Use of a Parasitic Wasp as a Biosensor
Abstract
:1. Introduction
2. Experimental Section
2.1. Conditioning and Testing of Response to Concentrations of Methyl Benzoate
2.1.1. Conditioning
2.1.2. Test
2.2. Sensitivity Tests
2.2.1. Conditioning
2.2.2. Test
2.3. Response to Methyl Benzoate with Green Tea or Ground Coffee
2.3.1. Conditioning
2.3.2. Test
2.3.3. Conditioning
2.3.4. Test
2.4. Statistics
3. Results and Discussion
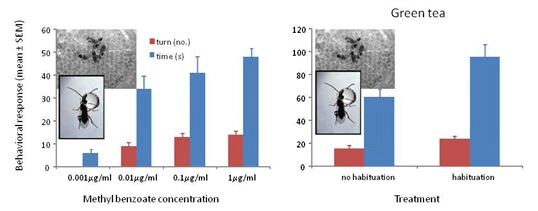
3.1. Response to Concentrations of Conditioned Odors

3.2. Sensitivity Tests

3.3. Response to Methyl Benzoate with Green Tea and Ground Coffee

4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Lai, H.; Corbin, I.; Almirall, J.R. Headspace sampling and detection of cocaine, MDMA, and marijuana via volatile markers in the presence of potential interferences by solid phase microextraction-ion mobility spectrometry (SPME-IMS). Anal. Bioanal. Chem. 2008, 392, 105–113. [Google Scholar] [CrossRef]
- Lorenzo, N.; Wan, T.L.; Harper, R.R.; Hsu, Y.; Chow, M.; Rose, S.; Furton, K.G. Laboratory and field experiments used to identify Canis lupus var. farmiliaris signature chemicals from drugs, explosives, and humans. Anal. Bioanal. Chem. 2003, 376, 1212–1224. [Google Scholar] [CrossRef]
- Furton, K.G.; Hong, Y.; Hsu, Y.; Lue, T.; Tose, S.; Walton, J. Identification of odor signature chemicals in cocaine using solid-phase microextraction-gas chromatography and detector-dog response to isolated compounds spiked on U.S. paper currency. J. Chrom. Sci. 2002, 40, 147–155. [Google Scholar] [CrossRef]
- Kanukstis, K.K.; van Hecke, G.R. Chemistry Connections: The Chemical Basis of Everyday Phenomena (Complementary Science Series), 2nd ed.; Academic: San Diego, CA, USA, 2003. [Google Scholar]
- Wäckers, F.L.; Lewis, W.J. Olfactory and visual learning and their combined influence on host site location by the parasitoid Microplitis croceipes (Cresson). Biol. Control 1994, 4, 105–112. [Google Scholar] [CrossRef]
- Lewis, W.J.; Takasu, K. Use of learned odours by a parasitic wasp in accordance with host and food needs. Nature 1990, 348, 635–636. [Google Scholar] [CrossRef]
- Takasu, K.; Lewis, W.J. Host-and food-foraging of the parasitoid Microplitis croceipes: Learning and physiological state effects. Biol. Control 1993, 3, 70–74. [Google Scholar]
- Wäckers, F.L.; Olson, D.M.; Rains, G.C.; Lunby, F.; Haugen, J.E. Boar taint detection using parasitoid biosensors. J. Food Sci. 2011, 76, 41–47. [Google Scholar]
- Olson, D.M.; Wäckers, F.L.; Haugen, J.-E. Threshold detection of boar taint chemicals using parasitic wasps. J. Food Science 2012, 77, 356–361. [Google Scholar] [CrossRef]
- Olson, D.M.; Rains, G.C.; Meiners, T.; Takasu, K.; Tertuliano, M.; Tumlinson, J.H.; Wäckers, F.L.; Lewis, W.J. Parasitic wasps learn and report diverse chemicals with unique conditionable behaviors. Chem. Senses 2003, 28, 545–549. [Google Scholar] [CrossRef]
- Meiners, T.; Wäckers, F.; Lewis, W.J. The effect of molecular structure on olfactory discrimination by the parasitoid Microplitis croceipes. Chem. Senses 2002, 27, 811–816. [Google Scholar] [CrossRef]
- Rains, G.C.; Lewis, W.J.; D’Alessandro, M.; Tomberlin, J.K. Limits of volatile chemical detection of a parasitoid wasp, Microplitis croceipes, and an electronic nose: A comparative study. Trans. ASAE 2004, 47, 2145–2152. [Google Scholar] [CrossRef]
- Meiners, T.; Wäckers, F.; Lewis, W.J. Associative learning of complex odors in parasitoid host location. Chem. Senses 2003, 28, 231–236. [Google Scholar] [CrossRef]
- Pelz, C.; Gerber, B.; Menzel, R. Odorant intensity as a determinant for olfactory conditioning in the honeybee: Roles in discrimination, overshadowing, and memory consolidation. J. Exp. Biol. 1997, 200, 837–847. [Google Scholar]
- Wright, G.A.; Thomson, M.G.A.; Smith, B.H. Odour concentration affects odour identity in honeybees. Proc. Royal Soc. B 2005, 272, 2417–2422. [Google Scholar] [CrossRef]
- Lieberman, D.A. Theories of conditioning. In Learning, Behavior and Cognition; Knight, V., Taflinger, M., Walker, J., Eds.; Wadsworth Pub. Co.: Elmont, CA, USA, 1990; p. 249. [Google Scholar]
- Utley, S.L.; Rains, G.C.; Lewis, W.J. Behavioral monitoring of Microplitis croceipes, a parasitoid wasp, for detecting target odorants using a computer vision system. Trans. ASABE 2007, 50, 1843–1849. [Google Scholar] [CrossRef]
- Lewis, W.J.; Burton, R.L. Rearing Microplitis croceipes in the laboratory with Heliothis zea as hosts. J. Econ. Entomol. 1970, 63, 656–658. [Google Scholar]
- Tomberlin, J.K.; Rains, C.C.; Sanford, M.R. Development of Microplitis croceipes as a biological sensor. Entomol. Exp. Appl. 2008, 128, 249–257. [Google Scholar] [CrossRef]
- Rains, G.C.; Tomberlin, J.K.; Kulasiri, D. Using insect sniffing devices for detection. Trends iBiotech. 2008, 26, 288–294. [Google Scholar] [CrossRef]
- Rains, G.C.; Utley, S.L.; Lewis, W.J. Behavioral monitoring of trained insects for chemical detection. Biotechnol. Prog. 2006, 22, 2–8. [Google Scholar] [CrossRef]
- Curio, E. The Ethiology of Predation; Springer-Verlag: Berlin, Germany, 1976. [Google Scholar]
- SAS/STAT User’s GuideVersion 6, 4th ed.; SAS Institute Inc.: Cary, NC, USA, 1998; Volume 1–2.
- Walker, D.B.; Walker, J.C.; Cavnar, P.J.; Taylor, J.L.; Pickel, D.H.; Hall, S.B.; Suarez, J.C. Naturalistic quantification of canine olfactory sensitivity. Appl. Anim. Behav. Sci. 2006, 97, 241–254. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Olson, D.; Rains, G. Use of a Parasitic Wasp as a Biosensor. Biosensors 2014, 4, 150-160. https://doi.org/10.3390/bios4020150
Olson D, Rains G. Use of a Parasitic Wasp as a Biosensor. Biosensors. 2014; 4(2):150-160. https://doi.org/10.3390/bios4020150
Chicago/Turabian StyleOlson, Dawn, and Glen Rains. 2014. "Use of a Parasitic Wasp as a Biosensor" Biosensors 4, no. 2: 150-160. https://doi.org/10.3390/bios4020150
APA StyleOlson, D., & Rains, G. (2014). Use of a Parasitic Wasp as a Biosensor. Biosensors, 4(2), 150-160. https://doi.org/10.3390/bios4020150