High-Contrast Fluorescence Microscopy for a Biomolecular Analysis Based on Polarization Techniques Using an Optical Interference Mirror Slide
Abstract
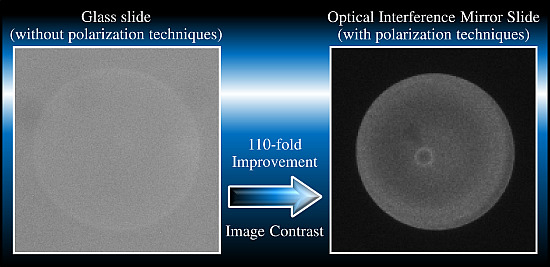
:1. Introduction
2. Experimental Section
2.1. Reagents and Proteins
2.2. Fabrication of an Optical Interference Mirror Slide
2.3. Preparation of Rhodamine B
2.4. Detection of Streptavidin
2.5. Optical System

2.6. Design of the Al2O3 Thickness
3. Results and Discussion
3.1. Polarizations of the Back-Scattered Excitation Light and Fluorescence Emission

3.2. Evaluation of the Image Contrast for Rhodamine B

3.3. High-Contrast Fluorescence Microscopy for Biomolecular Analysis

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Waters, J.C. Accuracy and precision in quantitative fluorescence microscopy. J. Cell Biol. 2009, 185, 1135–1148. [Google Scholar]
- Tawa, K.; Hori, H.; Kintaka, K.; Kiyosue, K.; Tatsu, Y.; Nishii, J. Optical microscopic observation of fluorescence enhanced by grating-coupled surface plasmon resonance. Opt. Express 2008, 16, 9781–9790. [Google Scholar]
- Matveeva, E.G.; Gryczynski, I.; Barnett, A.; Leonenko, Z.; Lakowicz, J.R.; Gryczynski, Z. Metal particle-enhanced fluorescent immunoassays on metal mirrors. Anal. Biochem. 2007, 363, 239–245. [Google Scholar]
- Kuwabara, K.; Ogino, M.; Ando, T.; Miyauchi, A. Enhancement of fluorescence intensity from an immunoassay chip using high-aspect-ratio nanopillars fabricated by nanoimprinting. Appl. Phys. Lett. 2008, 93. [Google Scholar] [CrossRef]
- Takmakov, P.; Vlassiouk, I.; Smirnov, S. Application of anodized aluminum in fluorescence detection of biological species. Anal. Bioanal. Chem. 2006, 385, 954–958. [Google Scholar]
- Grandin, H.M.; Städler, B.; Textor, M.; Vörös, J. Waveguide excitation fluorescence microscopy: A new tool for sensing and imaging the biointerface. Biosens. Bioelectron. 2006, 21, 1476–1482. [Google Scholar]
- Mathias, P.C.; Ganesh, N.; Chan, L.L.; Cunningham, B.T. Combined enhanced fluorescence and label-free biomolecular detection with a photonic crystal surface. Appl. Opt. 2007, 46, 2351–2360. [Google Scholar]
- Zubtsova, Z.I.; Zubtsov, D.A.; Savvateeva, E.N.; Stomakhin, A.A.; Chechetkin, V.R.; Zasedatelev, A.S.; Rubina, A.Y. Hydrogel-based protein and oligonucleotide microchips on metal-coated surfaces: Enhancement of fluorescence and optimization of immunoassay. J. Biotechnol. 2009, 144, 151–159. [Google Scholar]
- Holland, W.R.; Hall, D.G. Waveguide mode enhancement of molecular fluorescence. Opt. Lett. 1985, 10, 414–416. [Google Scholar]
- Sullivan, K.G.; King, O.; Sigg, C.; Hall, D.G. Directional, enhanced fluorescence from molecules near a periodic surface. Appl. Opt. 1994, 33, 2447–2454. [Google Scholar]
- Sullivan, K.G.; Hall, D.G. Enhancement and inhibition of electromagnetic radiation in plane-layered media. I. Plane-wave spectrum approach to modeling classical effects. J. Opt. Soc. Am. B 1997, 14, 1149–1159. [Google Scholar]
- Sullivan, K.G.; Hall, D.G. Enhancement and inhibition of electromagnetic radiation in plane-layered media. II. Enhanced fluorescence in optical waveguide sensors. J. Opt. Soc. Am. B 1997, 14, 1160–1166. [Google Scholar]
- Akimoto, T.; Yasuda, M. Fluorescence enhancement and reflection of the excitation light observed with a multilayered substrate. Appl. Opt. 2010, 49, 80–85. [Google Scholar]
- Yasuda, M.; Akimoto, T. Highly sensitive fluorescence detection of avidin/streptavidin with an optical interference mirror slide. Anal. Sci. 2012, 28, 947–952. [Google Scholar]
- Etoh, H.; Yasuda, M.; Akimoto, T. Signal enhancement by a multi-layered substrate for mutagen detection using an SOS response-induced green fluorescent protein in genetically modified Escherichia coli. Anal. Sci. 2011, 27, 1179–1183. [Google Scholar]
- Moal, E.L.; Fort, E.; Lévêque-Fort, S.; Cordelières, F.P.; Fontaine-Aupart, M.-P.; Ricolleau, C. Enhanced fluorescence cell imaging with metal-coated slides. Biophys. J. 2007, 92, 2150–2161. [Google Scholar]
- Moal, E.L.; Fort, E.; Lévêque-Fort, S.; Janin, A.; Murata, H.; Cordelières, F.P.; Fontaine-Aupart, M.-P.; Ricolleau, C. Mirror slides for high-sensitivity cell and tissue fluorescence imaging. J. Biomed. Opt. 2007, 12. [Google Scholar] [CrossRef]
- Akimoto, T.; Yasuda, M.; Karube, I. Effect of the polarization and incident angle of excitation light on the fluorescence enhancement observed with a multilayered substrate fabricated by Ag and Al2O3. Appl. Opt. 2008, 47, 3789–3794. [Google Scholar]
- Yasuda, M.; Akimoto, T. High contrast fluorescence imaging with an optical interference mirror slide based on polarization dependence of fluorescence enhancement. 2014; submitted. [Google Scholar]
- Eriksson, T.S.; Hjortsberg, A.; Niklasson, G.A.; Granqvist, C.G. Infrared optical properties of evaporated alumina films. Appl. Opt. 1981, 20, 2742–2746. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasuda, M.; Akimoto, T. High-Contrast Fluorescence Microscopy for a Biomolecular Analysis Based on Polarization Techniques Using an Optical Interference Mirror Slide. Biosensors 2014, 4, 513-522. https://doi.org/10.3390/bios4040513
Yasuda M, Akimoto T. High-Contrast Fluorescence Microscopy for a Biomolecular Analysis Based on Polarization Techniques Using an Optical Interference Mirror Slide. Biosensors. 2014; 4(4):513-522. https://doi.org/10.3390/bios4040513
Chicago/Turabian StyleYasuda, Mitsuru, and Takuo Akimoto. 2014. "High-Contrast Fluorescence Microscopy for a Biomolecular Analysis Based on Polarization Techniques Using an Optical Interference Mirror Slide" Biosensors 4, no. 4: 513-522. https://doi.org/10.3390/bios4040513