Regenerative, Highly-Sensitive, Non-Enzymatic Dopamine Sensor and Impact of Different Buffer Systems in Dopamine Sensing
Abstract
:1. Introduction
2. Materials and Methods
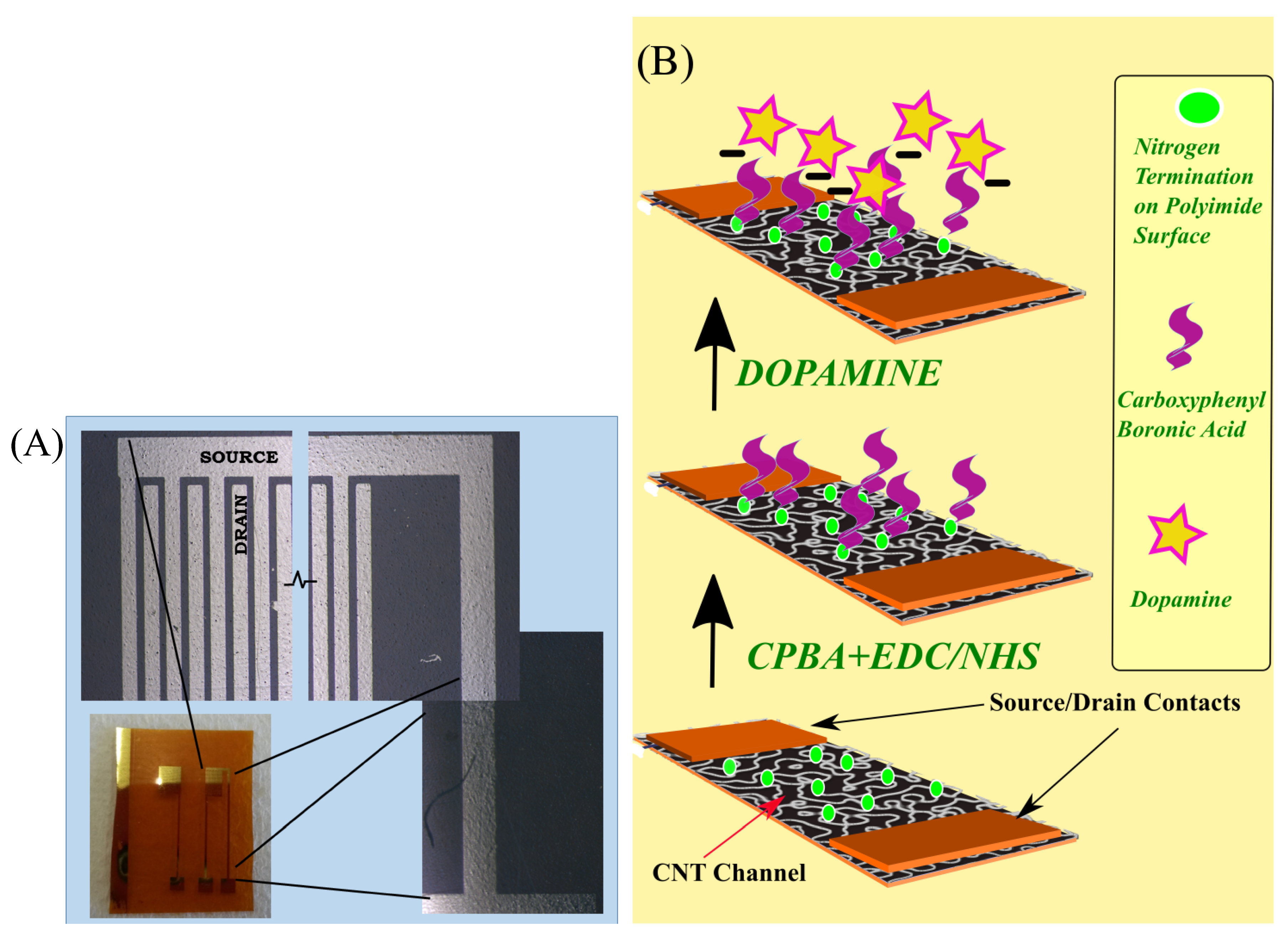
2.1. Fabrication of Flexible CNTFET
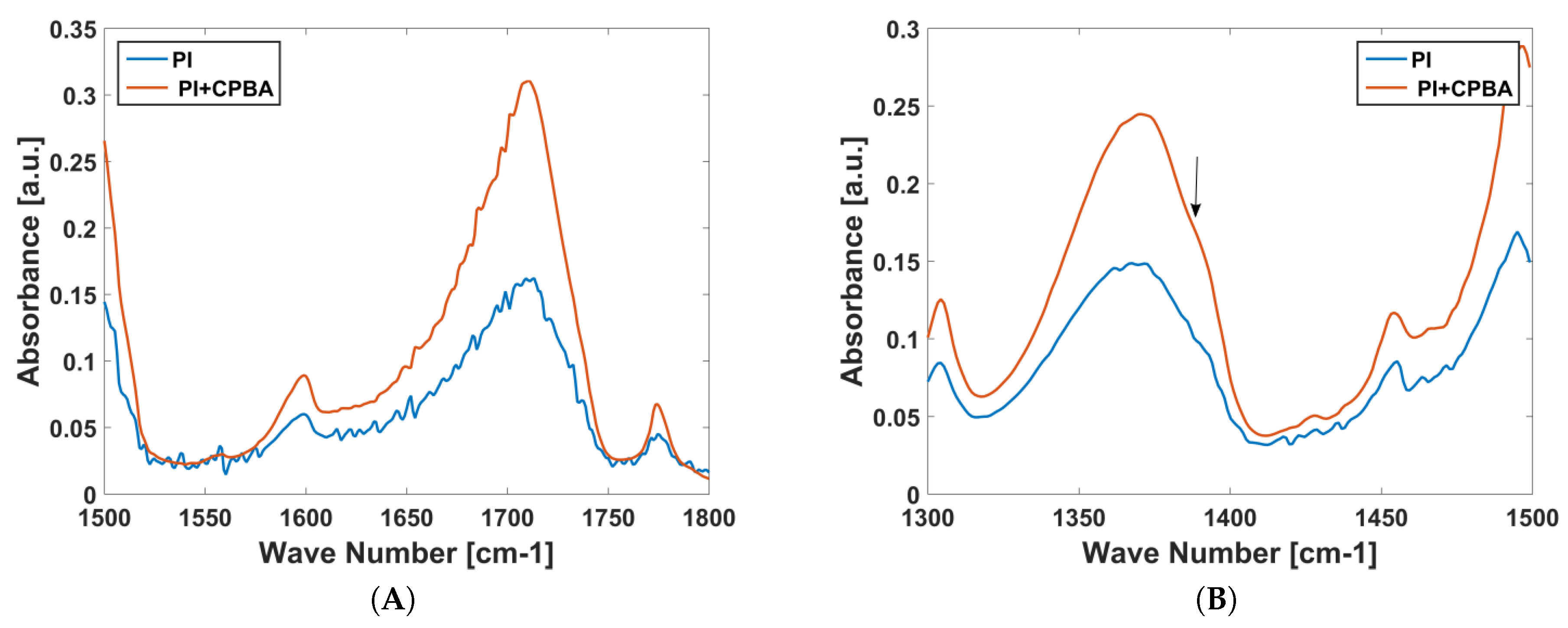
2.2. Functionalization of the CNTFETs for Dopamine Sensing
2.3. CNTFET Electrical Characterization
3. Results and Discussion
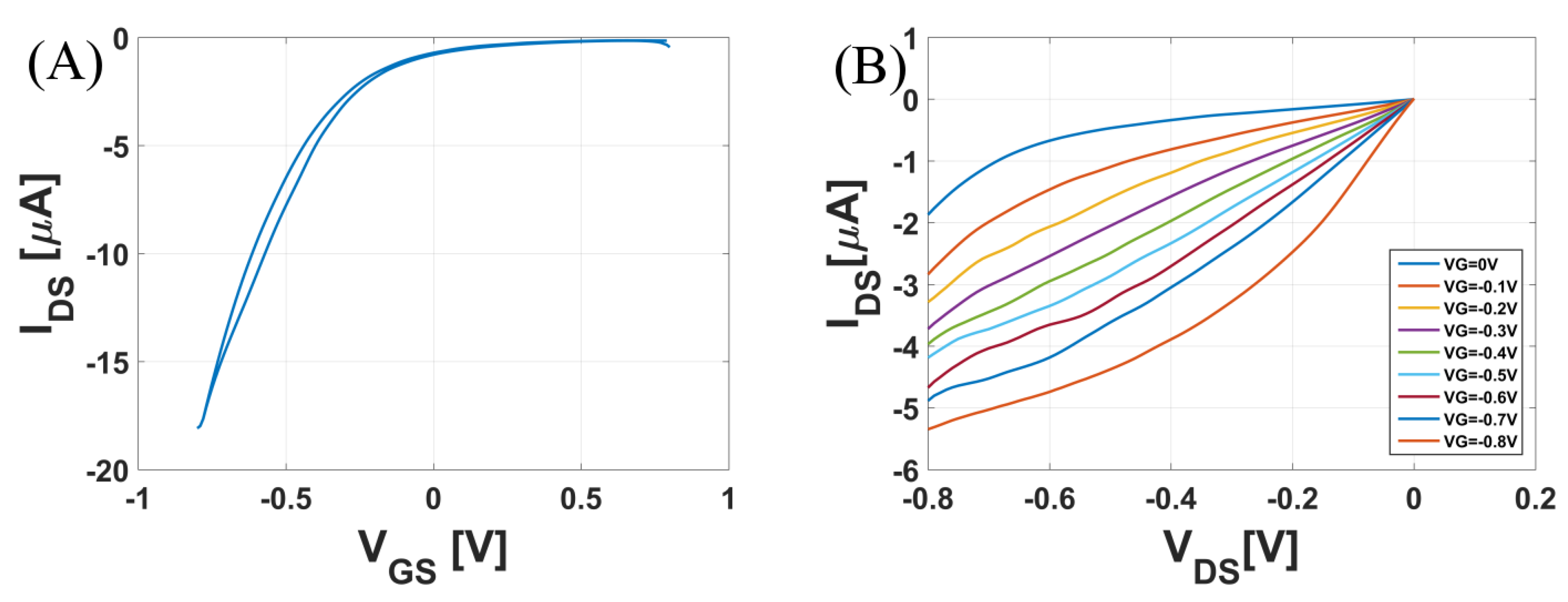
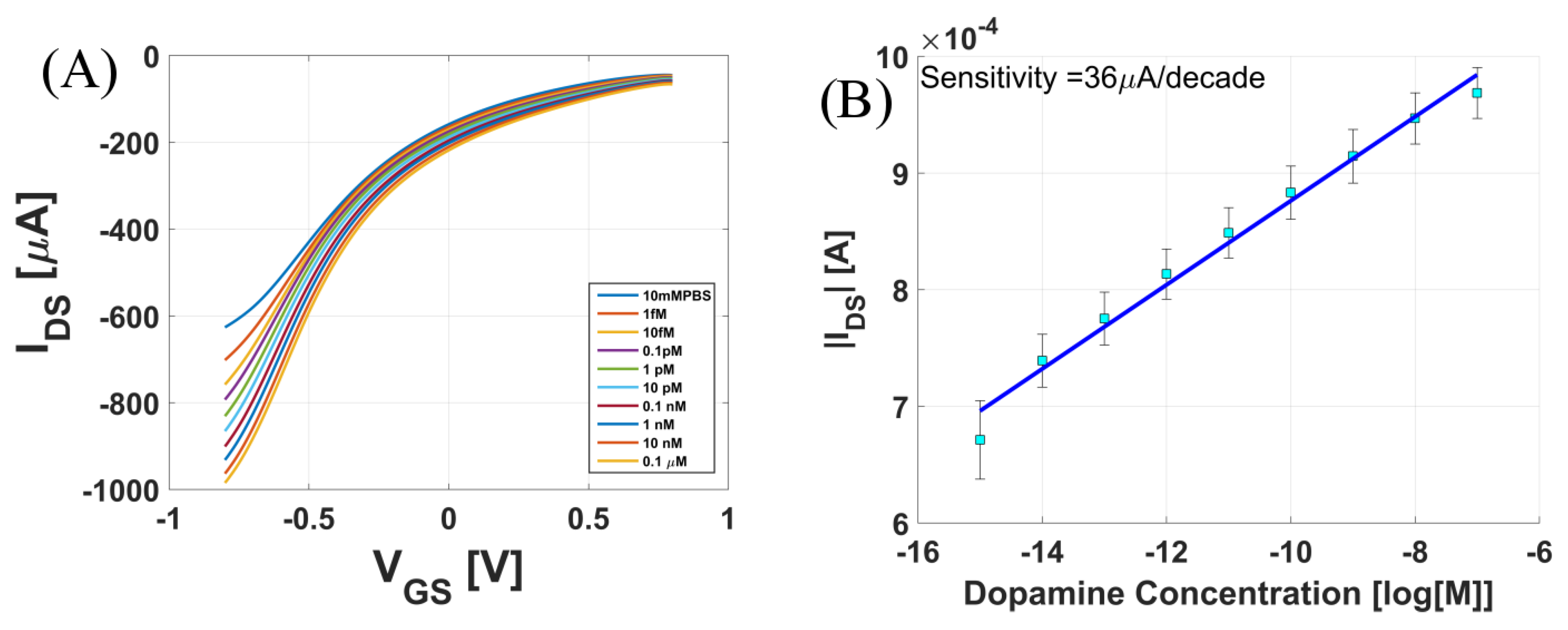
3.1. A Dopamine Sensor: Measured in Phosphate-Buffered Saline (PBS) Solution
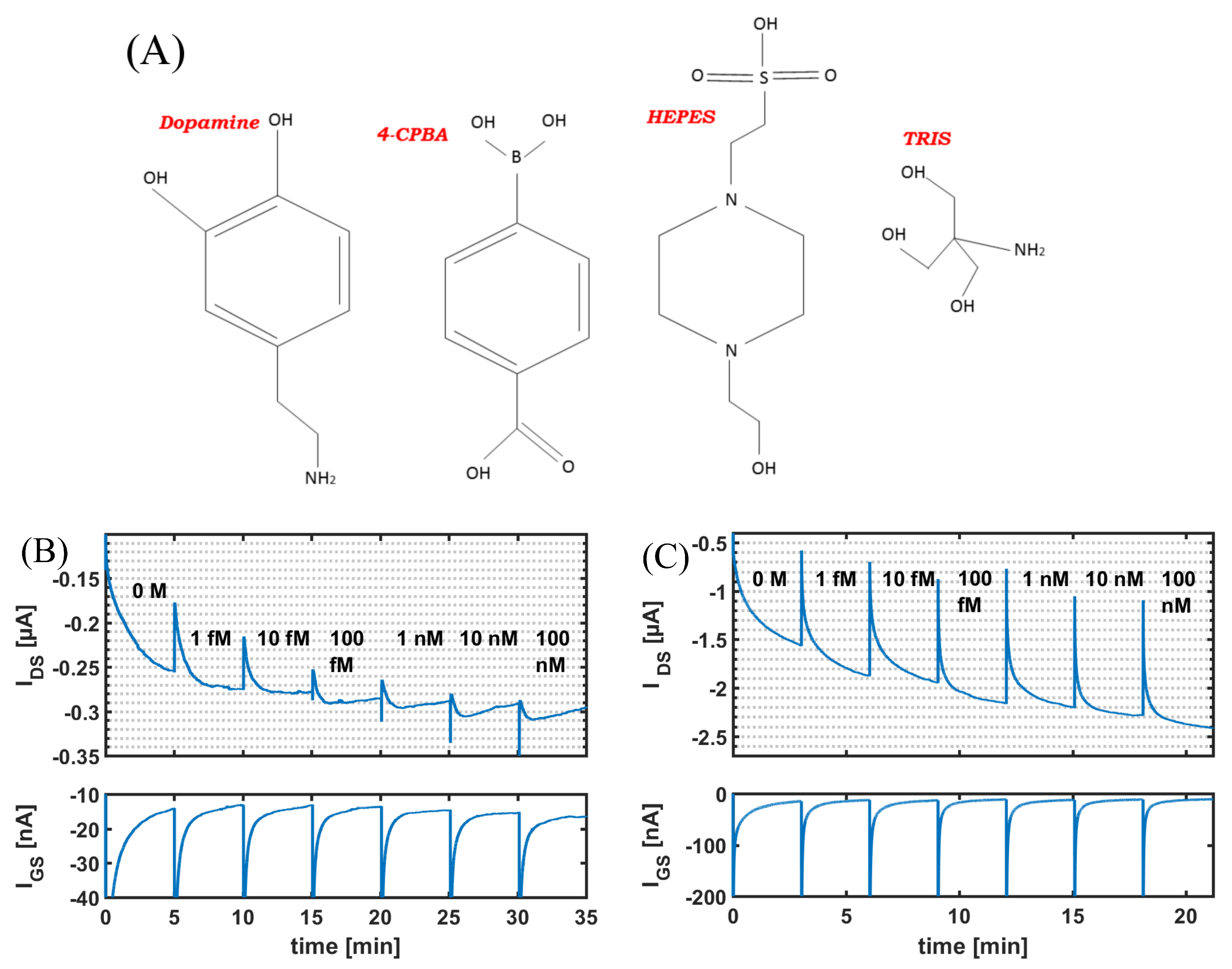
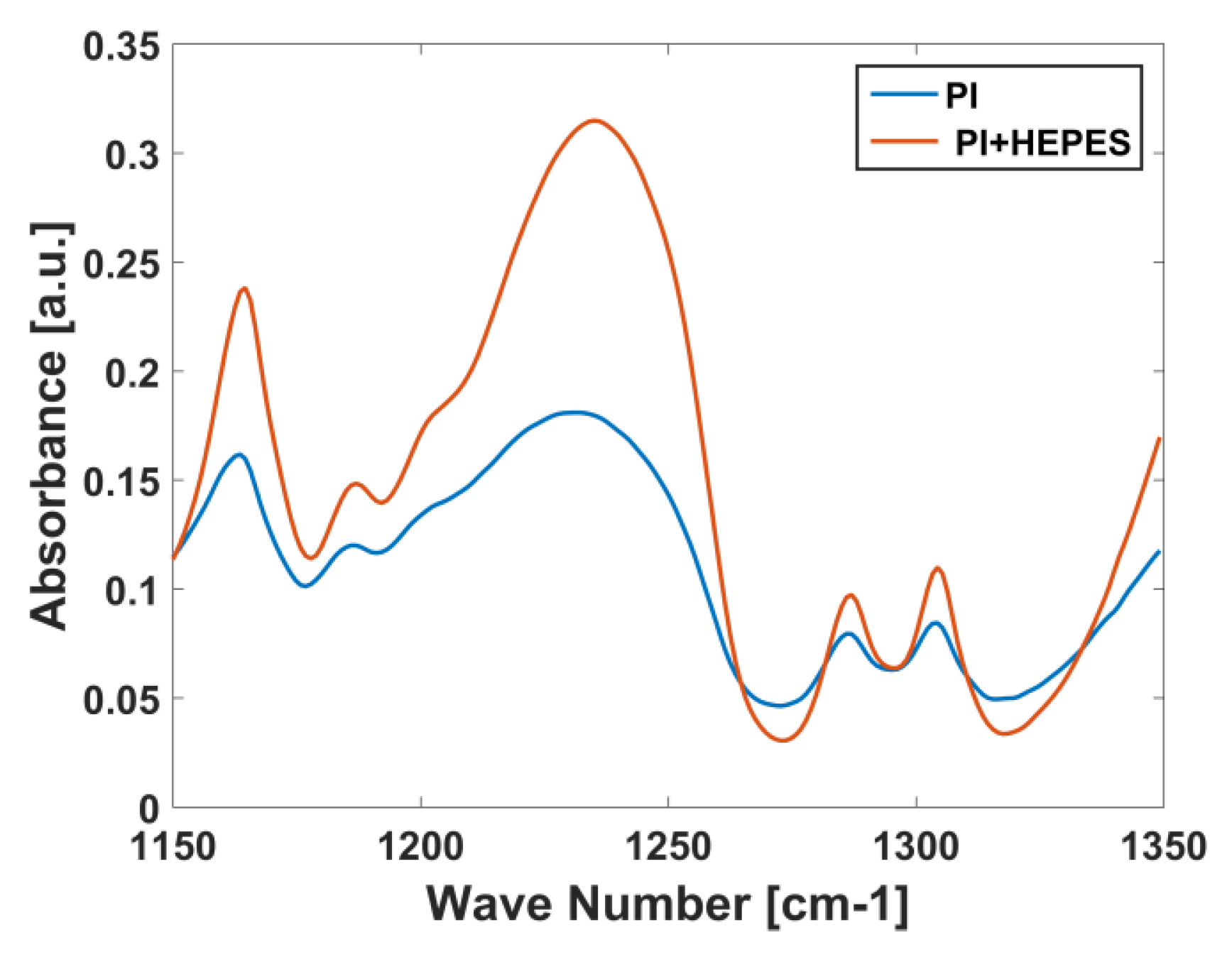
3.2. Comparison with Other Buffers
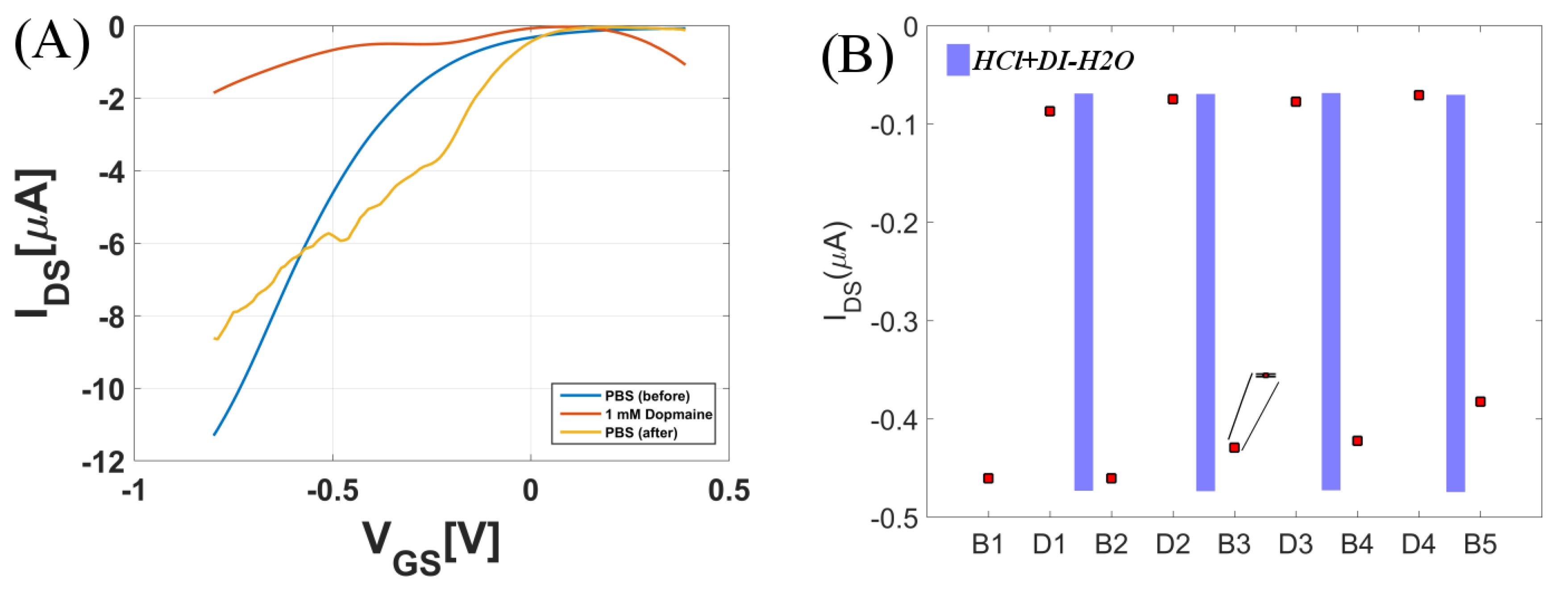
3.3. Regeneration Using Acidic Solution
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Park, S.J.; Yang, H.; Lee, S.H.; Song, H.S.; Park, C.S.; Bae, J.; Kwon, O.S.; Park, T.H.; Jang, J. Dopamine Receptor D1 Agonism and Antagonism Using a Field-Effect Transistor Assay. ACS Nano 2017, 11, 5950–5959. [Google Scholar] [CrossRef] [PubMed]
- Seeman, P. Brain dopamine receptors. Pharmacol. Rev. 1980, 32, 229–313. [Google Scholar] [PubMed]
- Clark, D.; White, F.J. Review: D1 Dopamine Receptor-The Search for a Function: A Critical Evaluation of the DUD2 Dopamine Receptor Classification and its Functional Implications. Synapse 1987, 1, 347–388. [Google Scholar] [CrossRef] [PubMed]
- Elsworth, J.D.; Roth, R.H. Dopamine Synthesis, Uptake, Metabolism, and Receptors: Relevance to Gene Therapy of Parkinson’s Disease. Exp. Neurol. 1997, 144, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Auerbach, J.M.; Rodríguez-Gómez, J.A.; Velasco, I.; Gavin, D.; Lumelsky, N.; Lee, S.H.; Nguyen, J.; Sánchez-Pernaute, R.; Bankiewicz, K.; et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature 2002, 418, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Hyman, B.T.; Hoesen, G.W.V.; Damasio, A.R.; Barnes, C.L. Alzheimer’s Disease: Cell-Specific Pathology Isolates the Hippocampal Formation Alzheimer’s Disease: Cell-Specific Pathology Isolates the Hippocampal Formation. Adv. Sci. 2008, 225, 1168–1170. [Google Scholar]
- Yoshitake, T.; Kehr, J.; Yoshitake, S.; Fujino, K.; Nohta, H.; Yamaguchi, M. Determination of serotonin, noradrenaline, dopamine and their metabolites in rat brain extracts and microdialysis samples by column liquid chromatography with fluorescence detection following derivatization with benzylamine and 1,2-diphenylethylenediamine. J. Chromatogr. B 2004, 807, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Flanigan, V.; Ma, Y. Simultaneous determination of polyamines and catecholamines in PC-12 tumor cell extracts by capillary electrophoresis with laser-induced fluorescence detection. Electrophoresis 2004, 25, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Hows, M.E.P.; Lacroix, L.; Heidbreder, C.; Organ, A.J.; Shah, A.J. High-performance liquid chromatography/ tandem mass spectrometric assay for the simultaneous measurement of dopamine, norepinephrine, 5-hydroxytryptamine and cocaine in biological samples. J. Neurosci. Methods 2004, 138, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Zhou, K.G.; Zhang, Y.H.; Wang, H.X.; Wang, X.D.; Guo, Y.F.; Zhang, H.L. Nanomolar detection of dopamine in the presence of ascorbic acid at β-cyclodextrin/graphene nanocomposite platform. Electrochem. Commun. 2010, 12, 557–560. [Google Scholar] [CrossRef]
- Casalini, S.; Leonardi, F.; Cramer, T.; Biscarini, F. Organic field-effect transistor for label-free dopamine sensing. Org. Electron. Phys. Mater. Appl. 2013, 14, 156–163. [Google Scholar] [CrossRef]
- Freeman, R.; Elbaz, J.; Gill, R.; Zayats, M.; Willner, I. Analysis of dopamine and tyrosinase activity on Ion-Sensitive Field-Effect Transistor (ISFET) devices. Chem. A Eur. J. 2007, 13, 7288–7293. [Google Scholar] [CrossRef] [PubMed]
- Someya, T.; Dodabalapur, A.; Huang, J.; See, K.C.; Katz, H.E. Chemical and physical sensing by organic field-effect transistors and related devices. Adv. Mater. 2010, 22, 3799–3811. [Google Scholar] [CrossRef] [PubMed]
- Bernards, D.A.; Macaya, D.J.; Nikolou, M.; DeFranco, J.A.; Takamatsu, S.; Malliaras, G.G. Enzymatic sensing with organic electrochemical transistors. J. Mater. Chem. 2008, 18, 116–120. [Google Scholar] [CrossRef]
- Biosensors, G.L.F.; Sarkar, D.; Liu, W.; Xie, X.; Anselmo, A.C.; Mitragotri, S.; Banerjee, K. MoS2 Field-Effect Transistor for Next-Generation Label-Free Biosensors. ACS Nano 2014, 8, 3992–4003. [Google Scholar]
- Qian, T.; Yu, C.; Zhou, X.; Ma, P.; Wu, S.; Xu, L.; Shen, J. Ultrasensitive dopamine sensor based on novel molecularly imprinted polypyrrole coated carbon nanotubes. Biosens. Bioelectron. 2014, 58, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Muguruma, H.; Hoshino, T.; Nowaki, K. Electronically type-sorted carbon nanotube-based electrochemical biosensors with glucose oxidase and dehydrogenase. ACS Appl. Mater. Interfaces 2015, 7, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, V.D.; Joshi, S.; Melzer, K.; Lugli, P. Flexible dopamine sensor based on electrolyte gated carbon nanotube field effect transistor. In Proceedings of the 2016 IEEE Biomedical Circuits and Systems Conference (BioCAS), Shanghai, China, 17–19 October 2017; Volume 1, pp. 38–41. [Google Scholar]
- Chung, R.J.; Wang, A.N.; Liao, Q.L.; Chuang, K.Y. Non-Enzymatic Glucose Sensor Composed of Carbon-Coated Nano-Zinc Oxide. Nanomaterials 2017, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Song, H.S.; Kwon, O.S.; Chung, J.H.; Lee, S.H.; An, J.H.; Ahn, S.R.; Lee, J.E.; Yoon, H.; Park, T.H.; et al. Human dopamine receptor nanovesicles for gate-potential modulators in high-performance field-effect transistor biosensors. Sci. Rep. 2014, 4, 4342. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Oh, J.; Kim, S.G.; Jang, J. Highly sensitive and selective field-effect-transistor nonenzyme dopamine sensors based on Pt/conducting polymer hybrid nanoparticles. Small 2015, 11, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liao, C.; Yao, Y.; Liu, Z.; Gong, F.; Yan, F. High-performance dopamine sensors based on whole-graphene solution-gated transistors. Adv. Funct. Mater. 2014, 24, 978–985. [Google Scholar] [CrossRef]
- Lloret, N.; Frederiksen, R.S.; Møller, T.C.; Rieben, N.I.; Upadhyay, S.; De Vico, L.; Jensen, J.H.; Nygård, J.; Martinez, K.L. Effects of buffer composition and dilution on nanowire field-effect biosensors. Nanotechnology 2013, 24, 035501. [Google Scholar] [CrossRef] [PubMed]
- Stern, E.; Wagner, R.; Sigworth, F.J.; Breaker, R.; Fahmy, T.M.; Reed, M.A. Importance of the debye screening length on nanowire field effect transistor sensors. Nano Lett. 2007, 7, 3405–3409. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, A.; Wipf, M.; Stoop, R.L.; Bedner, K.; Fu, W.; Guzenko, V.A.; Knopfmacher, O.; Calame, M.; Schönenberger, C. Understanding the electrolyte background for biochemical sensing with ion-sensitive field-effect transistors. ACS Nano 2012, 6, 9291–9298. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, F.N.; Chang, H.K.; Curreli, M.; Liao, H.I.; Olson, C.A.; Chen, P.C.; Zhang, R.; Roberts, R.W.; Sun, R.; Cote, R.J.; et al. Label-free, electrical detection of the SARS virus n-protein with nanowire biosensors utilizing antibody mimics as capture probes. ACS Nano 2009, 3, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Bjellqvist, B.; Ek, K.; Righetti, P.G.; Gianazza, E.; Görg, A.; Westermeier, R.; Postel, W. Isoelectric focusing in immobilized pH gradients: Principle, methodology and some applications. J. Biochem. Biophys. Methods 1982, 6, 317–339. [Google Scholar] [CrossRef]
- Goode, J.A.; Rushworth, J.V.; Millner, P.A. Biosensor Regeneration: A Review of Common Techniques and Outcomes. Langmuir 2015, 31, 6267–6276. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Bhatt, V.D.; Wu, H.; Becherer, M.; Lugli, P. Flexible Lactate and Glucose Sensors using Electrolyte-Gated Carbon Nanotube Field Effect Transistor for Non-invasive Real-time Monitoring. IEEE Sens. J. 2017, 17, 4315–4321. [Google Scholar] [CrossRef]
- Bhatt, V.; Joshi, S.; Becherer, M.; Lugli, P. Flexible, Low-Cost Sensor Based on Electrolyte Gated Carbon Nanotube Field Effect Transistor for Organo-Phosphate Detection. Sensors 2017, 17, 1147. [Google Scholar] [CrossRef] [PubMed]
- Li, D.C.; Yang, P.H.; Lu, M.S.C. CMOS open-gate ion-sensitive field-effect transistors for ultrasensitive dopamine detection. IEEE Trans. Electron Dev. 2010, 57, 2761–2767. [Google Scholar] [CrossRef]
- Li, B.R.; Chen, C.W.; Yang, W.L.; Lin, T.Y.; Pan, C.Y.; Chen, Y.T. Biomolecular recognition with a sensitivity-enhanced nanowire transistor biosensor. Biosens. Bioelectron. 2013, 45, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Hsiao, C.Y.; Hung, C.H.; Lo, Y.R.; Lee, C.C.; Su, C.J.; Lin, H.C.; Ko, F.H.; Huang, T.Y.; Yang, Y.S. Ultrasensitive detection of dopamine using a polysilicon nanowire field-effect transistor. Chem. Commun. 2008, 5749–5751. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, H.; Asghar, W.; Inci, F.; Yuksekkaya, M.; Jahangir, M.; Zhang, M.H.; Durmus, N.G.; Gurkan, U.A.; Kuritzkes, D.R.; Demirci, U. Paper and flexible substrates as materials for biosensing platforms to detect multiple biotargets. Sci. Rep. 2015, 5, 8719. [Google Scholar] [CrossRef] [PubMed]
- Nik, O.G.; Chen, X.Y.; Kaliaguine, S. Amine-functionalized zeolite FAU/EMT-polyimide mixed matrix membranes for CO2/CH4 separation. J. Membr. Sci. 2011, 379, 468–478. [Google Scholar] [CrossRef]
- Faniran, J.A.; Shurvell, H.F. Infrared spectra of phenylboronic acid (normal and deuterated) and diphenyl phenylboronate. Can. J. Chem. 1968, 46, 2089–2095. [Google Scholar] [CrossRef]
- Vehlow, D.; Schmidt, R.; Gebert, A.; Siebert, M.; Lips, K.; Müller, M. Polyelectrolyte Complex Based Interfacial Drug Delivery System with Controlled Loading and Improved Release Performance for Bone Therapeutics. Nanomaterials 2016, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xia, N.; Xing, Y.; Deng, D. Boronic acid-based electrochemical sensors for detection of biomolecules. Int. J. Electrochem. Sci. 2013, 8, 11161–11174. [Google Scholar]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, S.; Bhatt, V.D.; Märtl, A.; Becherer, M.; Lugli, P. Regenerative, Highly-Sensitive, Non-Enzymatic Dopamine Sensor and Impact of Different Buffer Systems in Dopamine Sensing. Biosensors 2018, 8, 9. https://doi.org/10.3390/bios8010009
Joshi S, Bhatt VD, Märtl A, Becherer M, Lugli P. Regenerative, Highly-Sensitive, Non-Enzymatic Dopamine Sensor and Impact of Different Buffer Systems in Dopamine Sensing. Biosensors. 2018; 8(1):9. https://doi.org/10.3390/bios8010009
Chicago/Turabian StyleJoshi, Saumya, Vijay Deep Bhatt, Andreas Märtl, Markus Becherer, and Paolo Lugli. 2018. "Regenerative, Highly-Sensitive, Non-Enzymatic Dopamine Sensor and Impact of Different Buffer Systems in Dopamine Sensing" Biosensors 8, no. 1: 9. https://doi.org/10.3390/bios8010009
APA StyleJoshi, S., Bhatt, V. D., Märtl, A., Becherer, M., & Lugli, P. (2018). Regenerative, Highly-Sensitive, Non-Enzymatic Dopamine Sensor and Impact of Different Buffer Systems in Dopamine Sensing. Biosensors, 8(1), 9. https://doi.org/10.3390/bios8010009




