Raman Computational and Experimental Studies of Dopamine Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. Computational Analysis
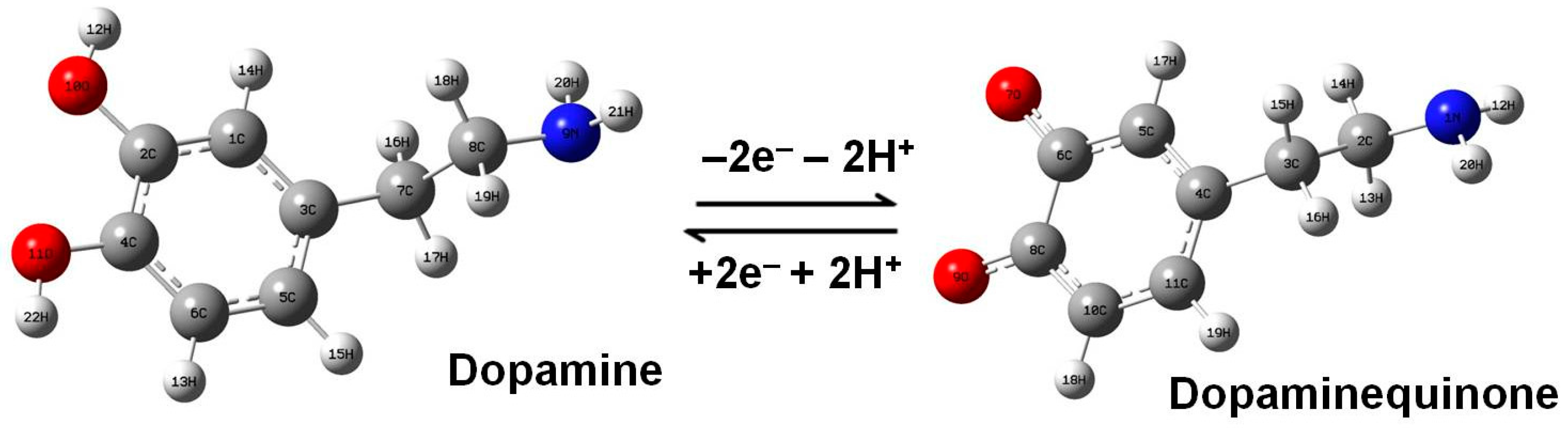
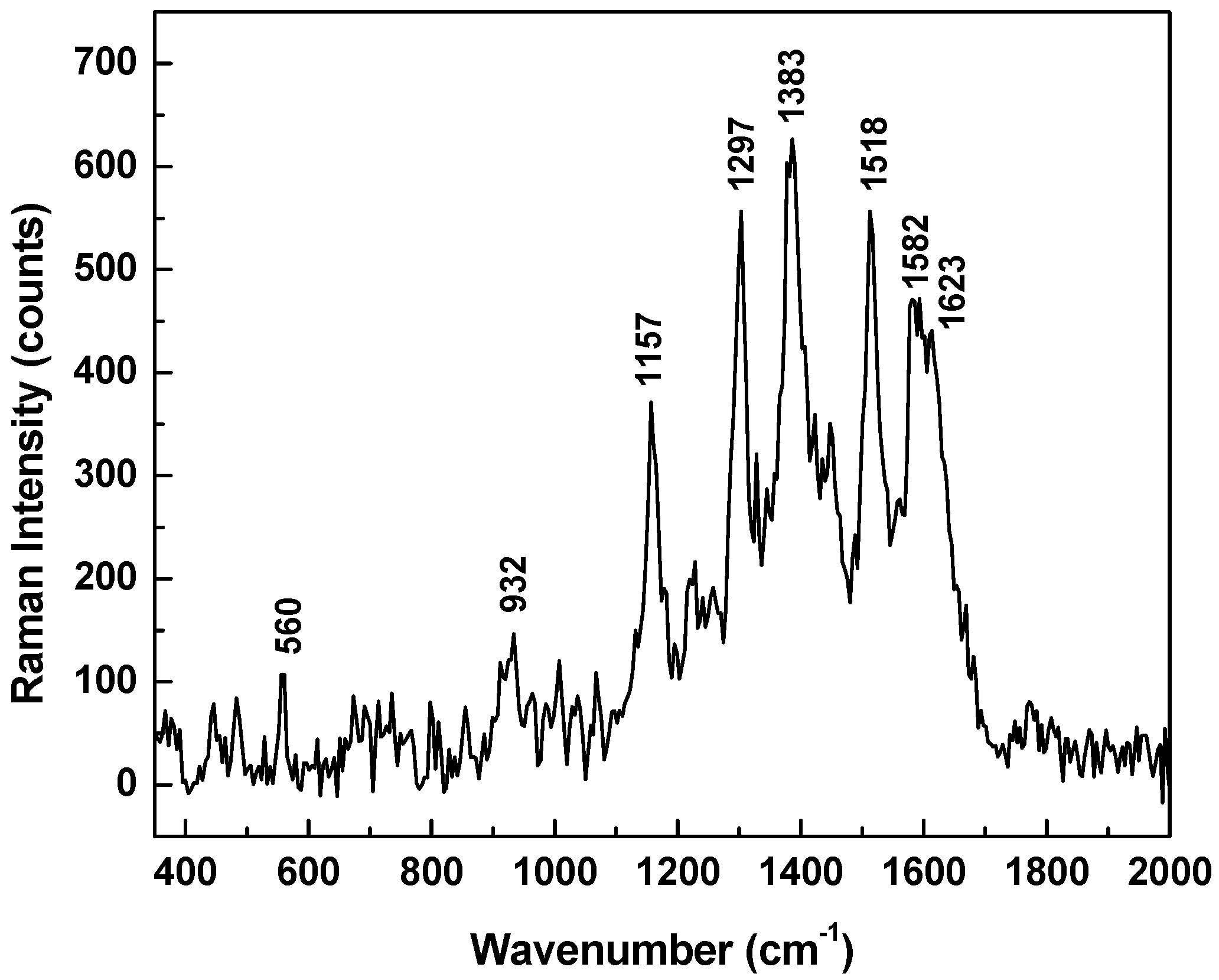
3. Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fleischmann, M.; Hendra, P.J.J.; McQuillan, A.J.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Dieringer, J.A.; Mcfarland, A.D.; Shah, N.C.; Stuart, D.A.; Whitney, A.V.; Yonzon, C.R.; Young, M.A.; Zhang, X.; Van Duyne, R.P. Introductory Lecture Surface enhanced Raman spectroscopy: New materials, concepts, characterization tools, and applications. Faraday Discuss. 2006, 132, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Virga, A.; Rivolo, P.; Frascella, F.; Angelini, A.; Descrovi, E.; Geobaldo, F.; Giorgis, F. Silver Nanoparticles on Porous Silicon: Approaching Single Molecule Detection in Resonant SERS Regime. J. Phys. Chem. C 2013, 117, 20139–20145. [Google Scholar] [CrossRef]
- Otto, A. The ‘chemical’ (electronic) contribution to surface-enhanced Raman scattering. J. Raman Spectrosc. 2005, 36, 497–509. [Google Scholar] [CrossRef]
- Qian, X.-M.; Nie, S.M. Single-molecule and single-nanoparticle SERS: From fundamental mechanisms to biomedical applications. Chem. Soc. Rev. 2008, 37, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Stranahan, S.M.; Willets, K.A. Super-resolution optical imaging of single-molecule SERS hot spots. Nano Lett. 2010, 10, 3777–3784. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Bennet, K.E.; Tomshine, J.R.; Hara, S.; Ciubuc, J.D.; Schmidt, U.; Durrer, W.G.; McIntosh, M.B.; Eastman, M.; Manciu, F.S. Ultrasensitive Detection of Neurotransmitters by Surface Enhanced Raman Spectroscopy for Biosensing Applications. Bionterface Res. Appl. Chem. 2017, 1, 1921–1926. [Google Scholar]
- Le Ru, E.C.; Meyer, M.; Etchegoin, P.G. Proof of single-molecule sensitivity in Surface Enhanced Raman Scattering (SERS) by means of a two-analyte technique. J. Phys. Chem. B 2006, 110, 1944–1948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Neumeyer, J.L.; Baldessarini, R.J. Recent progress in development of dopamine receptor subtype-selective agents: Potential therapeutics for neurological and psychiatric disorders. Chem. Rev. 2007, 107, 274–302. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente-Fernández, R.; Ruth, T.J.; Sossi, V.; Schulzer, M.; Calne, D.B.; Stoessl, A.J. Expectation and dopamine release: Mechanism of the placebo effect in Parkinson’s disease. Science 2001, 293, 1164–1166. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.J.; Hendry, J.; Dennany, L. Whole Blood Electrochemiluminescent Detection of Dopamine. Anal. Chem. 2015, 87, 11847–11853. [Google Scholar] [CrossRef] [PubMed]
- Meister, B. Neurotransmitters in key neurons of the hypothalamus that regulate feeding behavior and body weight. Physiol. Behav. 2007, 92, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Bennet, K.E.; Tomshine, J.R.; Min, H.-K.; Manciu, F.S.; Marsh, M.P.; Paek, S.B.; Settell, M.L.; Nicolai, E.N.; Blaha, C.D.; Kouzani, A.Z.; et al. A Diamond-Based Electrode for Detection of Neurochemicals in the Human Brain. Front. Hum. Neurosci. 2016, 10, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Yu, Z. Electrochemical biosensor for sensitively simultaneous determination of dopamine, uric acid, guanine, and adenosine based on poly-melamine and nano Ag hybridized film-modified electrode. J. Solid State Electrochem. 2014, 18, 105–113. [Google Scholar] [CrossRef]
- Heidbreder, C.A.; Lacroix, L.; Atkins, A.R.; Organ, A.J.; Murray, S.; West, A.; Shah, A.J. Development and application of a sensitive high performance ion-exchange chromatography method for the simultaneous measurement of dopamine, 5-hydroxytryptamine and norepinephrine in microdialysates from the rat brain. J. Neurosci. Methods 2001, 112, 135–144. [Google Scholar] [CrossRef]
- Fourati, N.; Seydou, M.; Zerrouki, C.; Singh, A.; Samanta, S.; Maurel, F.; Aswal, D.K.; Chehimi, M. Ultrasensitive and Selective Detection of Dopamine Using Cobalt-Phthalocyanine Nanopillar-Based Surface Acoustic Wave Sensor. ACS Appl. Mater. Interfaces 2014, 6, 22378–22386. [Google Scholar] [CrossRef] [PubMed]
- Maouche, N.; Ktari, N.; Bakas, I.; Fourati, N.; Zerrouki, C.; Seydou, M.; Maurel, F.; Chehimi, M.M. A surface acoustic wave sensor functionalized with a polypyrrole molecularly imprinted polymer for selective dopamine detection. J. Mol. Recognit. 2015, 28, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Meulemans, A.; Poulain, B.; Baux, G.; Tauc, L.; Henzel, D. Micro carbon electrode for intracellular voltammetry. Anal. Chem. 1986, 58, 2088–2091. [Google Scholar] [CrossRef]
- Pande, S.; Jana, S.; Sinha, A.K.; Sarkar, S.; Basu, M.; Pradhan, M.; Pal, A.; Chowdhury, J.; Pal, T. Dopamine molecules on Aucore-Agshell bimetallic nanocolloids: Fourier transform infrared, Raman, and surface-enhanced Raman spectroscopy study aided by density functional theory. J. Phys. Chem. C 2009, 113, 6989–7002. [Google Scholar] [CrossRef]
- Palanisamy, S.; Yan, L.; Zhang, X.; He, T. Surface enhanced Raman scattering-active worm-like Ag clusters for sensitive and selective detection of dopamine. Anal. Methods 2015, 7, 3438–3447. [Google Scholar] [CrossRef]
- Lee, N.-S.; Hsieh, Y.-Z.; Paisley, R.F.; Morris, M.D. Surface-Enhanced Raman Spectroscopy of the Catecholamine Neurotransmitters and Related Compounds. Anal. Chem. 1988, 60, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Menshykau, D.; Streeter, I.; Compton, R.G. Influence of Electrode Roughness on Cyclic Voltammetry. J. Phys. Chem. C 2008, 112, 14428–14438. [Google Scholar] [CrossRef]
- Wan, Y.; Guo, Z.; Jiang, X.; Fang, K.; Lu, X.; Zhang, Y.; Gu, N. Quasi-spherical silver nanoparticles: Aqueous synthesis and size control by the seed-mediated Lee-Meisel method. J. Colloid Interface Sci. 2013, 394, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Krishnakumar, V.; Keresztury, G.; Sundius, T.; Ramasamy, R. Simulation of IR and Raman spectra based on scaled DFT force fields: A case study of 2-(methylthio)benzonitrile, with emphasis on band assignment. J. Mol. Struct. 2004, 702, 9–21. [Google Scholar] [CrossRef]
- Polavarapu, P.L. Ab initio vibrational Raman and Raman optical activity spectra. J. Phys. Chem. 1990, 94, 8106–8112. [Google Scholar] [CrossRef]
- Keresztury, G. Handbook of Vibrational Spectroscopy; John Wiley & Sons, Ltd.: Chichester, UK, 2001; Volume 1. [Google Scholar]
- Pulay, P. Applications of Electronic Structure Theory; Plenum: New York, NY, USA, 1997; Volume 4. [Google Scholar]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Academic Press: San Diego, CA, USA, 1991. [Google Scholar]
- Lambert, J.B.; Shurvell, H.F.; Cooks, R.G. Introduction to Organic Spectroscopy; Collier Macmillan Cop.: New York, NY, USA, 1987; pp. 174–177. [Google Scholar]
- Mohammad-Shiri, H.; Ghaemi, M.; Riahi, S.; Akbari-Sehat, A. Computational and Electrochemical Studies on the Redox Reaction of Dopamine in Aqueous Solution. Int. J. Electrochem. Sci. 2011, 6, 317–336. [Google Scholar]
- Ciubuc, J.D.; Qiu, C.; Bennet, K.E.; Alonzo, M.; Durrer, W.G.; Manciu, F.S. Raman computational and experimental studies of dopamine molecules on silver nanocolloids. In Proceedings of the 2017 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rochester, MN, USA, 7–10 May 2017; pp. 153–158. [Google Scholar] [CrossRef]
- Yu, F.; Chen, S.; Chen, Y.; Li, H.; Yang, L.; Chen, Y.; Yin, Y. Experimental and theoretical analysis of polymerization reaction process on the polydopamine membranes and its corrosion protection properties for 304 Stainless Steel. J. Mol. Struct. 2010, 982, 152–161. [Google Scholar] [CrossRef]

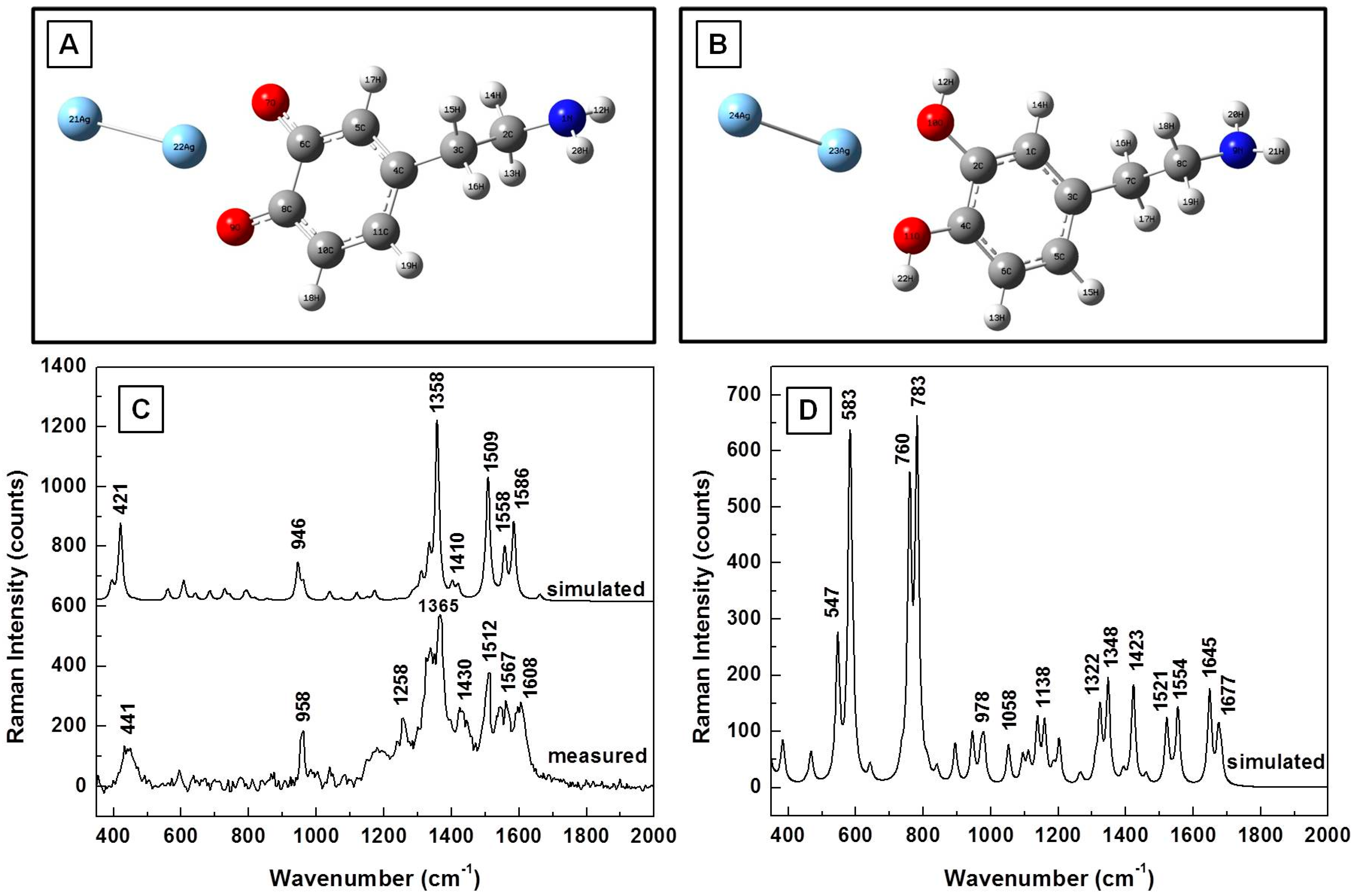
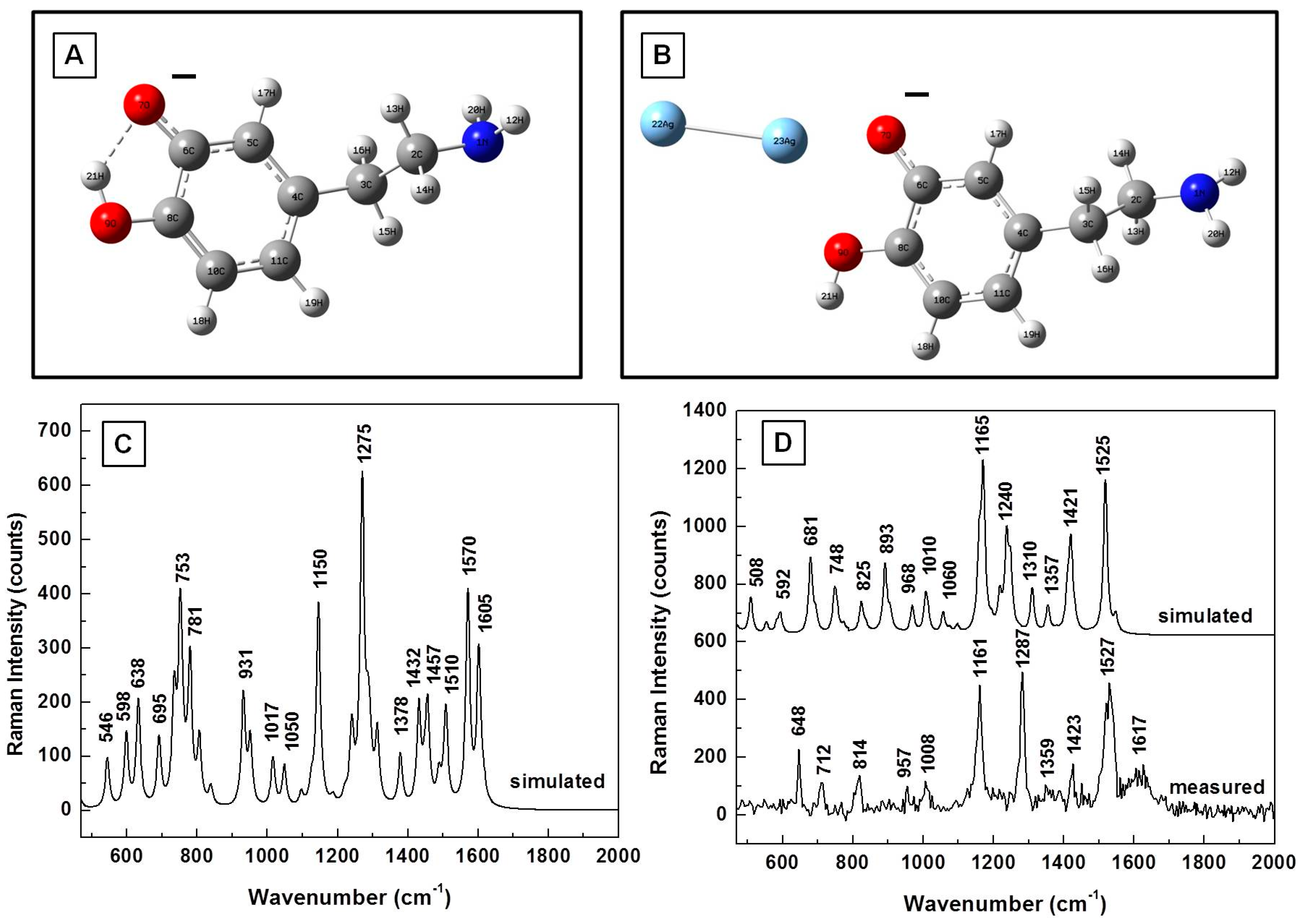
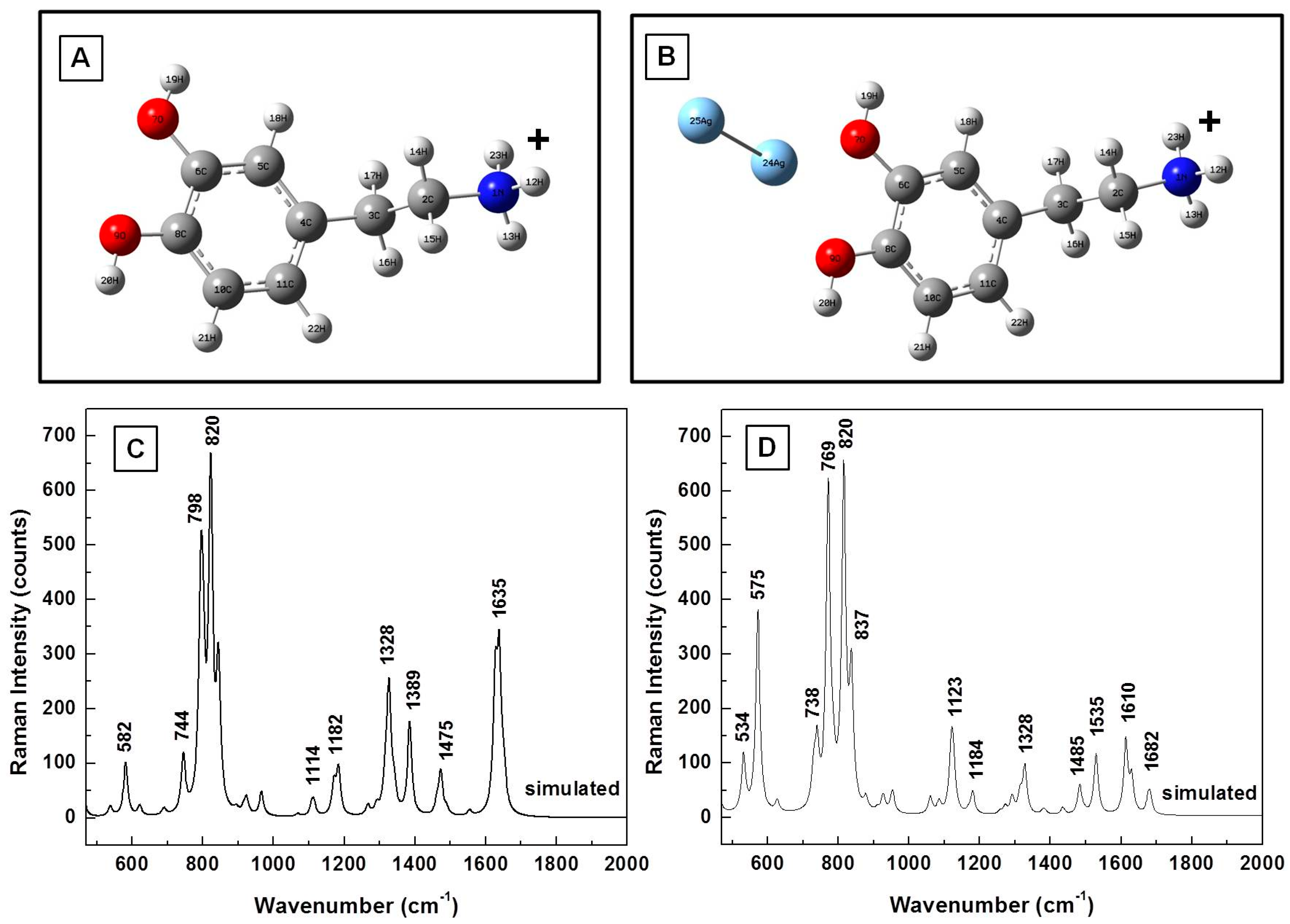
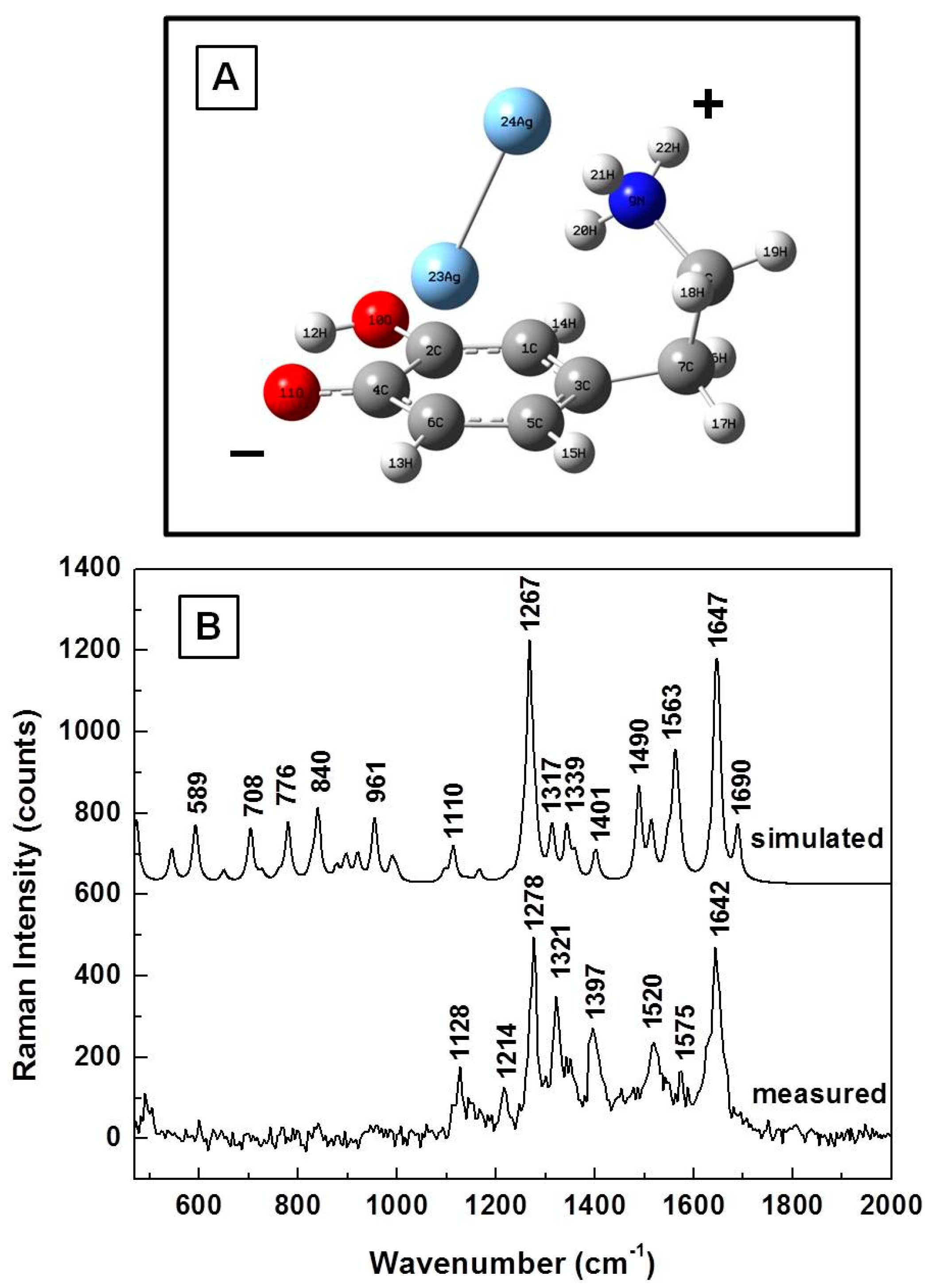
| Calculated (cm−1) | Measured (cm−1) | Assignment |
|---|---|---|
| 381 | 393 | CH wagging, ring deformation |
| 577 | 593 | CH in-plane ring deformation |
| 622 | 635 | CH wagging; aliphatic chain C–C vibrations |
| 744 | 750 | CH out-of-plane; ring deformation (two band response) |
| 782 | 795 | CH out-of-plane; ring deformation (two band response) |
| 934 | 931 | NH twisting |
| 961 | 962 | NH twisting; CH wagging; ring deformation |
| 1029 | CH wagging | |
| 1060 | C–C–N stretching; CH wagging | |
| 1123 | 1117 | CH twisting; NH twisting; CN stretching |
| 1156 | 1148 | OH rocking; CH aromatic rocking; weak ring breathing; CH wagging |
| 1209 | CO stretching | |
| 1294 | 1285 | Ring breathing; CH aromatic rocking; CH twisting |
| 1321 | Ring breathing; CH aromatic in-plane rocking; CH twisting | |
| 1355 | Ring deformation; OH scissoring; CH twisting | |
| 1387 | CH wagging; NH twisting | |
| 1466 | 1455 | CH scissoring |
| 1615 | 1617 | Ring deformation, OH scissoring |
| 1631 | NH2 scissoring | |
| 1634 | Benzene ring deformation |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciubuc, J.D.; Bennet, K.E.; Qiu, C.; Alonzo, M.; Durrer, W.G.; Manciu, F.S. Raman Computational and Experimental Studies of Dopamine Detection. Biosensors 2017, 7, 43. https://doi.org/10.3390/bios7040043
Ciubuc JD, Bennet KE, Qiu C, Alonzo M, Durrer WG, Manciu FS. Raman Computational and Experimental Studies of Dopamine Detection. Biosensors. 2017; 7(4):43. https://doi.org/10.3390/bios7040043
Chicago/Turabian StyleCiubuc, John D., Kevin E. Bennet, Chao Qiu, Matthew Alonzo, William G. Durrer, and Felicia S. Manciu. 2017. "Raman Computational and Experimental Studies of Dopamine Detection" Biosensors 7, no. 4: 43. https://doi.org/10.3390/bios7040043




