Customizable Nichrome Wire Heaters for Molecular Diagnostic Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. NiCr Wire Patterning Heater Fabrication
2.3. Bacteria Preparation
2.4. PCR Solution Preparation
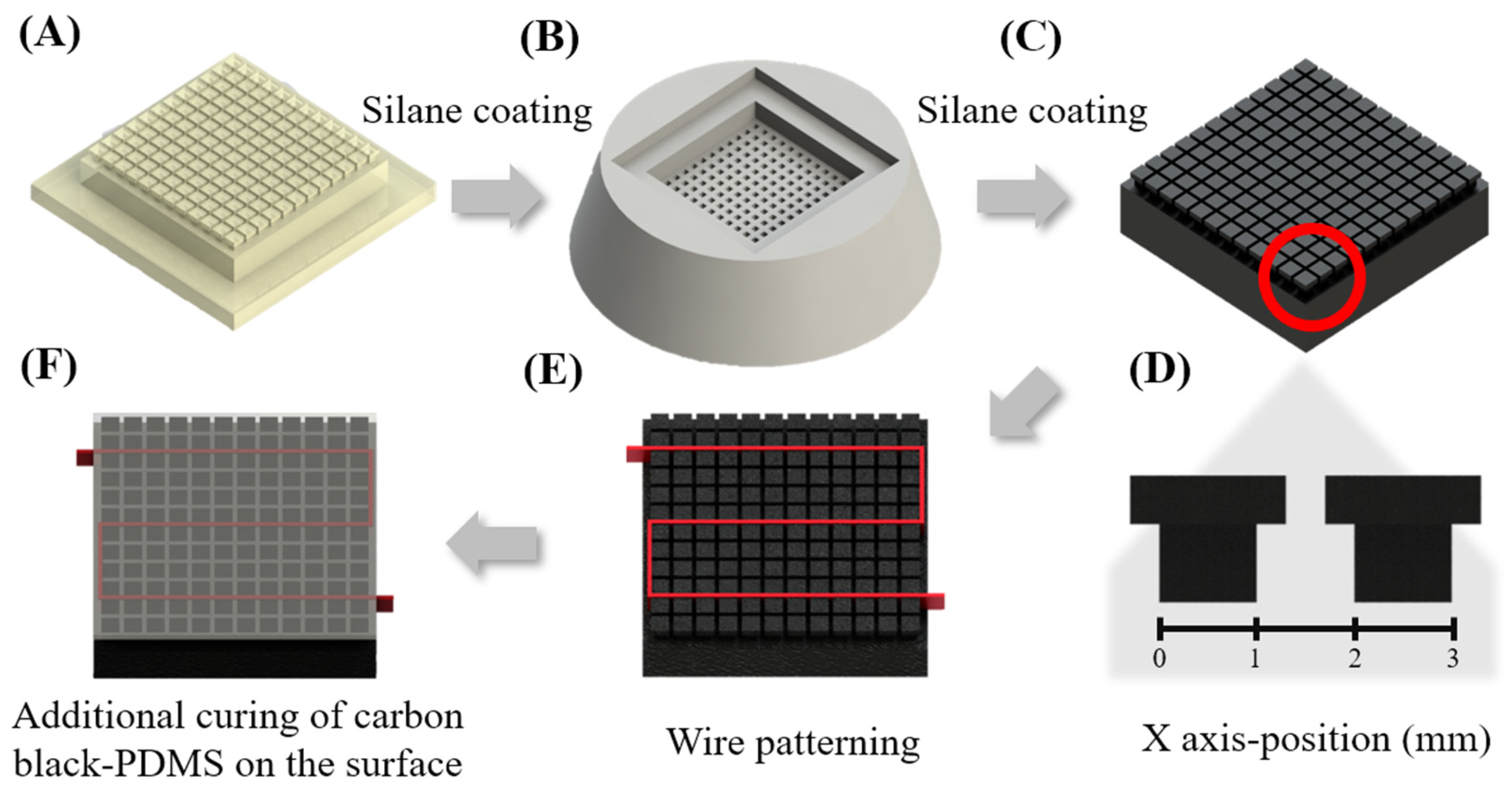
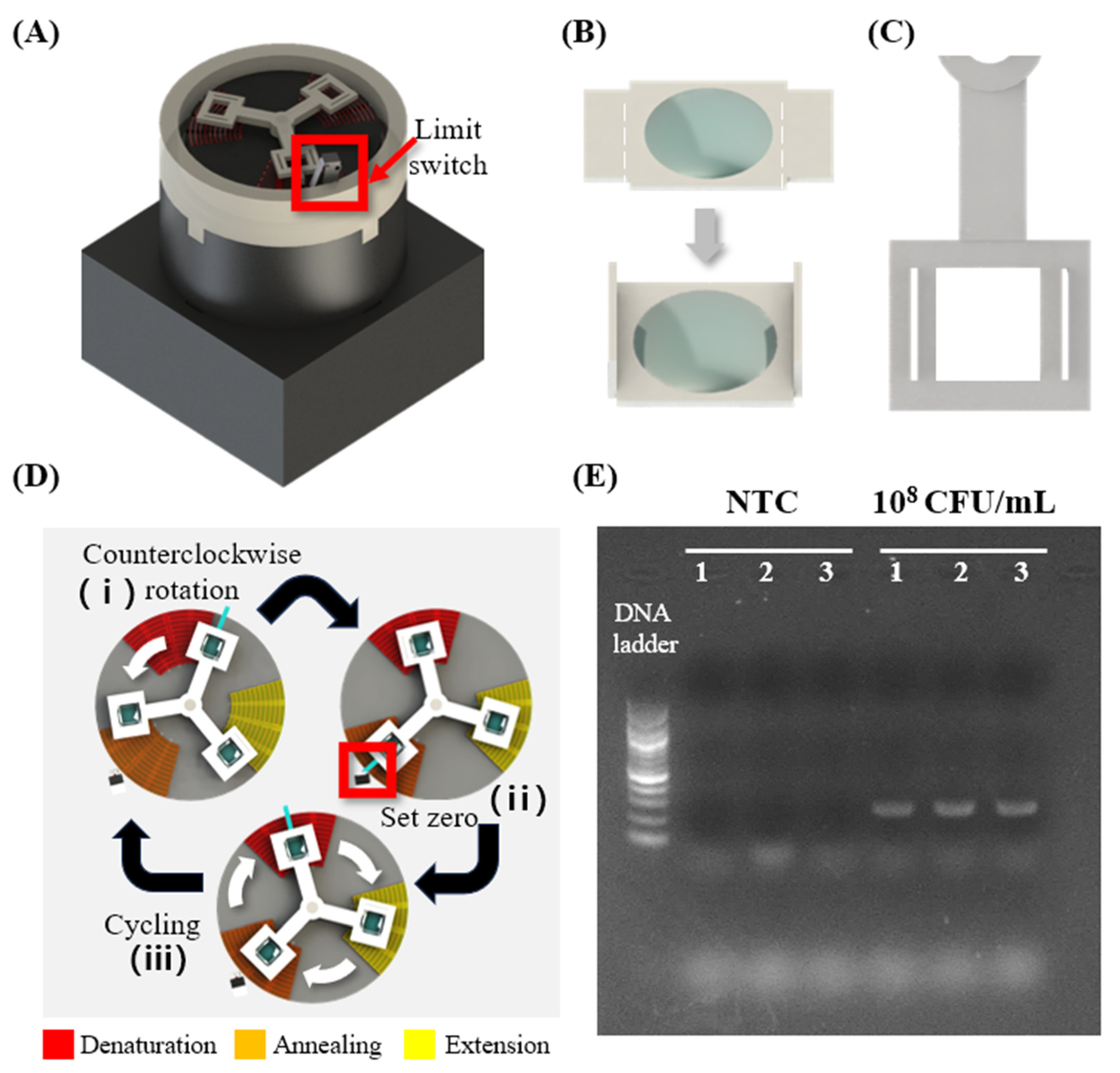
2.5. 2D Heater Patterning for the Fabrication of a Rotary-Type Thermocycler
2.6. PCR Chamber Fabrication
2.7. LAMP Solution Preparation
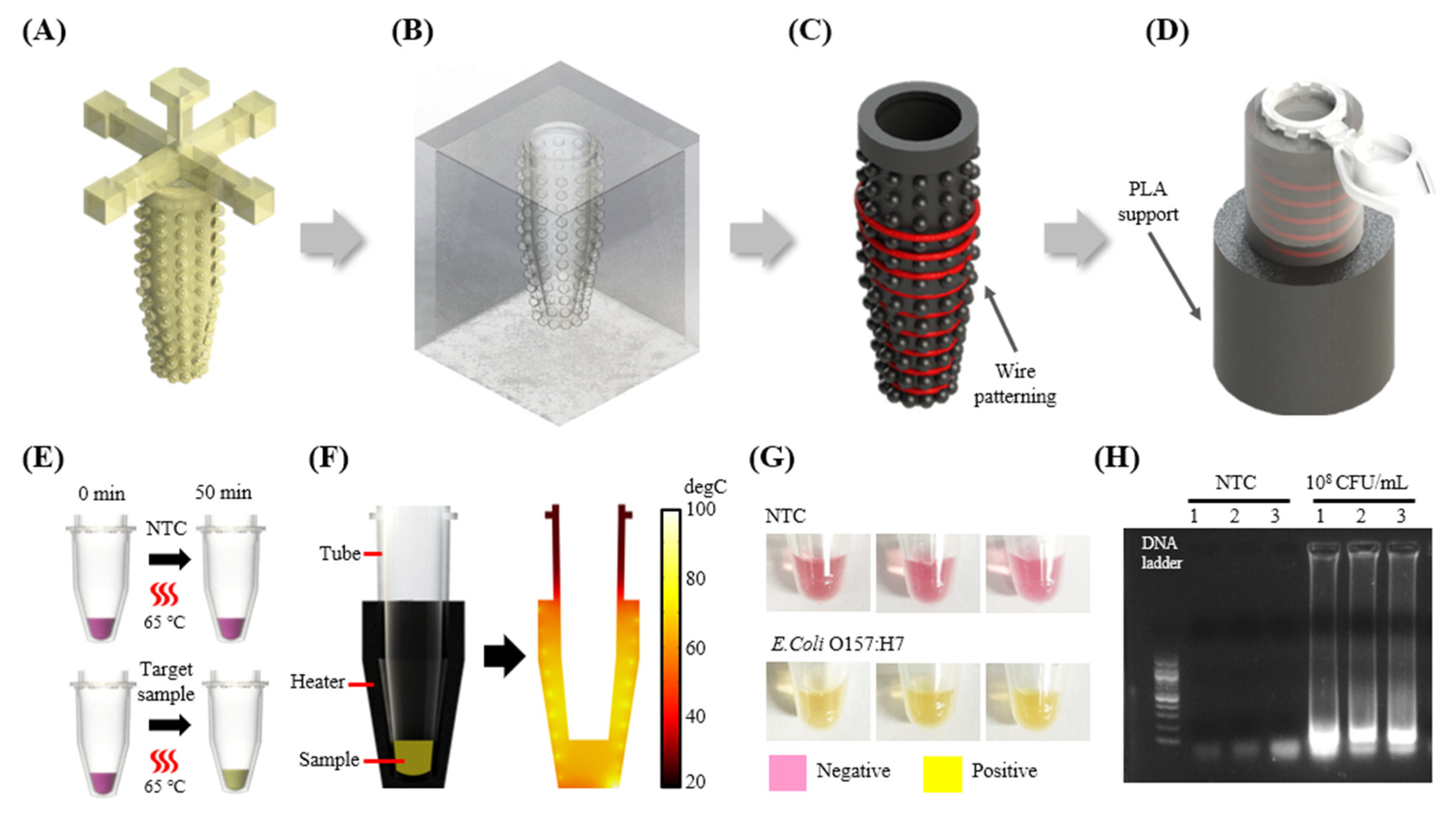
2.8. 3D Heater Patterning for the Fabrication of a Tube-Shaped Heater
3. Results and Discussions
3.1. The Selection of Material for Heater Fabrication
3.2. Thermal Profiling
3.2.1. Simulation for the Determination of the Wire Spacing
3.2.2. Observation of the Heater Surface Temperature with Respect to the Applied Voltage
3.3. Applying NAATs for on-Site Diagnosis Pathogenic Bacteria
3.3.1. Fabricating Thermal Cycling Device and Performing PCR
3.3.2. Fabrication and Application of 3D Heater for Isothermal Amplification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fox, T.; Geppert, J.; Dinnes, J.; Scandrett, K.; Bigio, J.; Sulis, G.; Hettiarachchi, D.; Mathangasinghe, Y.; Weeratunga, P.; Wickramasinghe, D. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2022, 11, CD013652. [Google Scholar] [CrossRef]
- Kim, H.; Huh, H.J.; Park, E.; Chung, D.-R.; Kang, M. Multiplex molecular point-of-care test for syndromic infectious diseases. BioChip J. 2021, 15, 14–22. [Google Scholar] [CrossRef]
- Dashti, A.A.; Jadaon, M.M.; Abdulsamad, A.M.; Dashti, H.M. Heat treatment of bacteria: A simple method of DNA extraction for molecular techniques. Kuwait Med. J. 2009, 41, 117–122. [Google Scholar]
- Kubista, M.; Andrade, J.M.; Bengtsson, M.; Forootan, A.; Jonák, J.; Lind, K.; Sindelka, R.; Sjöback, R.; Sjögreen, B.; Strömbom, L. The real-time polymerase chain reaction. Mol. Asp. Med. 2006, 27, 95–125. [Google Scholar] [CrossRef]
- Kim, Y.L.; Kim, D.; Park, J.; Kwak, M.; Shin, J.H. A carbon-black-embedded poly (dimethylsiloxane)-paper hybrid device for energy-efficient nucleic-acid amplification in point-of-care testing. Anal. Methods 2022, 14, 2569–2577. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.N.; Lee, J.; Kang, Y.K.; Lee, J.H.; Yang, S.; Chung, H.J. A lateral flow assay for nucleic acid detection based on rolling circle amplification using capture ligand-modified oligonucleotides. BioChip J. 2022, 16, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Liao, X.-J.; Chang, W.; Chiou, C.-C. Ultrafast DNA amplification using microchannel flow-through PCR device. Biosensors 2022, 12, 303. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.B.; Goyal, S.; Dhar, A.; Sriram, D.; Goel, S. Miniaturized and IoT enabled continuous-flow-based microfluidic PCR device for DNA amplification. IEEE Trans. Nanobiosci. 2021, 21, 97–104. [Google Scholar] [CrossRef]
- Costantini, F.; Petrucci, G.; Lovecchio, N.; Nardecchia, M.; Nascetti, A.; de Cesare, G.; Tedeschi, L.; Domenici, C.; Ruggi, A.; Placidi, P. Integrated sensor system for DNA amplification and separation based on thin film technology. IEEE Trans. Compon. Packag. Manuf. Technol. 2018, 8, 1141–1148. [Google Scholar] [CrossRef]
- Raddatz, B.W.; Kim, E.Y.S.; Imamura, L.M.; Steil, G.J.; Santiago, E.B.; Soares, S.P.T.; Ribeiro, V.H.A.; de Almeida, B.M.M.; Rogal, S.R., Jr.; Figueredo, M.V.M. Development of an optimized colorimetric RT-LAMP for SARS-CoV-2 assay with enhanced procedure controls for remote diagnostics. Sci. Rep. 2022, 12, 21424. [Google Scholar] [CrossRef]
- Velders, A.H.; Ossendrijver, M.; Keijser, B.J.; Saggiomo, V. T-Cup: A Cheap, Rapid, and Simple Home Device for Isothermal Nucleic Acid Amplification. Glob. Chall. 2022, 6, 2100078. [Google Scholar] [CrossRef]
- Trinh, T.N.D.; Lee, N.Y. Colorimetric detection of viable antibiotic resistant Enterococcus mediated by cordless operation of reverse transcription loop-mediated isothermal amplification. J. Biotechnol. 2022, 357, 92–99. [Google Scholar] [CrossRef]
- Song, J.; Mauk, M.G.; Hackett, B.A.; Cherry, S.; Bau, H.H.; Liu, C. Correction to “Instrument-free point-of-care molecular detection of Zika virus”. Anal. Chem. 2018, 90, 13814. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, S.; Xiang, F.; Wu, D.; Guo, M.; Ge, S.; Li, K.; Ye, X.; Xia, N.; Qian, S. Instrument-free point-of-care molecular diagnosis of H1N1 based on microfluidic convective PCR. Sens. Actuators B Chem. 2017, 243, 738–744. [Google Scholar] [CrossRef]
- Li, R.J.; Mauk, M.G.; Seok, Y.; Bau, H.H. Electricity-free chemical heater for isothermal nucleic acid amplification with applications in COVID-19 home testing. Analyst 2021, 146, 4212–4218. [Google Scholar] [CrossRef]
- Song, J.; Pandian, V.; Mauk, M.G.; Bau, H.H.; Cherry, S.; Tisi, L.C.; Liu, C. Smartphone-based mobile detection platform for molecular diagnostics and spatiotemporal disease mapping. Anal. Chem. 2018, 90, 4823–4831. [Google Scholar] [CrossRef]
- Zai, Y.; Min, C.; Wang, Z.; Ding, Y.; Zhao, H.; Su, E.; He, N. A sample-to-answer, quantitative real-time PCR system with low-cost, gravity-driven microfluidic cartridge for rapid detection of SARS-CoV-2, influenza A/B, and human papillomavirus 16/18. Lab Chip 2022, 22, 3436–3452. [Google Scholar] [CrossRef]
- Seder, I.; Kim, D.-M.; Hwang, S.-H.; Sung, H.; Kim, D.-E.; Kim, S.-J. Fluidic handling system for PCR-based sample-to-answer detection of viral nucleic acids. Sens. Actuators B Chem. 2021, 349, 130788. [Google Scholar] [CrossRef]
- Nguyen, P.Q.M.; Wang, M.; Ann Maria, N.; Li, A.Y.; Tan, H.Y.; Xiong, G.M.; Tan, M.-K.M.; Bhagat, A.A.S.; Ong, C.W.; Lim, C.T. Modular micro-PCR system for the onsite rapid diagnosis of COVID-19. Microsyst. Nanoeng. 2022, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.B.; Goel, S. Advances in continuous-flow based microfluidic PCR devices—A review. Eng. Res. Express 2020, 2, 042001. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, Z.; Yan, S.; Yin, F.; Feng, X.; Liu, B.-F. Identifying multiple bacterial pathogens by loop-mediated isothermal amplification on a rotate & react slipchip. Sens. Actuators B Chem. 2016, 228, 491–499. [Google Scholar] [CrossRef]
- Rane, T.D.; Chen, L.; Zec, H.C.; Wang, T.-H. Microfluidic continuous flow digital loop-mediated isothermal amplification (LAMP). Lab Chip 2015, 15, 776–782. [Google Scholar] [CrossRef]
- Kaygusuz, D.; Vural, S.; Aytekin, A.Ö.; Lucas, S.J.; Elitas, M. DaimonDNA: A portable, low-cost loop-mediated isothermal amplification platform for naked-eye detection of genetically modified organisms in resource-limited settings. Biosens. Bioelectron. 2019, 141, 111409. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Tan, G. A review of thermoelectric cooling: Materials, modeling and applications. Appl. Therm. Eng. 2014, 66, 15–24. [Google Scholar] [CrossRef]
- Rajendran, V.K.; Bakthavathsalam, P.; Bergquist, P.L.; Sunna, A. Smartphone detection of antibiotic resistance using convective PCR and a lateral flow assay. Sens. Actuators B Chem. 2019, 298, 126849. [Google Scholar] [CrossRef]
- Sreejith, K.R.; Gorgannezhad, L.; Jin, J.; Ooi, C.H.; Stratton, H.; Dao, D.V.; Nguyen, N.-T. Liquid marbles as biochemical reactors for the polymerase chain reaction. Lab Chip 2019, 19, 3220–3227. [Google Scholar] [CrossRef]
- Fernández-Carballo, B.L.; McGuiness, I.; McBeth, C.; Kalashnikov, M.; Borrós, S.; Sharon, A.; Sauer-Budge, A.F. Low-cost, real-time, continuous flow PCR system for pathogen detection. Biomed. Microdevices 2016, 18, 34. [Google Scholar] [CrossRef]
- Sheu, S.; Huang, C.; Chen, J. Portable molecular diagnostics device for identification of Asini Corii Colla by loop-mediated isothermal amplification. Inventions 2021, 6, 51. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Goel, S. Miniaturized DNA amplification platform with soft-lithographically fabricated continuous-flow PCR microfluidic device on a portable temperature controller. Microfluid. Nanofluidics 2021, 25, 69. [Google Scholar] [CrossRef]
- Shaw, K.J.; Docker, P.T.; Yelland, J.V.; Dyer, C.E.; Greenman, J.; Greenway, G.M.; Haswell, S.J. Rapid PCR amplification using a microfluidic device with integrated microwave heating and air impingement cooling. Lab Chip 2010, 10, 1725–1728. [Google Scholar] [CrossRef]
- Marchiarullo, D.J.; Sklavounos, A.H.; Oh, K.; Poe, B.L.; Barker, N.S.; Landers, J.P. Low-power microwave-mediated heating for microchip-based PCR. Lab Chip 2013, 13, 3417–3425. [Google Scholar] [CrossRef]
- Fermér, C.; Nilsson, P.; Larhed, M. Microwave-assisted high-speed PCR. Eur. J. Pharm. Sci. 2003, 18, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Suzuki, T.; Mineki, S.; Ohuchi, S. Controlled microwave heating accelerates rolling circle amplification. PLoS ONE 2015, 10, e0136532. [Google Scholar] [CrossRef]
- Seo, M.-J.; Yoo, J.-C. Fully automated lab-on-A-disc platform for loop-mediated isothermal amplification using micro-carbon-activated cell lysis. Sensors 2020, 20, 4746. [Google Scholar] [CrossRef]
- Son, J.H.; Cho, B.; Hong, S.; Lee, S.H.; Hoxha, O.; Haack, A.J.; Lee, L.P. Ultrafast photonic PCR. Light Sci. Appl. 2015, 4, e280. [Google Scholar] [CrossRef]
- Cao, L.; Guo, X.; Mao, P.; Ren, Y.; Li, Z.; You, M.; Hu, J.; Tian, M.; Yao, C.; Li, F. A portable digital loop-mediated isothermal amplification platform based on microgel array and hand-held reader. ACS Sens. 2021, 6, 3564–3574. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Chen, T.; Gao, J.; Dong, C.; Wong, A.H.-H.; Jia, Y.; Mak, P.-I.; Deng, C.-X.; Martins, R.P. A digital microfluidic system for loop-mediated isothermal amplification and sequence specific pathogen detection. Sci. Rep. 2017, 7, 14586. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yu, H.; Sun, F.; Ornob, A.; Brisbin, R.; Ganguli, A.; Vemuri, V.; Strzebonski, P.; Cui, G.; Allen, K.J. Mobile platform for multiplexed detection and differentiation of disease-specific nucleic acid sequences, using microfluidic loop-mediated isothermal amplification and smartphone detection. Anal. Chem. 2017, 89, 11219–11226. [Google Scholar] [CrossRef]
- Sheu, S.-C.; Song, Y.-S.; Chen, J.-J. A portable continuous-flow polymerase chain reaction chip device integrated with arduino boards for detecting colla corii asini. Micromachines 2022, 13, 1289. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Wang, P.; Tao, C.; Zheng, L.; Sekine, S.; Zhuang, S.; Zhang, D.; Yamaguchi, Y. Multiplex amplification of target genes of periodontal pathogens in continuous flow PCR microfluidic chip. Lab Chip 2021, 21, 3159–3164. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Nguyen, V.D.; Van Nguyen, H.; Seo, T.S. Quantification of colorimetric isothermal amplification on the smartphone and its open-source app for point-of-care pathogen detection. Sci. Rep. 2020, 10, 15123. [Google Scholar] [CrossRef]
- Liu, C.; Mauk, M.G.; Bau, H.H. A disposable, integrated loop-mediated isothermal amplification cassette with thermally actuated valves. Microfluid. Nanofluidics 2011, 11, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Mauk, M.G.; Hart, R.; Qiu, X.; Bau, H.H. A self-heating cartridge for molecular diagnostics. Lab Chip 2011, 11, 2686–2692. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Geva, E.; Mauk, M.; Qiu, X.; Abrams, W.R.; Malamud, D.; Curtis, K.; Owen, S.M.; Bau, H.H. An isothermal amplification reactor with an integrated isolation membrane for point-of-care detection of infectious diseases. Analyst 2011, 136, 2069–2076. [Google Scholar] [CrossRef]
- Talebi, F.; Ghafoorifard, H.; Ghafouri-Fard, S.; Jahanshahi, A. A straight-forward, inexpensive, low power continuous-flow µPCR chip using PCB-based heater electrodes with uniform temperature distribution. Sens. Actuators A Phys. 2022, 333, 113220. [Google Scholar] [CrossRef]
- Srikanth, S.; Dudala, S.; Jayapiriya, U.; Mohan, J.M.; Raut, S.; Dubey, S.K.; Ishii, I.; Javed, A.; Goel, S. Droplet-based lab-on-chip platform integrated with laser ablated graphene heaters to synthesize gold nanoparticles for electrochemical sensing and fuel cell applications. Sci. Rep. 2021, 11, 9750. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.-H.; Seok, H.-J.; Kim, D.-H.; Kim, S.-K.; Kim, H.-K. Thermally-evaporated C60/Ag/C60 multilayer electrodes for semi-transparent perovskite photovoltaics and thin film heaters. Sci. Technol. Adv. Mater. 2020, 21, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Bobinger, M.R.; Romero, F.J.; Salinas-Castillo, A.; Becherer, M.; Lugli, P.; Morales, D.P.; Rodríguez, N.; Rivadeneyra, A. Flexible and robust laser-induced graphene heaters photothermally scribed on bare polyimide substrates. Carbon 2019, 144, 116–126. [Google Scholar] [CrossRef]
- Fornells, E.; Murray, E.; Waheed, S.; Morrin, A.; Diamond, D.; Paull, B.; Breadmore, M. Integrated 3D printed heaters for microfluidic applications: Ammonium analysis within environmental water. Anal. Chim. Acta 2020, 1098, 94–101. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, H.; Niu, H.; Ren, Y.; Fang, H.; Fang, X.; Lv, R.; Maqbool, M.; Bai, S. Highly thermally conductive 3D printed graphene filled polymer composites for scalable thermal management applications. ACS Nano 2021, 15, 6917–6928. [Google Scholar] [CrossRef]
- Liang, Z.; Yao, Y.; Jiang, B.; Wang, X.; Xie, H.; Jiao, M.; Liang, C.; Qiao, H.; Kline, D.; Zachariah, M.R. 3D Printed Graphene-Based 3000 K Probe. Adv. Funct. Mater. 2021, 31, 2102994. [Google Scholar] [CrossRef]
- Roa, M.A.; Dogar, M.R.; Pages, J.; Vivas, C.; Morales, A.; Correll, N.; Gorner, M.; Rosell, J.; Foix, S.; Memmesheimer, R. Mobile manipulation hackathon: Moving into real world applications. IEEE Robot. Autom. Mag. 2021, 28, 112–124. [Google Scholar] [CrossRef]
- Yi, P.; Awang, R.A.; Rowe, W.S.; Kalantar-zadeh, K.; Khoshmanesh, K. PDMS nanocomposites for heat transfer enhancement in microfluidic platforms. Lab Chip 2014, 14, 3419–3426. [Google Scholar] [CrossRef]
- Vlassov, S.; Oras, S.; Timusk, M.; Zadin, V.; Tiirats, T.; Sosnin, I.M.; Lõhmus, R.; Linarts, A.; Kyritsakis, A.; Dorogin, L.M. Thermal, mechanical, and acoustic properties of polydimethylsiloxane filled with hollow glass microspheres. Materials 2022, 15, 1652. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Liao, M.; Ma, A.; Chen, Y.; Duan, Z.; Hou, X.; Li, M.; Jiang, N.; Yu, J. Enhanced thermal conductivity of polydimethylsiloxane composites with carbon fiber. Compos. Commun. 2020, 17, 141–146. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Zhang, Y.-F.; Wu, Z.-K.; Bai, S.-L. Synergic enhancement of thermal properties of polymer composites by graphene foam and carbon black. Compos. B. Eng. 2016, 84, 52–58. [Google Scholar] [CrossRef]
- Bartsch, M.S.; Edwards, H.S.; Lee, D.; Moseley, C.E.; Tew, K.E.; Renzi, R.F.; Van de Vreugde, J.L.; Kim, H.; Knight, D.L.; Sinha, A. The rotary zone thermal cycler: A low-power system enabling automated rapid PCR. PLoS ONE 2015, 10, e0118182. [Google Scholar] [CrossRef]
- Song, W.; Zhang, C.; Lin, H.; Zhang, T.; Liu, H.; Huang, X. Portable rotary PCR system for real-time detection of Pseudomonas aeruginosa in milk. Lab Chip 2023, 23, 4592–4599. [Google Scholar] [CrossRef]
- Jung, J.H.; Choi, S.J.; Park, B.H.; Choi, Y.K.; Seo, T.S. Ultrafast Rotary PCR system for multiple influenza viral RNA detection. Lab. Chip 2012, 12, 1598–1600. [Google Scholar] [CrossRef]
- Gaertig, C.; Niemann, K.; Berthold, J.; Giel, L.; Leitschuh, N.; Boehm, C.; Roussak, L.; Vetter, K.; Kuhlmeier, D. Development of a point-of-care-device for fast detection of periodontal pathogens. BMC Oral Health 2015, 15, 165. [Google Scholar] [CrossRef]
- Lee, P.Y.; Costumbrado, J.; Hsu, C.-Y.; Kim, Y.H. Agarose gel electrophoresis for the separation of DNA fragments. J. Vis. Exp. 2012, 20, e3923. [Google Scholar] [CrossRef]
- Cacho-Soblechero, M.; Malpartida-Cardenas, K.; Cicatiello, C.; Rodriguez-Manzano, J.; Georgiou, P. A dual-sensing thermo-chemical ISFET array for DNA-based diagnostics. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 477–489. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.; Han, W.; Thang, L.T.H.; Lee, Y.W.; Shin, J.H. Customizable Nichrome Wire Heaters for Molecular Diagnostic Applications. Biosensors 2024, 14, 152. https://doi.org/10.3390/bios14030152
Lim J, Han W, Thang LTH, Lee YW, Shin JH. Customizable Nichrome Wire Heaters for Molecular Diagnostic Applications. Biosensors. 2024; 14(3):152. https://doi.org/10.3390/bios14030152
Chicago/Turabian StyleLim, Juhee, Won Han, Le Tran Huy Thang, Yong Wook Lee, and Joong Ho Shin. 2024. "Customizable Nichrome Wire Heaters for Molecular Diagnostic Applications" Biosensors 14, no. 3: 152. https://doi.org/10.3390/bios14030152





