In Situ Synthesis and Electrophoretic Deposition of NiO/Ni Core-Shell Nanoparticles and Its Application as Pseudocapacitor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of NiO Platelet Seeds and Metallic Ni (NPs)
2.2. Preparation of NiO/Ni Core/shell Nanoparticles
2.3. Electrochemical Tests
3. Results and Discusion
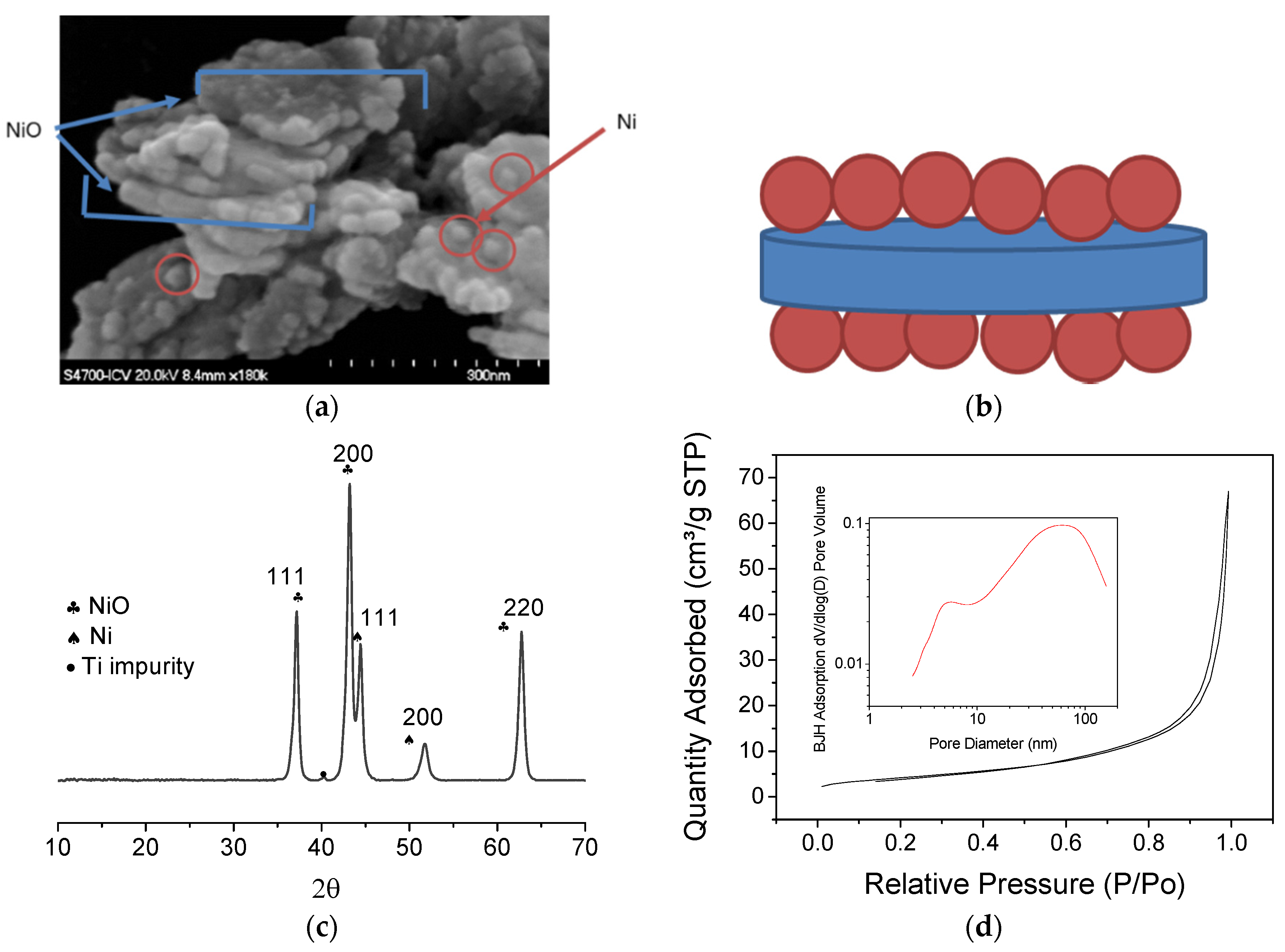
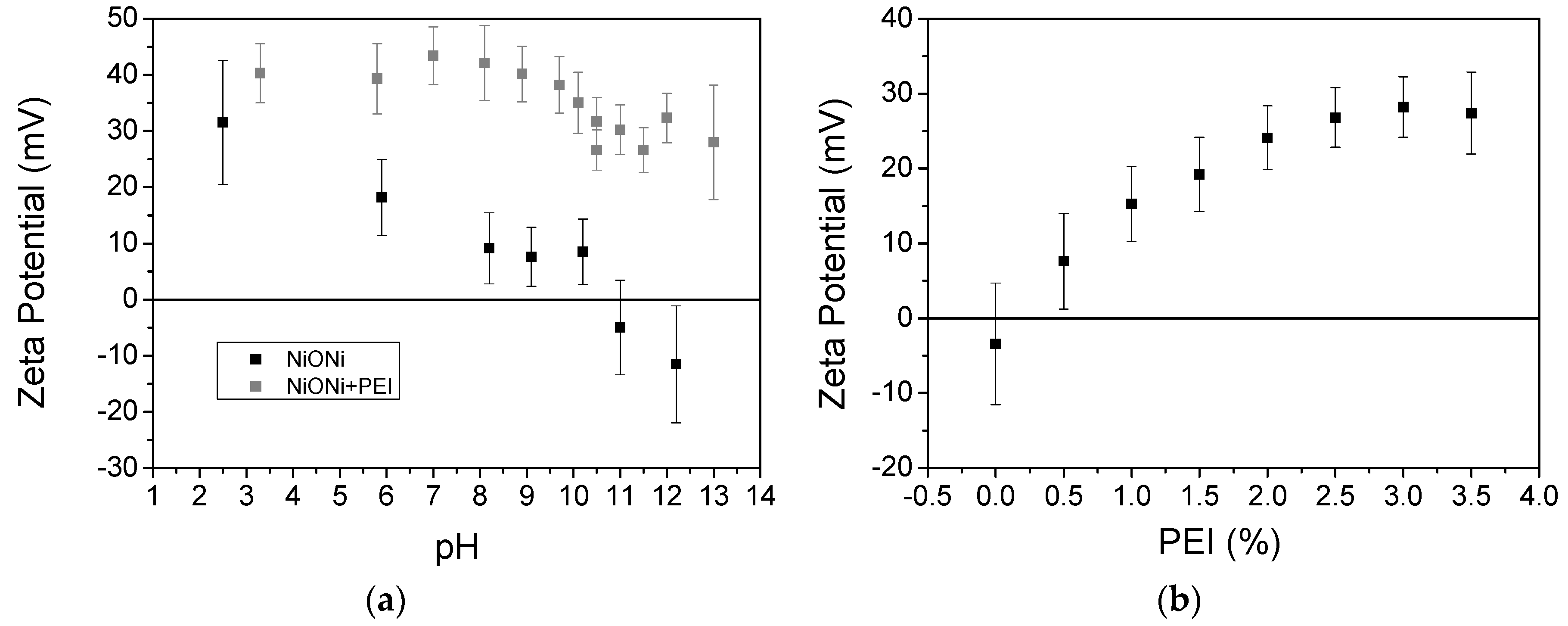
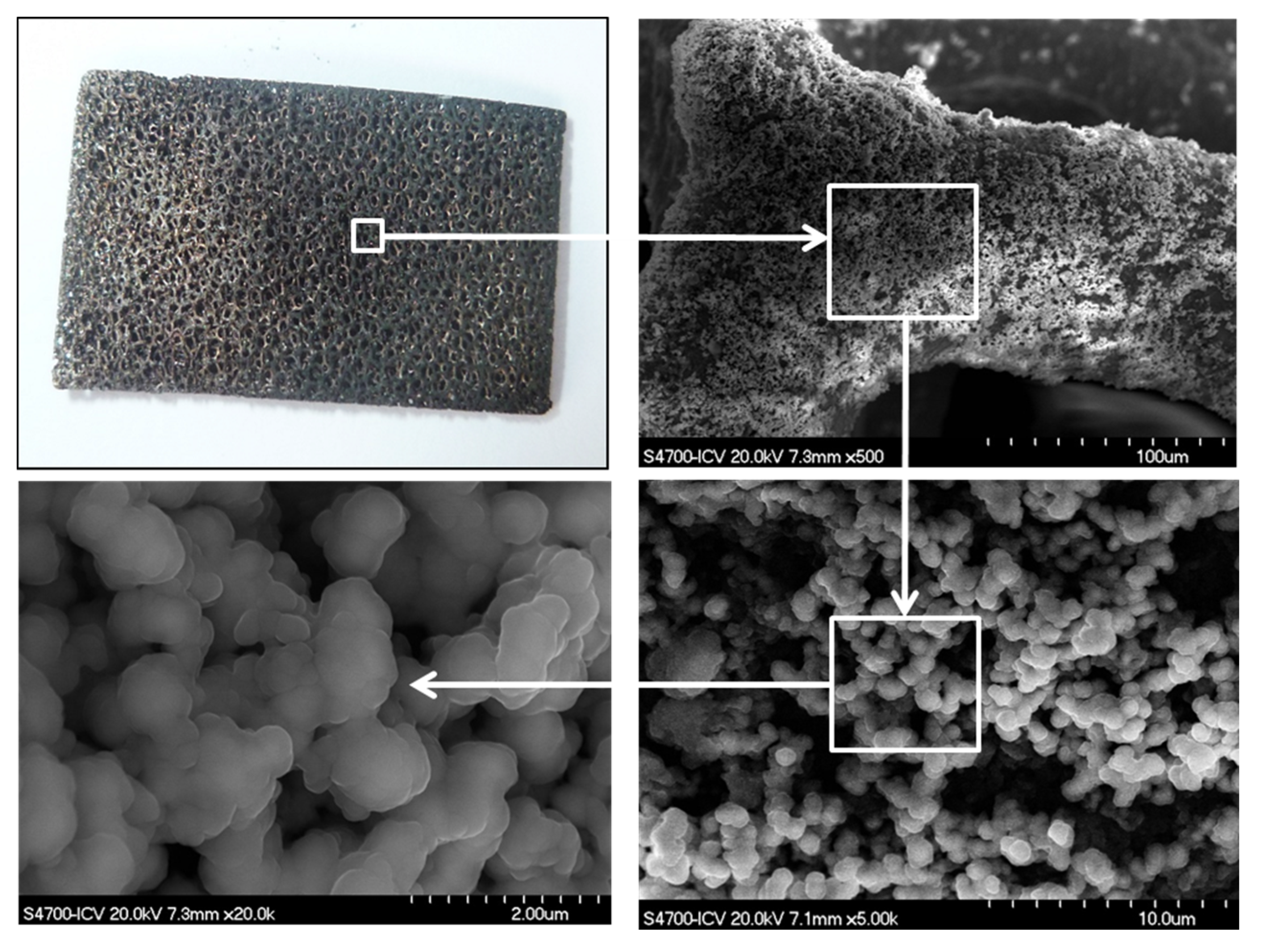
3.1. EPD Deposition
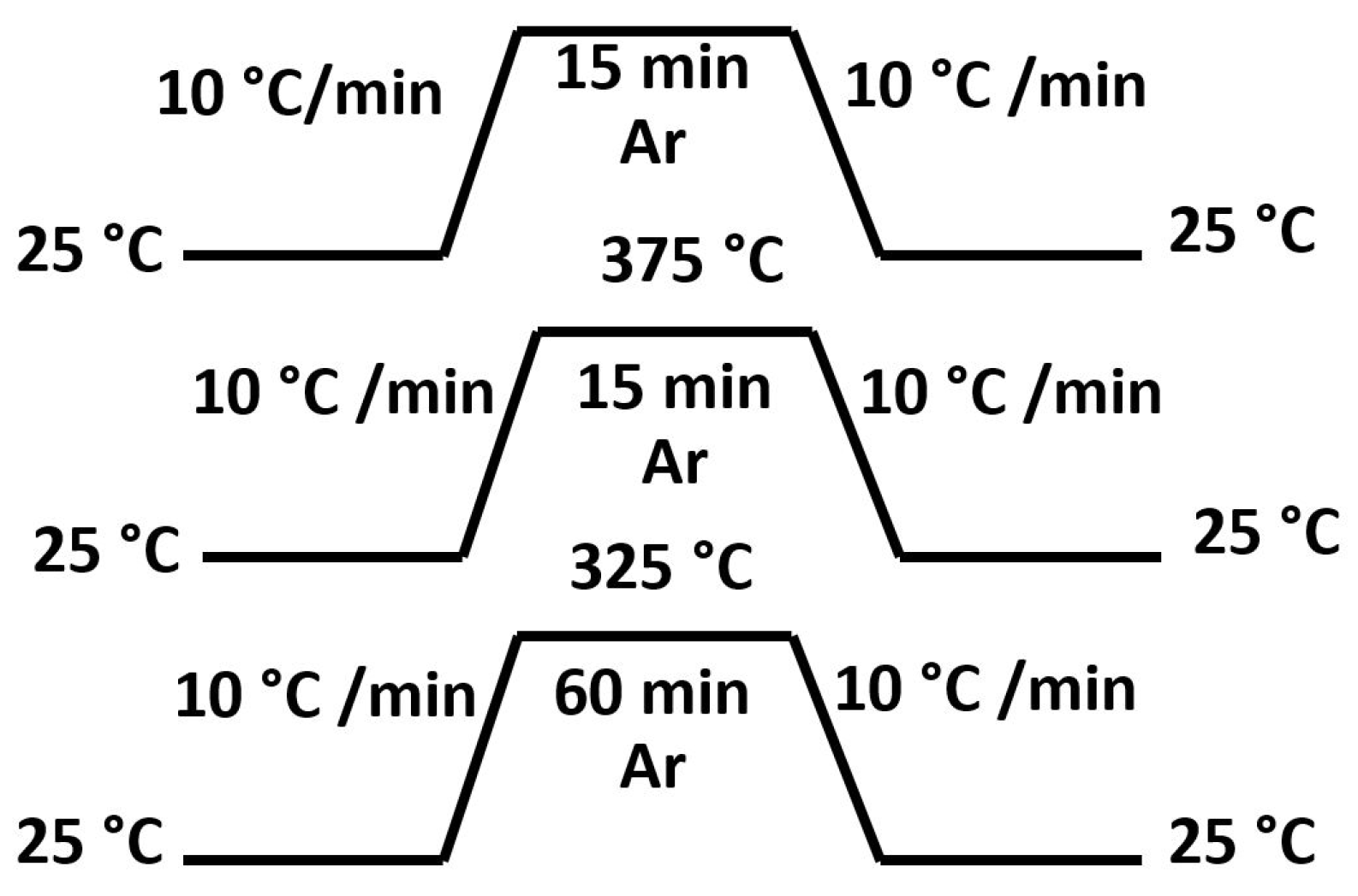
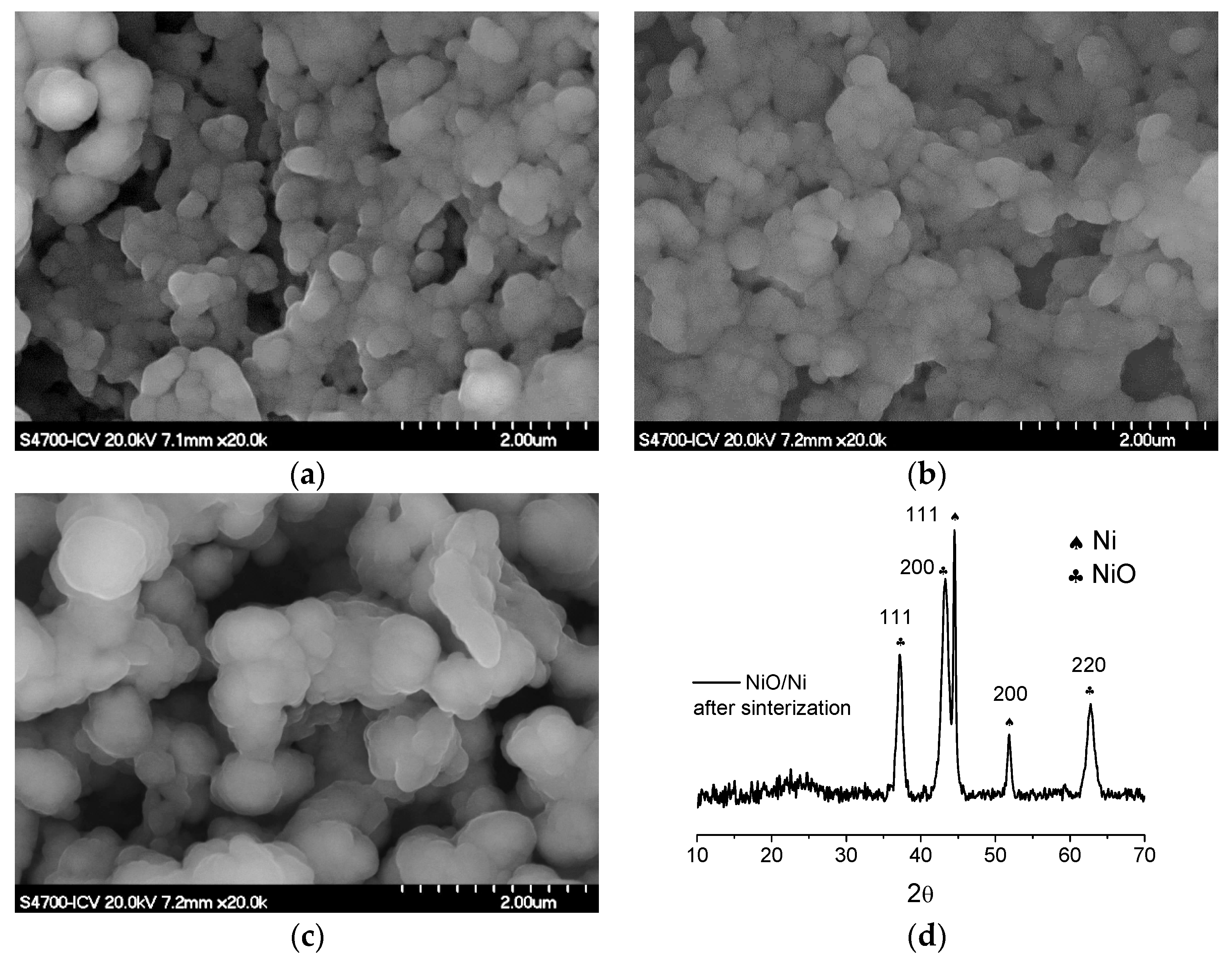
3.2. Sintering Study
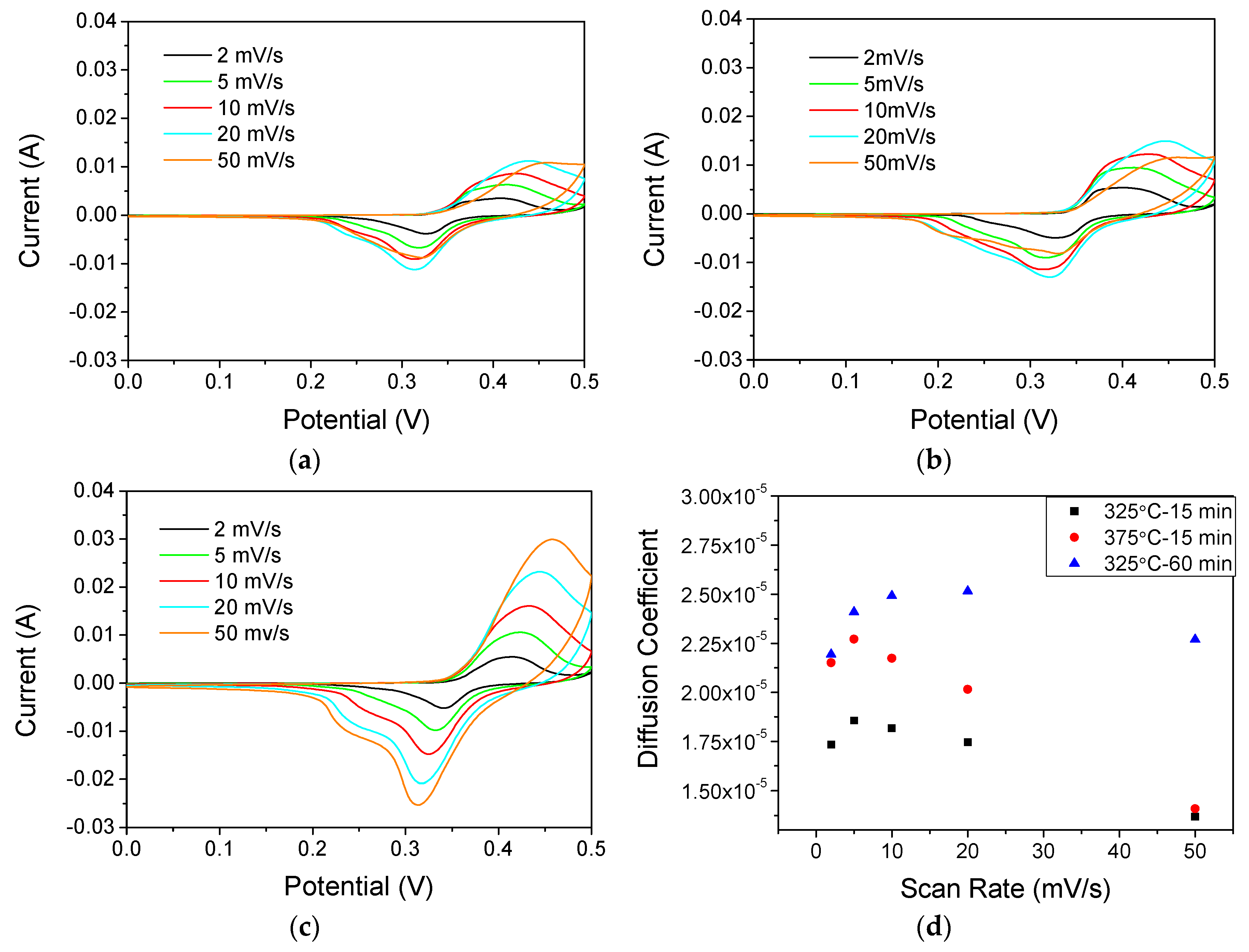
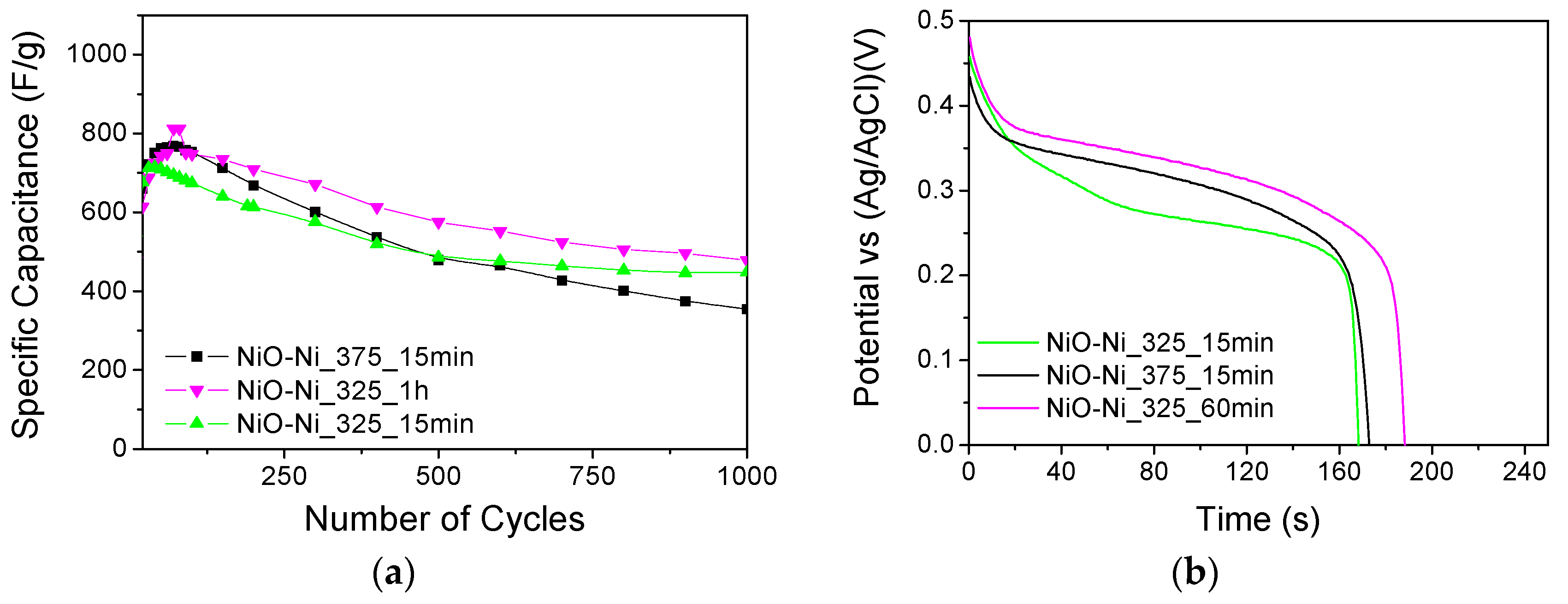
3.3. Electrochemical Response
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cuentas Gallegos, A.K.; Rincón, M.E. Carbon nanofiber and PEDOT-PSS bilayer systems as electrodes for symmetric and asymmetric electrochemical capacitor cells. J. Power Sources 2006, 162, 743–747. [Google Scholar] [CrossRef]
- Sidhu, N.K.; Rastogi, A.C. Electrochemical performance of supercapacitors based on carbon nanofoam composite and microporous poly(3,4-ethylenedioxythiophene) thin film asymmetric electrodes. Mater. Chem. Phys. 2016, 176, 75–86. [Google Scholar] [CrossRef]
- Xia, H.; Shirley Meng, Y.; Yuan, G.; Cui, C.; Lu, L. A symmetric RuO2/RuO2 supercapacitor operating at 1.6 V by using a neutral aqueous electrolyte. Electrochem. Solid State Lett. 2012, 15. [Google Scholar] [CrossRef]
- Yoo, C.Y.; Park, J.; Yun, D.S.; Yu, J.H.; Yoon, H.; Kim, J.N.; Yoon, H.C.; Kwak, M.; Kang, Y.C. Crucial role of a nickel substrate in Co3O4 pseudocapacitor directly grown on nickel and its electrochemical properties. J. Alloys Compd. 2016, 676, 407–413. [Google Scholar] [CrossRef]
- Argüello, J.A.; Cerpa, A.; Alanbari, M.H.; Fariñas, J.C.; Moreno, R. Preparation of manganese oxide-graphite electrodes by electrophoretic deposition. Ceram. Int. 2017, 43, 3231–3237. [Google Scholar] [CrossRef]
- Gonzalez, Z.; Ferrari, B.; Sanchez-Herencia, A.J.; Caballero, A.; Morales, J. Use of Polyelectrolytes for the Fabrication of Porous NiO Films by Electrophoretic Deposition for Supercapacitor Electrodes. Electrochim. Acta 2016, 211, 110–118. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Chen, C.; An, C.; Xu, Y.; Dong, Y.; Zhang, Q.; Wang, Y.; Jiao, L.; Yuan, H. Facile synthesis of diverse transition metal oxide nanoparticles and electrochemical properties. Inorg. Chem. Front. 2016, 3, 1048–1057. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, F.; Zhang, W.; Mei, Z.; Pei, B.; Zhu, X. Controllable preparation of multishelled NiO hollow nanospheres via layer-by-layer self-assembly for supercapacitor application. J. Power Sources 2014, 246, 24–31. [Google Scholar] [CrossRef]
- Bello, A.; Makgopa, K.; Fabiane, M.; Dodoo-Ahrin, D.; Ozoemena, K.I.; Manyala, N. Chemical adsorption of NiO nanostructures on nickel foam-graphene for supercapacitor applications. J. Mater. Sci. 2013, 48, 6707–6712. [Google Scholar] [CrossRef]
- Feng, F.; Zhao, S.; Liu, R.; Yang, Z.; Shen, Q. NiO Flowerlike porous hollow nanostructures with an enhanced interfacial storage capability for battery-to-pseudocapacitor transition. Electrochim. Acta 2016, 222, 1160–1168. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, Q.; Gao, H.; Shi, Z.; Wu, J.; Zhi, M.; Hong, Z. Nickel oxide aerogel for high performance supercapacitor electrodes. RSC Adv. 2016, 6. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Shi, J.; Du, N.; He, G. Multishelled Nickel-Cobalt Oxide Hollow Microspheres with Optimized Compositions and Shell Porosity for High-Performance Pseudocapacitors. ACS Appl. Mater. Interfaces 2016, 8, 17276–17283. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, Z.; Ferrari, B.; Sanchez-Herencia, A.J.; Caballero, A.; Morales, J. Relevance of the Semiconductor Microstructure in the Pseudocapacitance of the Electrodes Fabricated by EPD of Binder-Free β-Ni(OH)2 Nanoplatelets. J. Electrochem. Soc. 2015, 162, D3001–D3012. [Google Scholar] [CrossRef]
- Kurra, N.; Alhebshi, N.A.; Alshareef, H.N. Microfabricated pseudocapacitors using Ni(OH)2 electrodes exhibit remarkable volumetric capacitance and energy density. Adv. Energy Mater. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Liu, X.Y.; Gao, Y.Q.; Yang, G.W. A flexible, transparent and super-long-life supercapacitor based on ultrafine Co3O4 nanocrystal electrodes. Nanoscale 2016, 8, 4227–4235. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Q.; Wang, P.B.; Zang, X.N.; Yang, Y.; Kozinda, A.; Lin, L. Uniformly Embedded Metal Oxide Nanoparticles in Vertically Aligned Carbon Nanotube Forests as Pseudocapacitor Electrodes for Enhanced Energy Storage. Nano Lett. 2013, 13, 3524–3530. [Google Scholar] [CrossRef] [PubMed]
- Żołek-Tryznowska, Z. Additives for Ink Manufacture. In Printing on Polymers; Izdebska, J., Thomas, S., Eds.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 57–66. [Google Scholar]
- McManus, D.; Vranic, S.; Withers, F.; Sanchez-Romaguera, V.; Macucci, M.; Yang, H.; Sorrentino, R.; Parvez, K.; Son, S.K.; Iannaccone, G.; et al. Water-based and biocompatible 2D crystal inks for all-inkjet-printed heterostructures. Nat. Nanotechnol. 2017, 12, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, Z.; Yus, J.; Caballero, A.; Morales, J.; Sanchez-Herencia, A.J.; Ferrari, B. Electrochemical performance of pseudo-capacitor electrodes fabricated by Electrophoretic Deposition inducing Ni(OH)2 nanoplatelets agglomeration by Layer-by-Layer. Electrochim. Acta 2017, 247, 333–343. [Google Scholar] [CrossRef]
- Kazazi, M. Facile preparation of nanoflake-structured nickel oxide/carbon nanotube composite films by electrophoretic deposition as binder-free electrodes for high-performance pseudocapacitors. Curr. Appl. Phys. 2017, 17, 240–248. [Google Scholar] [CrossRef]
- Dios, M.; Gonzalez, Z.; Gordo, E.; Ferrari, B. Semiconductor-metal core-shell nanostructures by colloidal heterocoagulation in aqueous medium. Mater. Lett. 2016, 180, 327–331. [Google Scholar] [CrossRef]
- Wu, M.S.; Huang, C.Y.; Lin, K.H. Electrophoretic deposition of nickel oxide electrode for high-rate electrochemical capacitors. J. Power Sources 2009, 186, 557–564. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y.; Whangbo, M.-H. Facile in situ synthesis of visible-light plasmonic photocatalysts M@TiO2 (M = Au, Pt, Ag) and evaluation of their photocatalytic oxidation of benzene to phenol. J. Mater. Chem. 2011, 21, 9079. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, D.; Zhu, X.; Yang, C.; Liu, D.; Chen, X.; Song, Y.; Lu, L. High-performance and renewable supercapacitors based on TiO2 nanotube array electrodes treated by an electrochemical doping approach. Electrochim. Acta 2014, 116, 129–136. [Google Scholar] [CrossRef]
- Wen, W.; Wu, J.M. Eruption combustion synthesis of NiO/Ni nanocomposites with enhanced properties for dye-absorption and lithium storage. ACS Appl. Mater. Interfaces 2011, 3, 4112–4119. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Yao, S.; Cao, J.; Di, L.; Wu, G.; Guan, N.; Li, L. Heterostructured Ni/NiO composite as a robust catalyst for the hydrogenation of levulinic acid to γ-valerolactone. Appl. Catal. B Environ. 2017, 217, 115–124. [Google Scholar] [CrossRef]
- Lu, Q.; Lattanzi, M.W.; Chen, Y.P.; Kou, X.M.; Li, W.F.; Fan, X.; Unruh, K.M.; Chen, J.G.G.; Xiao, J.Q. Supercapacitor Electrodes with High-Energy and Power Densities Prepared from Monolithic NiO/Ni Nanocomposites. Angew. Chem. Int. Ed. 2011, 50, 6847–6850. [Google Scholar] [CrossRef] [PubMed]
- Dios, M.; Gonzalez, Z.; Gordo, E.; Ferrari, B. Chemical precipitation of nickel nanoparticles on Ti (C,N) suspensions focused on cermet processing. Int. J. Refract. Met. Hard Mater. 2017, 63, 2–8. [Google Scholar] [CrossRef]
- Ferrari, B.; Moreno, R. EPD kinetics: A review. J. Eur. Ceram. Soc. 2010, 30, 1069–1078. [Google Scholar] [CrossRef]
- Mishra, M.; Bhattacharjee, S.; Besra, L.; Sharma, H.S.; Uchikoshi, T.; Sakka, Y. Effect of pH localization on microstructure evolution of deposits during aqueous electrophoretic deposition (EPD). J. Eur. Ceram. Soc. 2010, 30, 2467–2473. [Google Scholar] [CrossRef]
- Besra, L.; Uchikoshi, T.; Suzuki, T.S.; Sakka, Y. Experimental verification of pH localization mechanism of particle consolidation at the electrode/solution interface and its application to pulsed DC electrophoretic deposition (EPD). J. Eur. Ceram. Soc. 2010, 30, 1187–1193. [Google Scholar] [CrossRef]
- Xu, P.; Miao, C.; Cheng, K.; Ye, K.; Yin, J.; Cao, D.; Wang, G.; Zhang, X. Preparation of binder-free porous ultrathin Ni(OH)2 nanoleafs using ZnO as pore forming agent displaying both high mass loading and excellent electrochemical energy storage performance. Electrochim. Acta 2016, 216, 499–509. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, D.; Xu, J.; Lu, Y.; Liu, Y.; Qiu, K.; Zhang, Y. Solution growth of NiO nanosheets supported on Ni foam as high-performance electrodes for supercapacitors. Nano Res. Lett. 2014, 9, 424. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Chang, Z.; Liu, J.; Sun, X. Stable ultrahigh specific capacitance of NiO nanorod arrays. Nano Res. 2011, 4, 658–665. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, J.; Tang, P.; Ye, X.; Peng, X.; Tang, H.; Sun, S.G.; Fransaer, J. A universal strategy for metal oxide anchored and binder-free carbon matrix electrode: A supercapacitor case with superior rate performance and high mass loading. Nano Energy 2017, 31, 311–321. [Google Scholar] [CrossRef]
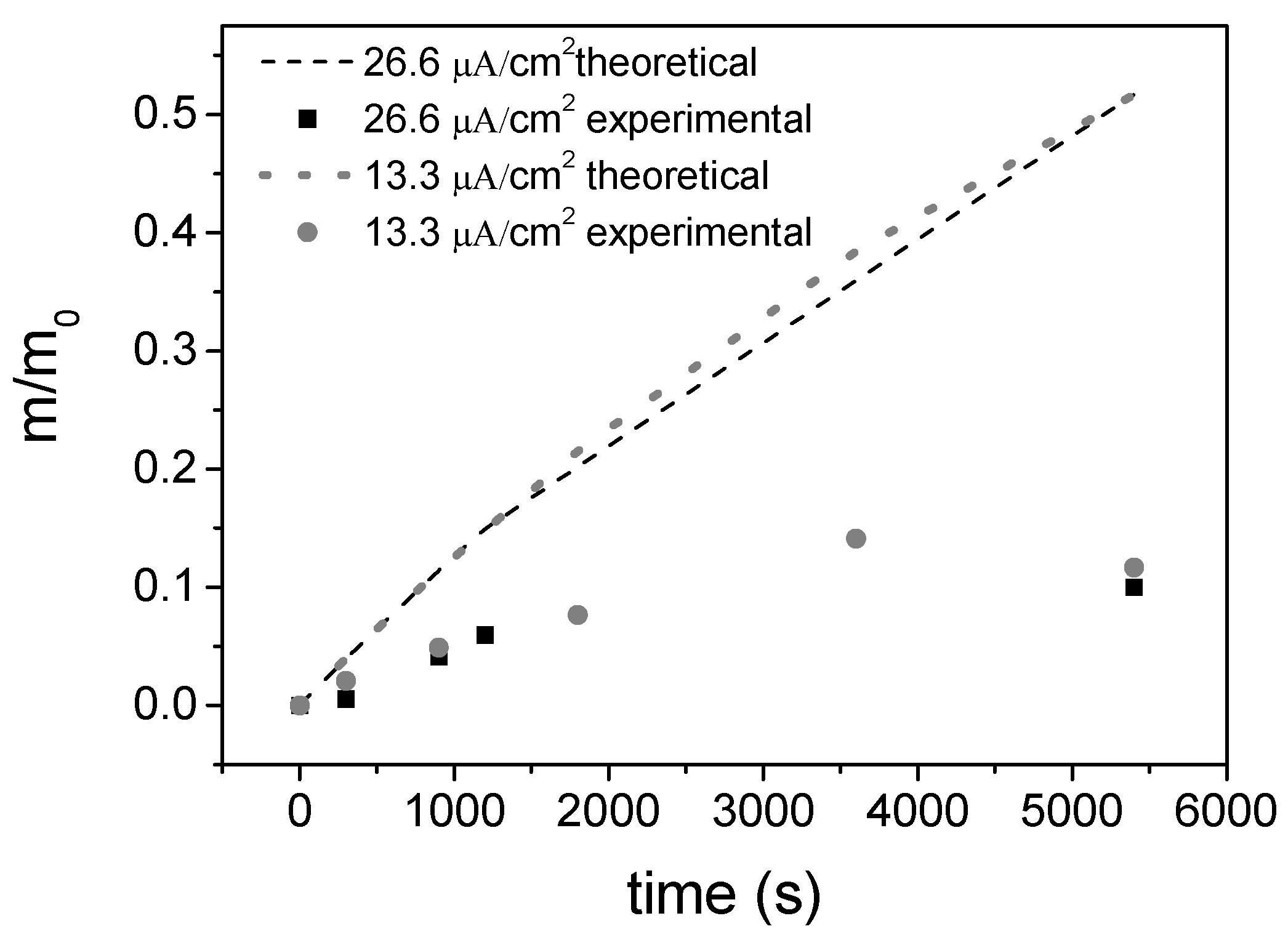
| Volume of Suspension, V | Electrophoretic Mobility, μe | Deposition Surface, S | Electric Field, E | Sticking Factor, f | Characteristic Time, T |
|---|---|---|---|---|---|
| 30 mL | 0.7186 × 10−4 cm2 V−1 s−1 | 3.75 cm2 | 15 V/cm | 1 | 7422 s |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yus, J.; Ferrari, B.; Sanchez-Herencia, A.J.; Caballero, A.; Morales, J.; Gonzalez, Z. In Situ Synthesis and Electrophoretic Deposition of NiO/Ni Core-Shell Nanoparticles and Its Application as Pseudocapacitor. Coatings 2017, 7, 193. https://doi.org/10.3390/coatings7110193
Yus J, Ferrari B, Sanchez-Herencia AJ, Caballero A, Morales J, Gonzalez Z. In Situ Synthesis and Electrophoretic Deposition of NiO/Ni Core-Shell Nanoparticles and Its Application as Pseudocapacitor. Coatings. 2017; 7(11):193. https://doi.org/10.3390/coatings7110193
Chicago/Turabian StyleYus, Joaquin, Begoña Ferrari, Antonio Javier Sanchez-Herencia, Alvaro Caballero, Julian Morales, and Zoilo Gonzalez. 2017. "In Situ Synthesis and Electrophoretic Deposition of NiO/Ni Core-Shell Nanoparticles and Its Application as Pseudocapacitor" Coatings 7, no. 11: 193. https://doi.org/10.3390/coatings7110193






