium in Chicken Carcass. J. Bionic Eng. 2008, 5, 239–246. [Google Scholar] [CrossRef]
- Yang, N.J.; Hinner, M.J. Getting across the cell membrane: An overview for small molecules, peptides, and proteins. Methods Mol. Biol. 2015, 1266, 29–53. [Google Scholar] [CrossRef] [PubMed]
- Shultz, M.D. Two Decades under the influence of the rule of five and the changing properties of approved oral drugs. J. Med. Chem. 2019, 62, 1701–1714. [Google Scholar] [CrossRef] [PubMed]
- Young, R.J.; Green, D.V.; Luscombe, C.N.; Hill, A.P. Getting physical in drug discovery II: The impact of chromatographic hydrophobicity measurements and aromaticity. Drug Discov. Today 2011, 16, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, A.; Townsend, C.E.; Schwochert, J.; Pye, C.R.; Bednarek, M.A.; Lokey, R.S. Passive membrane permeability in cyclic peptomer scaffolds is robust to extensive variation in side chain functionality and backbone geometry. J. Med. Chem. 2016, 59, 9503–9512. [Google Scholar] [CrossRef] [PubMed]
- Guha, R.; Dexheimer, T.S.; Kestranek, A.N.; Jadhav, A.; Chervenak, A.M.; Ford, M.G.; Simeonov, A.; Roth, G.P.; Thomas, C.J. Exploratory analysis of kinetic solubility measurements of a small molecule library. Bioorg Med. Chem. 2011, 19, 4127–4134. [Google Scholar] [CrossRef] [PubMed]
- Olguín, H.C.; Pisconti, A. Marking the tempo for myogenesis: pax7 and the regulation of muscle stem cell fate decisions. J. Cell Mol. Med. 2012, 16, 1013–1025. [Google Scholar] [CrossRef]
- Olguin, H.C.; Olwin, B.B. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: A potential mechanism for self-renewal. Dev. Biol. 2004, 275, 375–388. [Google Scholar] [CrossRef]
- Seale, P.; Sabourin, L.A.; Girgis-Gabardo, A.; Mansouri, A.; Gruss, P.; Rudnicki, M.A. Pax7 is required for the specification of myogenic satellite cells. Cell 2000, 102, 777–786. [Google Scholar] [CrossRef]
- Kim, J.A.; Shon, Y.H.; Lim, J.O.; Yoo, J.J.; Shin, H.-I.; Park, E.K. Myod mediates skeletal myogenic differentiation of human amniotic fluid stem cells and regeneration of muscle injury. Stem Cell Res. Ther. 2013, 4, 147. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, X.; Liu, Q.; Wang, Y.; Li, S.; Xu, S. Selenoprotein K protects skeletal muscle from damage and is required for satellite cells-mediated myogenic differentiation. Redox Biol. 2022, 50, 102255. [Google Scholar] [CrossRef] [PubMed]
- Freund, R.R.A.; Gobrecht, P.; Fischer, D.; Arndt, H.-D. Advances in chemistry and bioactivity of parthenolide. Nat. Prod. Rep. 2020, 37, 541–565. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Li, Y.; Lv, J.; Guo, X.; Zhang, J.; Zhou, D.; Zhang, Z.; Xue, Z.; Yang, G.; Xi, Q.; et al. Parthenolide regulates oxidative stress-induced mitophagy and suppresses apoptosis through p53 signaling pathway in C2C12 myoblasts. J. Cell. Biochem. 2019, 120, 15695–15708. [Google Scholar] [CrossRef] [PubMed]
- Imani, A.; Maleki, N.; Bohlouli, S.; Kouhsoltani, M.; Sharifi, S.; Maleki Dizaj, S. Molecular mechanisms of anticancer effect of rutin. Phytother. Res. 2021, 35, 2500–2513. [Google Scholar] [CrossRef]
- Semwal, R.; Joshi, S.K.; Semwal, R.B.; Semwal, D.K. Health benefits and limitations of rutin—A natural flavonoid with high nutraceutical value. Phytochem. Lett. 2021, 46, 119–128. [Google Scholar] [CrossRef]
- Liu, S.; Adewole, D.; Yu, L.; Sid, V.; Wang, B.; Karmin, O.; Yang, C. Rutin attenuates inflammatory responses induced by lipopolysaccharide in an in vitro mouse muscle cell (C2C12) model. Poult. Sci. 2019, 98, 2756–2764. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y.I.; Kim, M.J.; Hahm, J.H.; Seo, H.D.; Ha, T.Y.; Jung, C.H.; Ahn, J. Castor oil plant (Ricinus communis L.) leaves improve dexamethasone-induced muscle atrophy via Nrf2 activation. Front. Pharmacol. 2022, 13, 891762. [Google Scholar] [CrossRef]
- Ke, D.; Jorgensen, A.M.; Lee, S.J.; Yoo, J.J.; Murphy, S.V. Adenosine-treated bioprinted muscle constructs prolong cell survival and improve tissue formation. Bio-Des. Manuf. 2021, 4, 441–451. [Google Scholar] [CrossRef]
- Novitskiy, S.V.; Ryzhov, S.; Zaynagetdinov, R.; Goldstein, A.E.; Huang, Y.; Tikhomirov, O.Y.; Blackburn, M.R.; Biaggioni, I.; Carbone, D.P.; Feoktistov, I.; et al. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood 2008, 112, 1822–1831. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, I.-S.; Choi, Y.R.; Choi, J.; Kim, M.K.; Jung, C.H.; Um, M.Y.; Kim, M.J. Discovery of Novel Stimulators of Pax7 and/or MyoD: Enhancing the Efficacy of Cultured Meat Production through Culture Media Enrichment. Biosensors 2024, 14, 24. https://doi.org/10.3390/bios14010024
Yu I-S, Choi YR, Choi J, Kim MK, Jung CH, Um MY, Kim MJ. Discovery of Novel Stimulators of Pax7 and/or MyoD: Enhancing the Efficacy of Cultured Meat Production through Culture Media Enrichment. Biosensors. 2024; 14(1):24. https://doi.org/10.3390/bios14010024
Chicago/Turabian StyleYu, In-Sun, Yae Rim Choi, Jungseok Choi, Mina K. Kim, Chang Hwa Jung, Min Young Um, and Min Jung Kim. 2024. "Discovery of Novel Stimulators of Pax7 and/or MyoD: Enhancing the Efficacy of Cultured Meat Production through Culture Media Enrichment" Biosensors 14, no. 1: 24. https://doi.org/10.3390/bios14010024




