Carboxylated or Aminated Polyaniline—Multiwalled Carbon Nanotubes Nanohybrids for Immobilization of Cellobiose Dehydrogenase on Gold Electrodes
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Electrode Cleaning
2.3. Electrode Modification
2.4. Synthesis of Poly(3-Amino-4-Methoxybenzoic Acid-co-Aniline) P(AMB-A)
2.5. MWCNTs Functionalized with P(AMB-A)

2.6. Synthesis of MWNT-P(AMB-A)-PDA
2.7. Measurements
2.7.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.7.2. Cyclic Voltammetry (CV).
2.7.3. Lactose Detection
3. Results and Discussion
3.1. Synthesis of the P(AMB-A) and Coupling to the Surface of MWCNTs


3.2. Analysis of DET from Phanerochaete sordida CDH on Different Nanohybrid-Modified Gold Electrodes
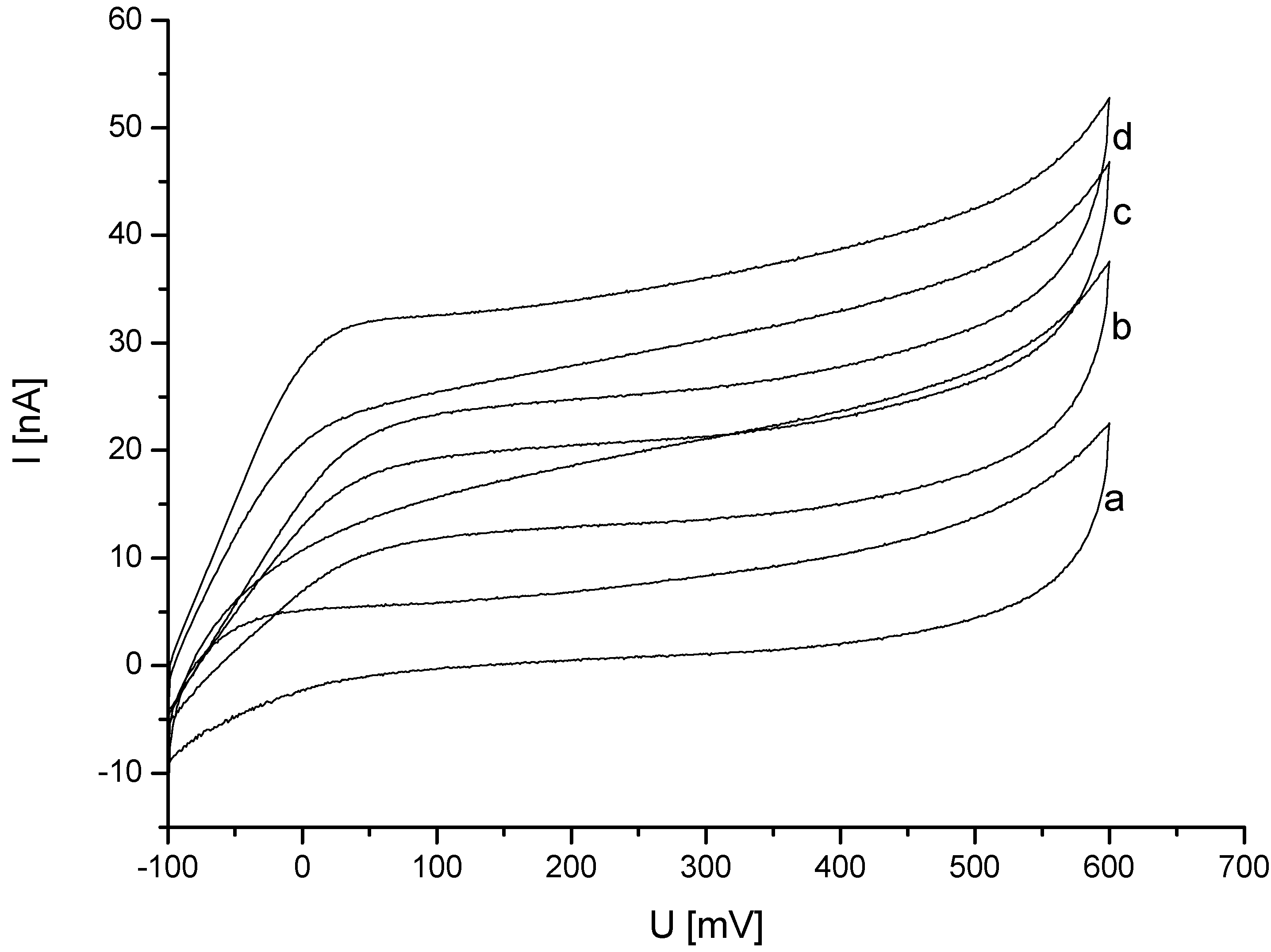


3.3. Study of Potential Ascorbic Acid Interference

| U [mV] | I [nA] |
|---|---|
| −100 | 0.04 ± 0.02 |
| ±0 | 1.92 ± 0.085 |
| +100 | 2.09 ± 0.086 |
3.4. Storage Stability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scheller, F.W.; Wollenberger, U.; Lei, C.; Jin, W.; Ge, B.; Lehmann, C.; Lisdat, F.; Fridman, V. Bioelectrocatalysis by redox enzymes at modified electrodes. Rev. Mol. Biotechnol. 2002, 82, 411–424. [Google Scholar] [CrossRef]
- Krylov, A.V.; Sczech, R.; Lisdat, F. Characterization of antioxidants using a fluidic chip in aqueous/organic media. Analyst 2007, 132, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Beissenhirtz, M.K.; Scheller, F.W.; Stöcklein, W.F.M.; Kurth, D.G.; Möhwald, H.; Lisdat, F. Electroactive cytochrome c multilayers within a polyelectrolyte assembly. Angew. Chem. Int. Ed. 2004, 43, 4357–4360. [Google Scholar] [CrossRef]
- Dronov, R.; Kurth, D.G.; Möhwald, H.; Scheller, F.W.; Lisdat, F. A self-assembled cytochrome c/xanthine oxidase multilayer arrangement on gold. Electrochim. Acta 2007, 53, 1107–1113. [Google Scholar] [CrossRef]
- Shen, L.; Huang, R.; Hu, N. Myoglobin in polyacrylamide hydrogel films: Direct electrochemistry and electrochemical catalysis. Talanta 2002, 56, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Kröning, S.; Scheller, F.W.; Wollenberger, U.; Lisdat, F. Myoglobin-clay electrode for nitric oxide (NO) detection in solution. Electroanalysis 2004, 16, 253–259. [Google Scholar] [CrossRef]
- Gow, A.J.; Luchsinger, B.P.; Pawloski, J.R.; Singel, D.J.; Stamler, J.S. The oxyhemoglobin reaction of nitric oxide. Proc. Natl. Acad. Sci. USA 1999, 96, 9027–9032. [Google Scholar] [CrossRef] [PubMed]
- Topoglidis, E.; Astuti, Y.; Duriaux, F.; Grätzel, M.; Durrant, J.R. Direct electrochemistry and nitric oxide interaction of heme proteins adsorbed on nanocrystalline tin oxide electrodes. Langmuir 2003, 19, 6894–6900. [Google Scholar] [CrossRef]
- Presnova, G.; Grigorenko, V.; Egorov, A.; Ruzgas, T.; Lindgren, A.; Gorton, L.; Börchers, T. Direct heterogeneous electron transfer of recombinant horseradish peroxidases on gold. Faraday Discuss. 2000, 116, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Ferapontova, E.; Schmengler, K.; Börchers, T.; Ruzgas, T.; Gorton, L. Effect of cysteine mutations on direct electron transfer of horseradish peroxidase on gold. Biosens. Bioelectron. 2002, 17, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Shi, L.; Chen, B.; Mayer, M.U.; Lower, B.H.; Londer, Y.; Bose, S.; Hochella, M.F.; Fredrickson, J.K.; Squier, T.C. High-affinity binding and direct electron transfer to solid metals by the shewanella oneidensis mr-1 outer membrane c-type cytochrome OmcA. J. Am. Chem. Soc. 2006, 128, 13978–13979. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Shin, H.-K.; Kwon, Y.-S. A hydrogen peroxide biosensor based on Langmuir–Blodgett technique: Direct electron transfer of hemoglobin in octadecylamine layer. Talanta 2005, 67, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, A.; Habermüller, K.; Csöregi, E.; Laurinavicius, V.; Schuhmann, W. Polypyrrole-entrapped quinohemoprotein alcohol dehydrogenase. Evidence for direct electron transfer via conducting-polymer chains. Anal. Chem. 1999, 71, 3581–3586. [Google Scholar] [CrossRef] [PubMed]
- Loew, N.; Scheller, F.W.; Wollenberger, U. Characterization of self-assembling of glucose dehydrogenase in mono- and multilayers on gold electrodes. Electroanalysis 2004, 16, 1149–1154. [Google Scholar] [CrossRef]
- Liu, G.; Paddon-Row, M.N.; Justin Gooding, J. A molecular wire modified glassy carbon electrode for achieving direct electron transfer to native glucose oxidase. Electrochem. Commun. 2007, 9, 2218–2223. [Google Scholar] [CrossRef]
- Vaddiraju, S.; Tomazos, I.; Burgess, D.J.; Jain, F.C.; Papadimitrakopoulos, F. Emerging synergy between nanotechnology and implantable biosensors: A review. Biosens. Bioelectron. 2010, 25, 1553–1565. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, V. Nanotechnology in glucose monitoring: Advances and challenges in the last 10 years. Biosens. Bioelectron. 2013, 47, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, P.A.; Sandhyarani, N. Femtomolar level detection of BRCA1 gene using a gold nanoparticle labeled sandwich type DNA sensor. Colloids Surf. B Biointerfaces 2014, 117, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Delfino, I.; Bizzarri, A.R.; Cannistraro, S. Single-molecule detection of yeast cytochrome c by surface-enhanced Raman spectroscopy. Biophys. Chem. 2005, 113, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P.; Maiyalagan, T.; Sahoo, N.G.; Opallo, M. Nitrogen doped graphene nanosheet supported platinum nanoparticles as high performance electrochemical homocysteine biosensors. J. Mater. Chem. B 2013, 1, 4655–4666. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhou, X.; Huang, X.; Wang, Z.; Yang, Y.; Zhang, Q.; Boey, F.; Zhang, H. Electrochemical deposition of Pt nanoparticles on carbon nanotube patterns for glucose detection. Analyst 2010, 135, 1726–1730. [Google Scholar] [CrossRef] [PubMed]
- Tanne, J.; Schäfer, D.; Khalid, W.; Parak, W.J.; Lisdat, F. Light-controlled bioelectrochemical sensor based on CdSe/ZnS quantum dots. Anal. Chem. 2011, 83, 7778–7785. [Google Scholar] [CrossRef] [PubMed]
- Khalid, W.; Göbel, G.; Hühn, D.; Montenegro, J.-M.; Rivera-Gil, P.; Lisdat, F.; Parak, W.J. Light triggered detection of aminophenyl phosphate with a quantum dot based enzyme electrode. J. Nanobiotechnol. 2011, 9. [Google Scholar] [CrossRef]
- Holzinger, M.; Bouffier, L.; Villalonga, R.; Cosnier, S. Adamantane/beta-cyclodextrin affinity biosensors based on single-walled carbon nanotubes. Biosens. Bioelectron. 2009, 24, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Janegitz, B.C.; Figueiredo-Filho, L.C.S.; Marcolino-Junior, L.H.; Souza, S.P.N.; Pereira-Filho, E.R.; Fatibello-Filho, O. Development of a carbon nanotubes paste electrode modified with crosslinked chitosan for cadmium(II) and mercury(II) determination. J. Electroanal. Chem. 2011, 660, 209–216. [Google Scholar] [CrossRef]
- Prévoteau, A.; Courjean, O.; Mano, N. Deglycosylation of glucose oxidase to improve biosensors and biofuel cells. Electrochem. Commun. 2010, 12, 213–215. [Google Scholar] [CrossRef]
- Courjean, O.; Gao, F.; Mano, N. Deglycosylation of glucose oxidase for direct and efficient glucose electrooxidation on a glassy carbon electrode. Angew. Chem. 2009, 121, 6011–6013. [Google Scholar] [CrossRef]
- Sarauli, D.; Xu, C.; Dietzel, B.; Schulz, B.; Lisdat, F. Differently substituted sulfonated polyanilines: The role of polymer compositions in electron transfer with pyrroloquinoline quinone-dependent glucose dehydrogenase. Acta Biomater. 2013, 9, 8290–8298. [Google Scholar] [CrossRef] [PubMed]
- Schubart, I.W.; Göbel, G.; Lisdat, F. A pyrroloquinolinequinone-dependent glucose dehydrogenase (PQQ-GDH)-electrode with direct electron transfer based on polyaniline modified carbon nanotubes for biofuel cell application. Electrochim. Acta 2012, 82, 224–232. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, R.; Zhai, J.; Tian, C. Bienzymatic glucose biosensor based on co-immobilization of peroxidase and glucose oxidase on a carbon nanotubes electrode. Biosens. Bioelectron. 2007, 23, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Silveira, C.M.; Baur, J.; Holzinger, M.; Moura, J.J.G.; Cosnier, S.; Almeida, M.G. Enhanced direct electron transfer of a multihemic nitrite reductase on single-walled carbon nanotube modified electrodes. Electroanalysis 2010, 22, 2973–2978. [Google Scholar] [CrossRef]
- Korkut, S.; Keskinler, B.; Erhan, E. An amperometric biosensor based on multiwalled carbon nanotube-poly(pyrrole)-horseradish peroxidase nanobiocomposite film for determination of phenol derivatives. Talanta 2008, 76, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Dai, L.; Wallace, G.G. Biosensors based on aligned carbon nanotubes coated with inherently conducting polymers. Electroanalysis 2003, 15, 1089–1094. [Google Scholar] [CrossRef]
- Zhou, D.-M.; Dai, Y.-Q.; Shiu, K.-K. Poly(phenylenediamine) film for the construction of glucose biosensors based on platinized glassy carbon electrode. J. Appl. Electrochem. 2010, 40, 1997–2003. [Google Scholar] [CrossRef]
- Le Goff, A.; Holzinger, M.; Cosnier, S. Enzymatic biosensors based on SWCNT-conducting polymer electrodes. Analyst 2011, 136, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Hu, H.; Yu, A.; Perea, D.; Haddon, R.C. Synthesis and characterization of water soluble single-walled carbon nanotube graft copolymers. J. Am. Chem. Soc. 2005, 127, 8197–8203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Hu, H.; Haddon, R.C. Synthesis and properties of a water-soluble single-walled carbon nanotube–poly(m-aminobenzene sulfonic acid) graft copolymer. Adv. Funct. Mater. 2004, 14, 71–76. [Google Scholar] [CrossRef]
- Tanne, J.; Dietzel, B.; Scheller, F.W.; Bier, F. Nanohybrid materials consisting of poly[(3-aminobenzoic acid)-co-(3-aminobenzenesulfonic acid)-co-aniline] and multiwalled carbon nanotubes for immobilization of redox active cytochrome c. Electroanalysis 2014, 26, 732–738. [Google Scholar] [CrossRef]
- Safina, G.; Ludwig, R.; Gorton, L. A simple and sensitive method for lactose detection based on direct electron transfer between immobilised cellobiose dehydrogenase and screen-printed carbon electrodes. Electrochim. Acta 2010, 55, 7690–7695. [Google Scholar] [CrossRef]
- Guiseppi-Elie, A.; Lei, C.; Baughman, R.H. Direct electron transfer of glucose oxidase on carbon nanotubes. Nanotechnology 2002, 13. [Google Scholar] [CrossRef]
- Patolsky, F.; Weizmann, Y.; Willner, I. Long-Range Electrical Contacting of Redox Enzymes by SWCNT Connectors. Angew. Chem. Int. Ed. 2004, 43, 2113–2117. [Google Scholar] [CrossRef]
- Liu, J.; Chou, A.; Rahmat, W.; Paddon-Row, M.N.; Gooding, J.J. Achieving direct electrical connection to glucose oxidase using aligned single walled carbon nanotube arrays. Electroanalysis 2005, 17, 38–46. [Google Scholar] [CrossRef]
- Ludwig, R.; Ortiz, R.; Schulz, C.; Harreither, W.; Sygmund, C.; Gorton, L. Cellobiose dehydrogenase modified electrodes: advances by materials science and biochemical engineering. Anal. Bioanal. Chem. 2013, 405, 3637–3658. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, R.; Salamon, A.; Varga, J.; Zámocky, M.; Peterbauer, C.K.; Kulbe, K.D.; Haltrich, D. Characterisation of cellobiose dehydrogenases from the white-rot fungi Trametes pubescens and Trametes villosa. Appl. Microbiol. Biotechnol. 2004, 64, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, P.N.; Wallace, E.N.K. The oxidation of ascorbate at poly(aniline)–poly(vinylsulfonate)composite coated electrodes. Phys. Chem. Chem. Phys. 2001, 3, 1491–1496. [Google Scholar] [CrossRef]
- Mirmohseni, A.; Houjaghan, M.R. Synthesis and characterization of water soluble conducting poly (3-amino-4-methoxybenzenesulfonic acid). Mol. Cryst. Liq. Cryst. 2008, 484, 722–727. [Google Scholar] [CrossRef]
- Sarauli, D.; Xu, C.; Dietzel, B.; Schulz, B.; Lisdat, F. A multilayered sulfonated polyaniline network with entrapped pyrroloquinoline quinone-dependent glucose dehydrogenase: Tunable direct bioelectrocatalysis. J. Mater. Chem. B 2014, 2, 3196–3203. [Google Scholar] [CrossRef]
- Dash, M.P.; Tripathy, M.; Sasmal, A.; Mohanty, G.C.; Nayak, P.L. Poly(anthranilic acid)/multi-walled carbon nanotube composites: Spectral, morphological, and electrical properties. J. Mater. Sci. 2010, 45, 3858–3865. [Google Scholar] [CrossRef]
- Gu, L.; Liang, Y.; Zhou, T.; Tang, X.; Shi, G. A novel electrochemical sensor based on boronic acid-functionalized multi-walled carbon nanotubes for astragaloside IV determination using ARS as the current indicator. Anal. Methods 2012, 4, 492–495. [Google Scholar] [CrossRef]
- Tasca, F.; Harreither, W.; Ludwig, R.; Gooding, J.J.; Gorton, L. Cellobiose dehydrogenase aryl diazonium modified single walled carbon nanotubes: Enhanced direct electron transfer through a positively charged surface. Anal. Chem. 2011, 83, 3042–3049. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, G.; Pettersson, G.; Johansson, G.; Ruiz, A.; Uzcategui, E. Cellobiose oxidase from Phanerochaete chrysosporium can be cleaved by papain into two domains. Eur. J. Biochem. 1991, 196, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Tasca, F.; Gorton, L.; Harreither, W.; Haltrich, D.; Ludwig, R.; Nöll, G. Comparison of direct and mediated electron transfer for cellobiose dehydrogenase from Phanerochaete sordida. Anal. Chem. 2009, 81, 2791–2798. [Google Scholar] [CrossRef] [PubMed]
- Tasca, F.; Gorton, L.; Harreither, W.; Haltrich, D.; Ludwig, R.; Nöll, G. Direct electron transfer at cellobiose dehydrogenase modified anodes for biofuel cells. J. Phys. Chem. C 2008, 112, 9956–9961. [Google Scholar] [CrossRef]
- Matsumura, H.; Ortiz, R.; Ludwig, R.; Igarashi, K.; Samejima, M.; Gorton, L. Direct electrochemistry of Phanerochaete chrysosporium cellobiose dehydrogenase covalently attached onto gold nanoparticle modified solid gold electrodes. Langmuir ACS J. Surf. Colloids 2012, 28, 10925–10933. [Google Scholar] [CrossRef]
- Lindgren, A.; Gorton, L.; Ruzgas, T.; Baminger, U.; Haltrich, D.; Schülein, M. Direct electron transfer of cellobiose dehydrogenase from various biological origins at gold and graphite electrodes. J. Electroanal. Chem. 2001, 496, 76–81. [Google Scholar] [CrossRef]
- Stoica, L.; Dimcheva, N.; Haltrich, D.; Ruzgas, T.; Gorton, L. Electrochemical investigation of cellobiose dehydrogenase from new fungal sources on Au electrodes. Biosens. Bioelectron. 2005, 20, 2010–2018. [Google Scholar] [CrossRef] [PubMed]
- Stoica, L.; Ludwig, R.; Haltrich, D.; Gorton, L. Third-generation biosensor for lactose based on newly discovered cellobiose dehydrogenase. Anal. Chem. 2006, 78, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Trashin, S.A.; Haltrich, D.; Ludwig, R.; Gorton, L.; Karyakin, A.A. Improvement of direct bioelectrocatalysis by cellobiose dehydrogenase on screen printed graphite electrodes using polyaniline modification. Bioelectrochem. Amst. Neth. 2009, 76, 87–92. [Google Scholar] [CrossRef]
- Chen, J.; Gorton, L.; Åkesson, B. Electrochemical studies on antioxidants in bovine milk. Anal. Chim. Acta 2002, 474, 137–146. [Google Scholar] [CrossRef]
- Xu, J.-J.; Zhou, D.-M.; Chen, H.-Y. Amperometric determination of ascorbic acid at a novel “self-doped” polyaniline modified microelectrode. Fresenius J. Anal. Chem. 1998, 362, 234–238. [Google Scholar] [CrossRef]
- Zhou, D.-M.; Xu, J.-J.; Chen, H.-Y.; Fang, H.-Q. Ascorbate sensor based on “self-doped” polyaniline. Electroanalysis 1997, 9, 1185–1188. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanne, J.; Kracher, D.; Dietzel, B.; Schulz, B.; Ludwig, R.; Lisdat, F.; Scheller, F.W.; Bier, F.F. Carboxylated or Aminated Polyaniline—Multiwalled Carbon Nanotubes Nanohybrids for Immobilization of Cellobiose Dehydrogenase on Gold Electrodes. Biosensors 2014, 4, 370-386. https://doi.org/10.3390/bios4040370
Tanne J, Kracher D, Dietzel B, Schulz B, Ludwig R, Lisdat F, Scheller FW, Bier FF. Carboxylated or Aminated Polyaniline—Multiwalled Carbon Nanotubes Nanohybrids for Immobilization of Cellobiose Dehydrogenase on Gold Electrodes. Biosensors. 2014; 4(4):370-386. https://doi.org/10.3390/bios4040370
Chicago/Turabian StyleTanne, Johannes, Daniel Kracher, Birgit Dietzel, Burkhard Schulz, Roland Ludwig, Fred Lisdat, Frieder W. Scheller, and Frank F. Bier. 2014. "Carboxylated or Aminated Polyaniline—Multiwalled Carbon Nanotubes Nanohybrids for Immobilization of Cellobiose Dehydrogenase on Gold Electrodes" Biosensors 4, no. 4: 370-386. https://doi.org/10.3390/bios4040370




