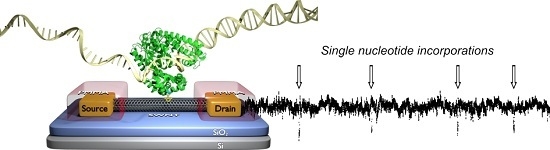
Single Molecule Bioelectronics and Their Application to Amplification-Free Measurement of DNA Lengths
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication and Functionalization of SWNT-FETs
2.2. Operation of Single-Molecule SWNT-FETs
3. Results
3.1. Single-Molecule Activity of DNA Polymerase (KF)
3.2. Accuracy of Amplification-Free Measurements of DNA Template Lengths
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| KF | Klenow Fragment of DNA polymerase I |
| DNA | deoxyribonucleic acid |
| smFRET | single-molecule Förster resonance energy transfer |
| SWNT | single-walled carbon nanotube |
| FET | field effect transistor |
| dNTP | deoxynucleoside triphosphate |
References and Notes
- Casas-Selves, M.; Degregori, J. How cancer shapes evolution, and how evolution shapes cancer. Evolution 2011, 4, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Weinberg, R.A. One Renegade Cell: The Quest for the Origins of Cancer; Basic Books: New York, NY, USA, 1999. [Google Scholar]
- Wu, Z.Q.; Brabletz, T.; Fearon, E.; Willis, A.L.; Hu, C.Y.; Li, X.Y.; Weiss, S.J. Canonical wnt suppressor, axin2, promotes colon carcinoma oncogenic activity. Proc. Natl. Acad. Sci. USA 2012, 109, 11312–11317. [Google Scholar] [CrossRef] [PubMed]
- Dravis, C.; Spike, B.T.; Harrell, J.C.; Johns, C.; Trejo, C.L.; Southard-Smith, E.M.; Perou, C.M.; Wahl, G.M. Sox10 regulates stem/progenitor and mesenchymal cell states in mammary epithelial cells. Cell Rep. 2015, 12, 2035–2048. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.N. Single-molecule approach to enzymology. Single Mol. 2001, 2, 229–236. [Google Scholar] [CrossRef]
- Min, W.; English, B.; Luo, G.; Cherayil, B.; Kou, S.; Xie, X. Fluctuating enzymes: Lessons from single-molecule studies. Acc. Chem. Res. 2005, 38, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Hohlbein, J.; Gryte, K.; Heilemann, M.; Kapanidis, A.N. Surfing on a new wave of single-molecule fluorescence methods. Phys. Biol. 2010, 7, 031001. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.K.; Jeon, K.S.; Hwang, J.H.; Kim, H.; Kwon, S.; Suh, Y.D.; Nam, J.M. Highly uniform and reproducible surface-enhanced raman scattering from DNA-tailorable nanoparticles with 1-nm interior gap. Nat. Nanotechnol. 2011, 6, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Qin, Y.; He, Y.; Huang, Q.; Fan, C.; Chen, H.Y. Functional nanoprobes for ultrasensitive detection of biomolecules. Chem. Soc. Rev. 2010, 39, 4234–4243. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Luo, G.B.; Karnchanaphanurach, P.; Louie, T.M.; Rech, I.; Cova, S.; Xun, L.Y.; Xie, X.S. Protein conformational dynamics probed by single-molecule electron transfer. Science 2003, 302, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Pudney, C.R.; Lane, R.S.K.; Fielding, A.J.; Magennis, S.W.; Hay, S.; Scrutton, N.S. Enzymatic single-molecule kinetic isotope effects. J. Am. Chem. Soc. 2013, 135, 3855–3864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagannathan, B.; Marqusee, S. Protein folding and unfolding under force. Biopolymers 2013, 99, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Quinlan, M.E.; Forkey, J.N.; Goldman, Y.E. Rotational motions of macromolecules by single-molecule fluorescence microscopy. Acc. Chem. Res. 2005, 38, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Vrtis, K.B.; Markiewicz, R.P.; Romano, L.J.; Rueda, D. Carcinogenic adducts induce distinct DNA polymerase binding orientations. Nucleic Acids Res. 2013, 41, 7843–7853. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, K.R.; Dahl, J.M.; Mai, A.H.; Cox, A.; Akeson, M.; Wang, H. Kinetic mechanism of translocation and dNTP binding in individual DNA polymerase complexes. J. Am. Chem. Soc. 2013, 135, 9149–9155. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.S.; Lu, H.P. Single-molecule enzymatic conformational dynamics: Spilling out the product molecules. J. Phys. Chem. B 2014, 118, 9128–9140. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Pardo, J.A.; Alegre-Cebollada, J.; Ramirez-Sarmiento, C.A.; Fernandez, J.M.; Guixe, V. Identifying sequential substrate binding at the single-molecule level by enzyme mechanical stabilization. ACS Nano 2015, 9, 3996–4005. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.B.; Eggertson, M.J.; Chikamatsu, M.; Horwood, C.A. Single molecule assay of Escherichia coli beta-galactosidase using two competing substrates simultaneously, ddao-beta-d-galactoside and resorufin-beta-d-galactoside. Anal. Lett. 2011, 44, 1835–1841. [Google Scholar] [CrossRef]
- Choi, Y.; Moody, I.S.; Sims, P.C.; Hunt, S.R.; Corso, B.L.; Seitz, D.E.; Blaszcazk, L.C.; Collins, P.G.; Weiss, G.A. Single molecule dynamics of lysozyme processing distinguishes linear and cross-linked peptidoglycan substrates. J. Am. Chem. Soc. 2012, 134, 2032–2035. [Google Scholar] [CrossRef] [PubMed]
- Betous, R.; Couch, F.B.; Mason, A.C.; Eichman, B.F.; Manosas, M.; Cortez, D. Substrate-selective repair and restart of replication forks by DNA translocases. Cell Rep. 2013, 3, 1958–1969. [Google Scholar] [CrossRef] [PubMed]
- Joo, C.; Balci, H.; Ishitsuka, Y.; Buranachai, C.; Ha, T. Advances in single-molecule fluorescence methods for molecular biology. Annu. Rev. Biochem. 2008, 77, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Blank, K.; De Cremer, G.; Hofkens, J. Fluorescence-based analysis of enzymes at the single-molecule level. Biotechnol. J. 2009, 4, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Eid, J.; Fehr, A.; Gray, J.; Luong, K.; Lyle, J.; Otto, G.; Peluso, P.; Rank, D.; Baybayan, P.; Bettman, B.; et al. Real-time DNA sequencing from single polymerase molecules. Science 2009, 323, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Neuman, K.C.; Nagy, A. Single-molecule force spectroscopy: Optical tweezers, magnetic tweezers and atomic force microscopy. Nat. Methods 2008, 5, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Deniz, A.A.; Mukhopadhyay, S.; Lemke, E.A. Single-molecule biophysics: At the interface of biology, physics and chemistry. J. R. Soc. Interface 2008, 5, 15–45. [Google Scholar] [CrossRef] [PubMed]
- Martina, M.; Taverna, S. Patch-Clamp Methods and Protocols, 2nd ed.; Humana Press: New York, NY, USA, 2014; p. 403. [Google Scholar]
- Iqbal, S.M.; Bashir, R. Nanopores; Springer US: New York, NY, USA, 2011; p. 369. [Google Scholar]
- Healy, K.; Schiedt, B.; Morrison, A.P. Solid-state nanopore technologies for nanopore-based DNA analysis. Nanomedicine 2007, 2, 875–897. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, B.M.; Bashir, R. Nanopore sensors for nucleic acid analysis. Nat. Nanotechnol. 2011, 6, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Branton, D.; Deamer, D.W.; Marziali, A.; Bayley, H.; Benner, S.A.; Butler, T.; Di Ventra, M.; Garaj, S.; Hibbs, A.; Huang, X.H.; et al. The potential and challenges of nanopore sequencing. Nat. Biotechnol. 2008, 26, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, Y.; Ying, C.; Wang, D.; Du, C. Nanopore-based fourth-generation DNA sequencing technology. Genom. Proteom. Bioinform. 2015, 13, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; He, J.; Chang, S.; Zhang, P.; Liang, F.; Li, S.; Tuchband, M.; Fuhrmann, A.; Ros, R.; Lindsay, S. Identifying single bases in a DNA oligomer with electron tunnelling. Nat. Nanotechnol. 2010, 5, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ashcroft, B.; Zhang, P.; Liu, H.; Sen, S.; Song, W.; Im, J.; Gyarfas, B.; Manna, S.; Biswas, S.; et al. Single-molecule spectroscopy of amino acids and peptides by recognition tunnelling. Nat. Nano 2014, 9, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wei, Q.Q.; Park, H.K.; Lieber, C.M. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 2001, 293, 1289–1292. [Google Scholar] [CrossRef] [PubMed]
- Patolsky, F.; Zheng, G.; Hayden, O.; Lakadamyali, M.; Zhuang, X.; Lieber, C.M. Electrical detection of single viruses. Proc. Natl. Acad. Sci. USA 2004, 101, 14017–14022. [Google Scholar] [CrossRef] [PubMed]
- Patolsky, F.; Zheng, G.F.; Lieber, C.M. Nanowire-based biosensors. Anal. Chem. 2006, 78, 4260–4269. [Google Scholar] [CrossRef] [PubMed]
- Patolsky, F.; Zheng, G.F.; Lieber, C.M. Fabrication of silicon nanowire devices for ultrasensitive, label-free, real-time detection of biological and chemical species. Nat. Protoc. 2006, 1, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.F.; Gao, X.P.A.; Lieber, C.M. Frequency domain detection of biomolecules using silicon nanowire biosensors. Nano Lett. 2010, 10, 3179–3183. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Franklin, N.R.; Zhou, C.W.; Chapline, M.G.; Peng, S.; Cho, K.J.; Dai, H.J. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.G.; Bradley, K.; Ishigami, M.; Zettl, A. Extreme oxygen sensitivity of electronic properties of carbon nanotubes. Science 2000, 287, 1801–1804. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Small, J.P.; Klare, J.E.; Want, Y.; Purewal, M.S.; Tam, I.W.; Hong, B.H.; Caldwell, R.; Huang, L.; O'Brien, S.; et al. Covalently bridging gaps in single-walled carbon nanotubes with conducting molecules. Science 2006, 311, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, B.R.; Coroneus, J.G.; Kane, A.A.; Weiss, G.A.; Collins, P.G. Monitoring single molecule reactivity on a carbon nanotube. Nano Lett. 2008, 8, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Sorgenfrei, S.; Chiu, C.-Y.; Gonzalez, R.L.; Yu, Y.-J.; Kim, P.; Nuckolls, C.; Shepard, K.L. Label-free single-molecule detection of DNA-hybridization kinetics with a carbon nanotube field-effect transistor. Nat. Nanotechnol. 2011, 6, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Moody, I.S.; Sims, P.C.; Hunt, S.R.; Corso, B.L.; Weiss, G.A.; Collins, P.G. Single-molecule lysozyme dynamics monitored by an electronic circuit. Science 2012, 335, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Olsen, T.J.; Sims, P.C.; Moody, I.S.; Corso, B.L.; Dang, M.N.; Weiss, G.A.; Collins, P.G. Dissecting single-molecule signal transduction in carbon nanotube circuits with protein engineering. Nano Lett. 2013, 13, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Sims, P.C.; Moody, I.S.; Choi, Y.; Dong, C.; Iftikhar, M.; Corso, B.L.; Gul, O.T.; Collins, P.G.; Weiss, G.A. Electronic measurements of single-molecule catalysis by camp-dependent protein kinase A. J. Am. Chem. Soc. 2013, 135, 7861–7868. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.J.; Choi, Y.; Sims, P.C.; Gul, O.T.; Corso, B.L.; Dong, C.; Brown, W.A.; Collins, P.G.; Weiss, G.A. Electronic measurements of single-molecule processing by DNA polymerase I (klenow fragment). J. Am. Chem. Soc. 2013, 135, 7855–7860. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, K.M.; Gul, O.T.; Olsen, T.J.; Choi, Y.; Collins, P.G.; Weiss, G.A. Incorporation of deoxynucleoside triphosphate analogs by single-molecule DNA polymerase I (klenow fragment) nanocircuits. J. Am. Chem. Soc. 2015, 137, 9587–9594. [Google Scholar] [CrossRef] [PubMed]
- Martel, R.; Schmidt, T.; Shea, H.R.; Hertel, T.; Avouris, P. Single- and multi-wall carbon nanotube field-effect transistors. Appl. Phys. Lett. 1998, 73, 2447–2449. [Google Scholar] [CrossRef]
- Tans, S.J.; Verschueren, A.R.M.; Dekker, C. Room-temperature transistor based on a single carbon nanotube. Nature 1998, 393, 49–52. [Google Scholar]
- Star, A.; Gabriel, J.C.P.; Bradley, K.; Gruner, G. Electronic detection of specific protein binding using nanotube fet devices. Nano Lett. 2003, 3, 459–463. [Google Scholar] [CrossRef]
- Goldsmith, B.R.; Coroneus, J.G.; Khalap, V.R.; Kane, A.A.; Weiss, G.A.; Collins, P.G. Conductance-controlled point functionalization of single-walled carbon nanotubes. Science 2007, 315, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Sorgenfrei, S.; Chiu, C.-Y.; Johnston, M.; Nuckolls, C.; Shepard, K.L. Debye screening in single-molecule carbon nanotube field-effect sensors. Nano Lett. 2011, 11, 3739–3743. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Das, S.K.; Kogerler, P.; Bogge, H.; Schmidtmann, M.; Trautwein, A.X.; Schunemann, V.; Krickemeyer, E.; Preetz, W. A new type of supramolecular compound: Molybdenum-oxide-based composites consisting of magnetic nanocapsules with encapsulated keggin-ion electron reservoirs cross-linked to a two-dimensional network. Angew. Chem. Int. Ed. 2000, 39, 3413–3417. [Google Scholar] [CrossRef]
- An, L.; Owens, J.M.; McNeil, L.E.; Liu, J. Synthesis of nearly uniform single-walled carbon nanotubes using identical metal-containing molecular nanoclusters as catalysts. J. Am. Chem. Soc. 2002, 124, 13688–13689. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Axup, J.Y.; Schultz, P.G. Protein conjugation with genetically encoded unnatural amino acids. Curr. Opin. Chem. Biol. 2013, 17, 412–419. [Google Scholar] [CrossRef] [PubMed]
- England, C.G.; Luo, H.; Cai, W. Halotag technology: A versatile platform for biomedical applications. Bioconj. Chem. 2015, 26, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Roeder, R.G. Purification of his-tagged proteins in non-denaturing conditions suggests a convenient method for protein interaction studies. Nucleic Acids Res. 1991, 19, 6337–6338. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Zhan, Y.G.; Wang, D.W.; Dai, H.J. Noncovalent sidewall functionalization of single-walled carbon nanotubes for protein immobilization. J. Am. Chem. Soc. 2001, 123, 3838–3839. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Bioconjugate Techniques, 2nd ed.; Academic Press, Inc.: Chicago, IL, USA, 2008. [Google Scholar]
- Shulaker, M.M.; Hills, G.; Patil, N.; Wei, H.; Chen, H.-Y.; Wong, H.S.P.; Mitra, S. Carbon nanotube computer. Nature 2013, 501, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Franklin, A.D.; Tulevski, G.S.; Han, S.-J.; Shahrjerdi, D.; Cao, Q.; Chen, H.-Y.; Wong, H.S.P.; Haensch, W. Variability in carbon nanotube transistors: Improving device-to-device consistency. ACS Nano 2012, 6, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hierold, C.; Haluska, M. Electrical contacts to individual swcnts: A review. Beilstein J. Nanotechnol. 2014, 5, 2202–2215. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Han, S.-J.; Tersoff, J.; Franklin, A.D.; Zhu, Y.; Zhang, Z.; Tulevski, G.S.; Tang, J.; Haensch, W. End-bonded contacts for carbon nanotube transistors with low, size-independent resistance. Science 2015, 350, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.G.; Fuhrer, M.S.; Zettl, A. 1/f noise in carbon nanotubes. Appl. Phys. Lett. 2000, 76, 894–896. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Appenzeller, J.; Zhihong, C.; Avouris, P. Electrical transport and 1/f noise in semiconducting carbon nanotubes. Phys. E 2007, 37, 72–77. [Google Scholar] [CrossRef]
- Bronson, J.E.; Fei, J.; Hofman, J.M.; Gonzalez, R.L., Jr.; Wiggins, C.H. Learning rates and states from biophysical time series: A bayesian approach to model selection and single-molecule fret data. Biophys. J. 2009, 97, 3196–3205. [Google Scholar] [CrossRef] [PubMed]
- Reuel, N.F.; Bojo, P.; Zhang, J.; Boghossian, A.A.; Ahn, J.-H.; Kim, J.-H.; Strano, M.S. Norse: Noise reduction and state evaluator for high-frequency single event traces. Bioinformatics 2012, 28, 296–297. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Kennedy, R.A. Forward-backward nonlinear filtering technique for extracting small biological signals from noise. J. Neurosci. Methods 1991, 40, 71–86. [Google Scholar] [CrossRef]
- Akhterov, M.V.; Choi, Y.; Olsen, T.J.; Sims, P.C.; Iftikhar, M.; Gul, O.T.; Corso, B.L.; Weiss, G.A.; Collins, P.G. Observing lysozyme closing and opening motions by high-resolution single molecule enzymology. ACS Chem. Biol. 2015, 10, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, V.; Freemont, P.; Sanderson, M.; Beese, L.; Friedman, J.; Joyce, C.; Steitz, T. Genetic and crystallographic studies of the 3′,5′-exonucleolytic site of DNA polymerase I. Science 1988, 240, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Aposhian, H.V.; Kornberg, A. Enzymatic synthesis of deoxyribonucleic acid 9. The Polymerase formed after T2 bacteriophage infection of Escherichia coli—A new enzyme. J. Biol. Chem. 1962, 237, 519–525. [Google Scholar] [PubMed]
- Joyce, C.M. Techniques used to study the DNA polymerase reaction pathway. Biochim. Biophys. Acta Proteins Proteom. 2010, 1804, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.J.; Benkovic, S.J. Resonance energy-transfer measurements between substrate binding-sites within the large (klenow) fragment of Escherichia-coli DNA-polymerase-I. Biochemistry 1989, 28, 9586–9593. [Google Scholar] [CrossRef] [PubMed]
- Christian, T.D.; Romano, L.J.; Rueda, D. Single-molecule measurements of synthesis by DNA polymerase with base-pair resolution. Proc. Natl. Acad. Sci. USA 2009, 106, 21109–21114. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.J.; Quake, S.R. Single molecule measurement of the “speed limit” of DNA polymerase. Proc. Natl. Acad. Sci. USA 2009, 106, 20294–20299. [Google Scholar] [CrossRef] [PubMed]
- Torella, J.P.; Holden, S.J.; Santoso, Y.; Hohlbein, J.; Kapanidis, A.N. Identifying molecular dynamics in single-molecule fret experiments with burst variance analysis. Biophys. J. 2011, 100, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Santoso, Y.; Joyce, C.M.; Potapova, O.; Le Reste, L.; Hohlbein, J.; Torella, J.P.; Grindley, N.D.F.; Kapanidis, A.N. Conformational transitions in DNA polymerase I revealed by single-molecule fret. Proc. Natl. Acad. Sci. USA 2010, 107, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, R.P.; Vrtis, K.B.; Rueda, D.; Romano, L.J. Single-molecule microscopy reveals new insights into nucleotide selection by DNA polymerase I. Nucleic Acids Res. 2012, 40, 7975–7984. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.P.; Wang, J.; Millar, D.P. DNA polymerase activity at the single-molecule level. Biochem. Soc. Trans. 2011, 39, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Berezhna, S.Y.; Gill, J.P.; Lamichhane, R.; Millar, D.P. Single-molecule förster resonance energy transfer reveals an innate fidelity checkpoint in DNA polymerase I. J. Am. Chem. Soc. 2012, 134, 11261–11268. [Google Scholar] [CrossRef] [PubMed]
- Kohlstaedt, L.A.; Wang, J.; Friedman, J.M.; Rice, P.A.; Steitz, T.A. Crystal-structure at 3.5 angstrom resolution of HIV-1 reverse-transcriptase complexed with an inhibitor. Science 1992, 256, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.J.; Taylor, J.S.; Beese, L.S. Processive DNA synthesis observed in a polymerase crystal suggests a mechanism for the prevention of frameshift mutations. Proc. Natl. Acad. Sci. USA 2003, 100, 3895–3900. [Google Scholar] [CrossRef] [PubMed]
- Polesky, A.H.; Steitz, T.A.; Grindley, N.D.F.; Joyce, C.M. Identification of residues critical for the polymerase-activity of the klenow fragment of DNA-polymerase-I from Escherichia-coli. J. Biol. Chem. 1990, 265, 14579–14591. [Google Scholar] [PubMed]
- Purohit, V.; Grindley, N.D.F.; Joyce, C.M. Use of 2-aminopurine fluorescence to examine conformational changes during nucleotide incorporation by DNA polymerase I (klenow fragment). Biochemistry 2003, 42, 10200–10211. [Google Scholar] [CrossRef] [PubMed]
- Delagoutte, E. DNA polymerases: Mechanistic insight from biochemical and biophysical studies. Front. Biosci. Landmark 2012, 17, 509–544. [Google Scholar] [CrossRef]
- Bryant, F.R.; Johnson, K.A.; Benkovic, S.J. Elementary steps in the DNA-polymerase-I reaction pathway. Biochemistry 1983, 22, 3537–3546. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.; Pandey, V.N.; Modak, M.J. Significance of the O-helix residues of Escherichia coli DNA polymerase I in DNA synthesis: Dynamics of the dNTP binding pocket. Biochemistry 1996, 35, 7256–7266. [Google Scholar] [CrossRef] [PubMed]
- Joyce, C.M.; Potapova, O.; DeLucia, A.M.; Huang, X.; Basu, V.P.; Grindley, N.D.F. Fingers-closing and other rapid conformational changes in DNA polymerase I (klenow fragment) and their role in nucleotide selectivity. Biochemistry 2008, 47, 6103–6116. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Cain, S. Bound on range precision for shot-noise limited ladar systems. Appl. Opt. 2008, 47, 5147–5154. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Nir, E.; Hamadani, K.; Weiss, S. Photobleaching pathways in single-molecule fret experiments. J. Am. Chem. Soc. 2007, 129, 4643–4654. [Google Scholar] [CrossRef] [PubMed]
- Kuchta, R.D.; Mizrahi, V.; Benkovic, P.A.; Johnson, K.A.; Benkovic, S.J. Kinetic mechanism of DNA-polymerase-I (klenow). Biochemistry 1987, 26, 8410–8417. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, M.E.; Benkovic, S.J. Kinetic mechanism of DNA-polymerase-I (klenow fragment)—Identification of a 2nd conformational change and evaluation of the internal equilibrium-constant. Biochemistry 1991, 30, 4835–4843. [Google Scholar] [CrossRef] [PubMed]
- The fast motion of ions in the surrounding electrolyte contribute to the overall noise measured in dNTP-free buffer, but they do not produce ΔI(t) excursions in defect-free SWNTs.
- Bermek, O.; Grindley, N.D.F.; Joyce, C.M. Prechemistry nucleotide selection checkpoints in the reaction pathway of DNA polymerase I and roles of glu710 and tyr766. Biochemistry 2013, 52, 6258–6274. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.M. Forensic DNA Typing: Biology, Technology, and Genetics of Str Markers, 2nd ed.; Academic Press: San Diego, CA, USA, 2010; p. 688. [Google Scholar]







| Template | Nucleotide | τclosed (ms) | τopen (ms) | k (1/s) |
|---|---|---|---|---|
| poly(dT)42 | dATP | 0.33 ± 0.08 | 71.4 ± 1.4 | 14.4 ± 2.9 |
| poly(dA)42 | dTTP | 0.42 ± 0.09 | 63.7 ± 1.1 | 16.0 ± 2.9 |
| poly(dG)42 | dCTP | 0.32 ± 0.07 | 39.0 ± 5.6 | 26.2 ± 4.4 |
| poly(dC)42 | dGTP | 0.33 ± 0.05 | 38.0 ± 5.8 | 28.5 ± 3.5 |
| Template Sequence | Length(s) | N 2 | Peak Position(s) (bp) | FWHM (bp) |
|---|---|---|---|---|
| (CTTT)11 | 44 | 27 | 42.3 ± 0.3 | 7.6 ± 0.8 |
| (B10A)4 | 44 | 45 | 44.2 ± 0.1 | 6.4 ± 0.4 |
| (CTTT)11 and (B10A)4 | 44 and 44 | 72 | 43.6 ± 0.2 | 7.8 ± 0.6 |
| (CTTT)9 and (CTTT)10 | 36 and 40 | 38 | 34.5 ± 1.1 40.0 ± 0.2 | 10.1 ± 3.6 3.6 ± 0.4 |
| (CTTT)10 and (CTTT)11 | 40 and 44 | 31 | 38.8 ± 0.3 43.5 ± 0.3 | 7.1 ± 1.0 4.0 ± 1.0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gül, O.T.; Pugliese, K.M.; Choi, Y.; Sims, P.C.; Pan, D.; Rajapakse, A.J.; Weiss, G.A.; Collins, P.G. Single Molecule Bioelectronics and Their Application to Amplification-Free Measurement of DNA Lengths. Biosensors 2016, 6, 29. https://doi.org/10.3390/bios6030029
Gül OT, Pugliese KM, Choi Y, Sims PC, Pan D, Rajapakse AJ, Weiss GA, Collins PG. Single Molecule Bioelectronics and Their Application to Amplification-Free Measurement of DNA Lengths. Biosensors. 2016; 6(3):29. https://doi.org/10.3390/bios6030029
Chicago/Turabian StyleGül, O. Tolga, Kaitlin M. Pugliese, Yongki Choi, Patrick C. Sims, Deng Pan, Arith J. Rajapakse, Gregory A. Weiss, and Philip G. Collins. 2016. "Single Molecule Bioelectronics and Their Application to Amplification-Free Measurement of DNA Lengths" Biosensors 6, no. 3: 29. https://doi.org/10.3390/bios6030029