The Effect of Post-Baking Temperature and Thickness of ZnO Electron Transport Layers for Efficient Planar Heterojunction Organometal-Trihalide Perovskite Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of ZnO Precursor
2.2. Preparation of Perovskite Solar Cells
2.3. Characterization
3. Results and Discussion
3.1. Influence of ZnO Electron Transport Layer Thickness
3.2. Influence of Baking Temperature
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Burschka, J.; Pellet, N.; Moon, S.-J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Graetzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Graetzel, M.; Mhaisalkar, S.; Sum, T.C. Long-range balanced electron- and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; He, M.; Peng, J.; Sun, Y.; Liang, Z. Structure and growth control of organic-inorganic halide perovskites for optoelectronics: From polycrystalline films to single crystals. Adv. Sci. 2016, 3, 1500392. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Zhu, W.; Bao, C.; Wang, Y.; Li, F.; Zhou, X.; Yang, J.; Lv, B.; Wang, X.Y.; Yu, T.; Zou, Z. Coarsening of one-step deposited organolead triiodide perovskite films via Ostwald ripening for high efficiency planar-heterojunction solar cells. Dalton Trans. 2016, 45, 7856–7865. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Im, J.-H.; Lee, C.-R.; Lee, J.-W.; Park, S.-W.; Park, N.-G. 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 2011, 3, 4088–4093. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Park, B.-W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide-based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [PubMed]
- Matas Adams, A.; Marin-Beloqui, J.M.; Stoica, G.; Palomares, E. The influence of the mesoporous TiO2 scaffold on the performance of methyl ammonium lead iodide (MAPI) perovskite solar cells: Charge injection, charge recombination and solar cell efficiency relationship. J. Mater. Chem. A 2015, 3, 22154–22161. [Google Scholar] [CrossRef]
- Wang, H.-H.; Chen, Q.; Zhou, H.; Song, L.; Louis, Z.S.; Marco, N.D.; Fang, Y.; Sun, P.; Song, T.-B.; Chen, H.; et al. Improving the TiO2 electron transport layer in perovskite solar cells using acetylacetonate-based additives. J. Mater. Chem. A 2015, 3, 9108–9115. [Google Scholar] [CrossRef]
- Hadouchi, W.; Rousset, J.; Tondelier, D.; Geffroy, B.; Bonnassieux, Y. Zinc oxide as a hole blocking layer for perovskite solar cells deposited in atmospheric conditions. RSC Adv. 2016, 6, 67715–67723. [Google Scholar] [CrossRef]
- Ahmed, M.I.; Hussain, Z.; Mujahid, M.; Khan, A.N.; Javaid, S.S.; Habib, A. Low resistivity ZnO-GO electron transport layer based CH3NH3PbI3 solar cells. AIP Adv. 2016, 6, 065303. [Google Scholar] [CrossRef]
- Li, C.; Zang, Z.; Han, C.; Hu, Z.; Tang, X.; Du, J.; Leng, Y.; Sun, K. Highly compact CsPbBr3 perovskite thin films decorated by ZnO nanoparticles for enhanced random lasing. Nano Energy 2017, 40, 195–202. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Jiang, Q.; Wang, P.; Yin, Z.; Zhang, X.; Tan, H.; Yang, Y.; Wei, M.; Sutherland, B.R.; et al. Ultra-bright and highly efficient inorganic based perovskite light-emitting diodes. Nat. Commun. 2017, 8, 15640. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-Y.; Lim, D.; Kim, K.-D.; Jang, S.-Y. Performance optimization of low-temperature-annealed solution-processable zno buffer layers for inverted polymer solar cells. J. Mater. Chem. A 2013, 1, 6327–6334. [Google Scholar] [CrossRef]
- Xu, L.; Guo, Y.; Liao, Q.; Zhang, J.; Xu, D. Morphological control of ZnO nanostructures by electrodeposition. J. Phys. Chem. B 2005, 109, 13519–13522. [Google Scholar] [CrossRef] [PubMed]
- Carcia, P.; McLean, R.; Reilly, M. High-performance ZnO thin-film transistors on gate dielectrics grown by atomic layer deposition. Appl. Phys. Lett. 2006, 88, 3509. [Google Scholar] [CrossRef]
- Dong, X.; Hu, H.; Lin, B.; Ding, J.; Yuan, N. The effect of ALD-ZnO layers on the formation of CH3NH3PbI3 with different perovskite precursors and sintering temperatures. Chem. Commun. 2014, 50, 14405–14408. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-M.; Chang, S.H.; Wang, K.-H.; Chang, C.-M.; Cheng, H.-M.; Kei, C.-C.; Tseng, Z.-L.; Wu, C.-G. Thickness effects of ZnO thin film on the performance of tri-iodide perovskite absorber based photovoltaics. Sol. Energy 2015, 120, 117–122. [Google Scholar] [CrossRef]
- Liu, D.; Yang, J.; Kelly, T.L. Compact layer free perovskite solar cells with 13.5% efficiency. J. Am. Chem. Soc. 2014, 136, 17116–17122. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Liu, L.; Wang, X.-F.; Chen, G.; Tian, W.; Miyasaka, T. Highly efficient and stable low-temperature processed ZnO solar cells with triple cation perovskite absorber. J. Mater. Chem. A 2017, 5, 13439–13447. [Google Scholar] [CrossRef]
- Han, G.S.; Shim, H.-W.; Lee, S.; Duff, M.L.; Lee, J.-K. Low-temperature modification of ZnO nanoparticles film for electron-transport layers in perovskite solar cells. Chemsuschem 2017, 10, 2425–2430. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Kelly, T.L. Perovskite solar cells with a planar heterojunction structure prepared using room-temperature solution processing techniques. Nat. Photonics 2014, 8, 133–138. [Google Scholar] [CrossRef]
- Zhang, J.; Pauporté, T. Effects of oxide contact layer on the preparation and properties of CH3NH3PbI3 for perovskite solar cell application. J. Phys. Chem. C 2015, 119, 14919–14928. [Google Scholar] [CrossRef]
- Shin, S.S.; Yang, W.S.; Noh, J.H.; Suk, J.H.; Jeon, N.J.; Park, J.H.; Kim, J.S.; Seong, W.M.; Seok, S.I. High-performance flexible perovskite solar cells exploiting Zn2SnO4 prepared in solution below 100 °C. Nat. Commun. 2015, 6, 7410. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, M.A.; Elumalai, N.K.; Upama, M.B.; Wang, D.; Chan, K.H.; Wright, M.; Xu, C.; Haque, F.; Uddin, A. Low temperature processed ZnO thin film as electron transport layer for efficient perovskite solar cells. Sol. Energy Mater. Sol. Cells 2017, 159, 251–264. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, Q.-D.; Xiao, J.; Xue, Q.; Li, H.-W.; Guan, Z.; Yip, H.-L.; Tsang, S.-W. Decomposition of organometal halide perovskite films on zinc oxide nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 19986–19993. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Siempelkamp, B.D.; Mosconi, E.; De Angelis, F.; Kelly, T.L. Origin of the thermal instability in CH3NH3PbI3 thin films deposited on ZnO. Chem. Mater. 2015, 27, 4229–4236. [Google Scholar] [CrossRef]
- Zang, Y.; Li, C.-Z.; Chueh, C.-C.; Williams, S.T.; Jiang, W.; Wang, Z.-H.; Yu, J.-S.; Jen, A.K.Y. Integrated molecular, interfacial, and device engineering towards high-performance non-fullerene based organic solar cells. Adv. Mater. 2014, 26, 5708–5714. [Google Scholar] [CrossRef] [PubMed]
- IEC 60904:2017 Series Photovoltaic Devices—All Parts; International Electrotechnical Commission: London, UK, 2017.
- Song, J.; Bian, J.; Zheng, E.; Wang, X.-F.; Tian, W.; Miyasaka, T. Efficient and environmentally stable perovskite solar cells based on ZnO electron collection layer. Chem. Lett. 2015, 44, 610–612. [Google Scholar] [CrossRef]
- Sum, T.C.; Mathews, N. Advancements in perovskite solar cells: Photophysics behind the photovoltaics. Energy Environ. Sci. 2014, 7, 2518–2534. [Google Scholar] [CrossRef]
- David, C.L.; Kevin, D.L.; Darren, B.T.; Buguo, W. High conductance in ultrathin films of ZnO. Jpn. J. Appl. Phys. 2014, 53, 05FJ01. [Google Scholar]
- Li, Y.; Yan, W.; Li, Y.; Wang, S.; Wang, W.; Bian, Z.; Xiao, L.; Gong, Q. Direct observation of long electron-hole diffusion distance in CH3NH3PbI3 perovskite thin film. Sci. Rep. 2015, 5, 14485. [Google Scholar] [CrossRef] [PubMed]
- Deibel, C.; Strobel, T.; Dyakonov, V. Origin of the efficient polaron-pair dissociation in polymer-fullerene blends. Phys. Rev. Lett. 2009, 103, 036402. [Google Scholar] [CrossRef] [PubMed]


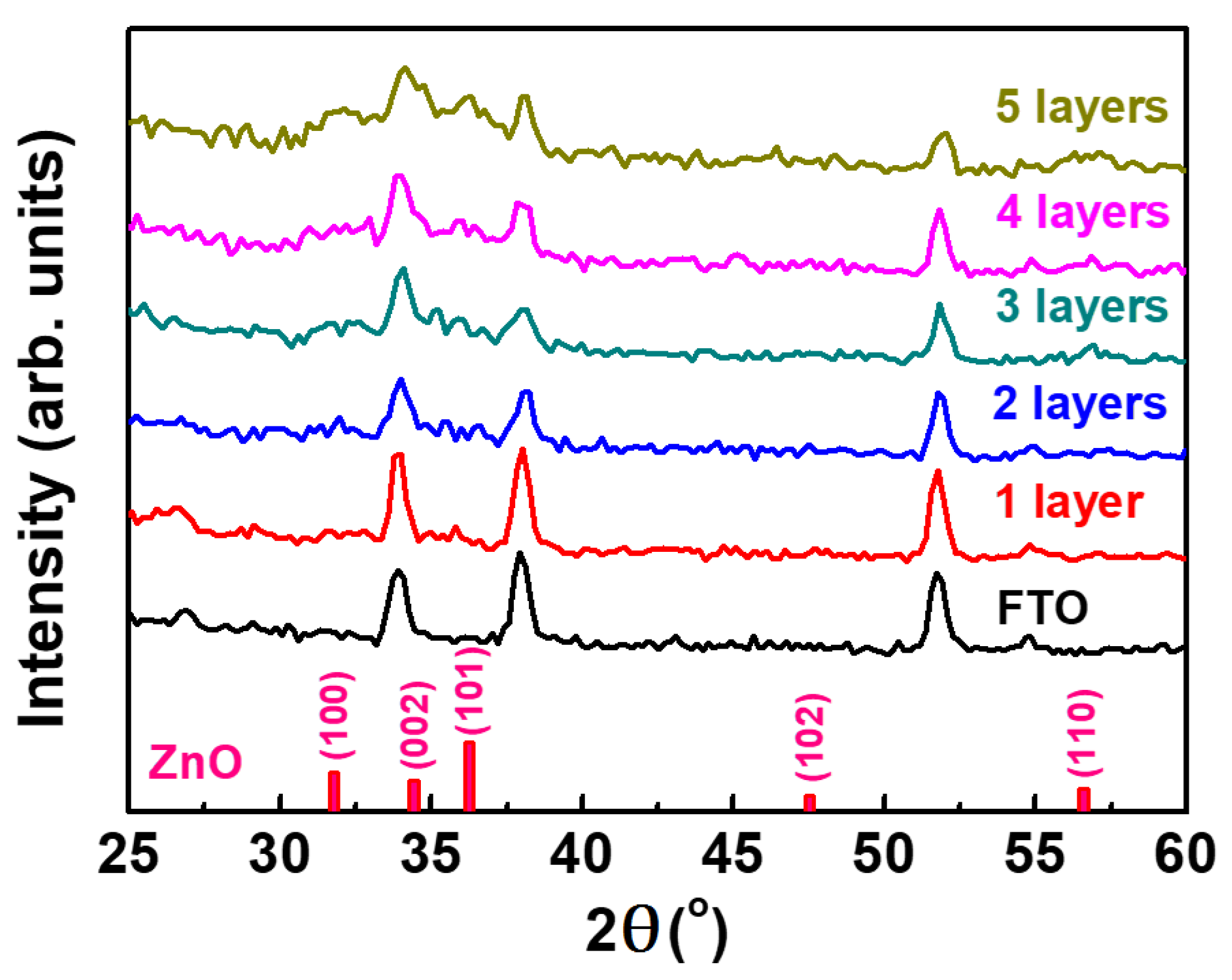
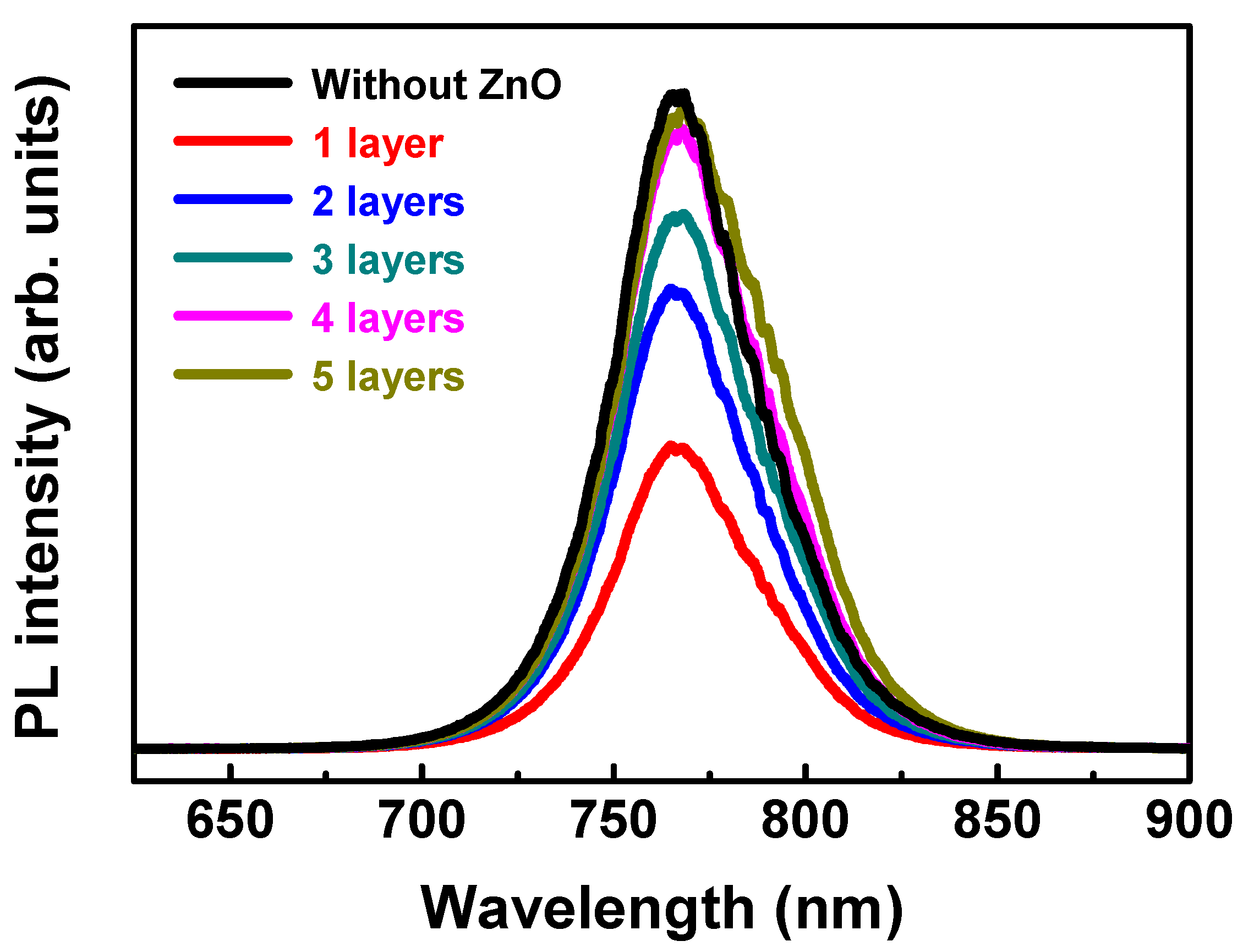
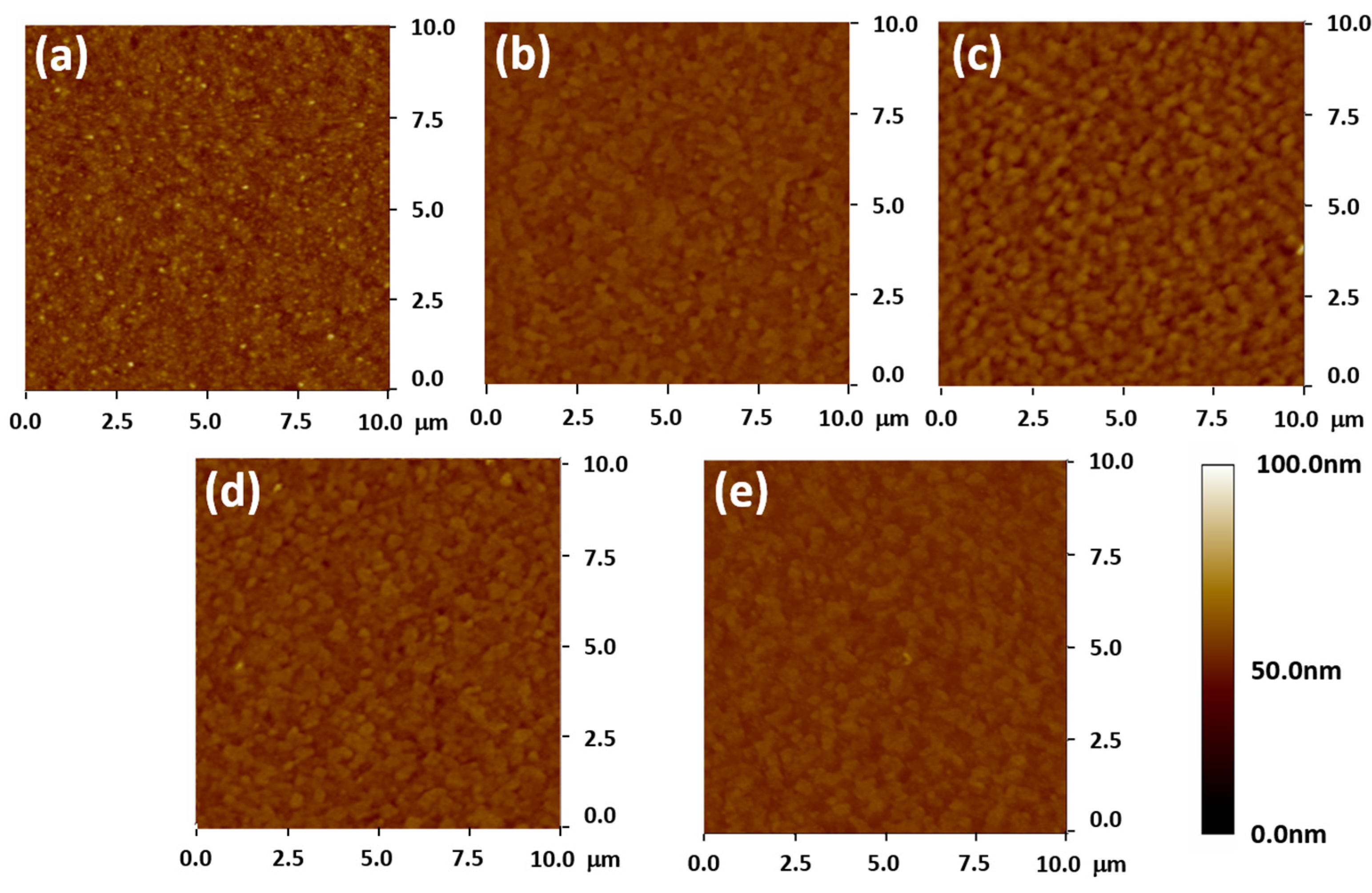
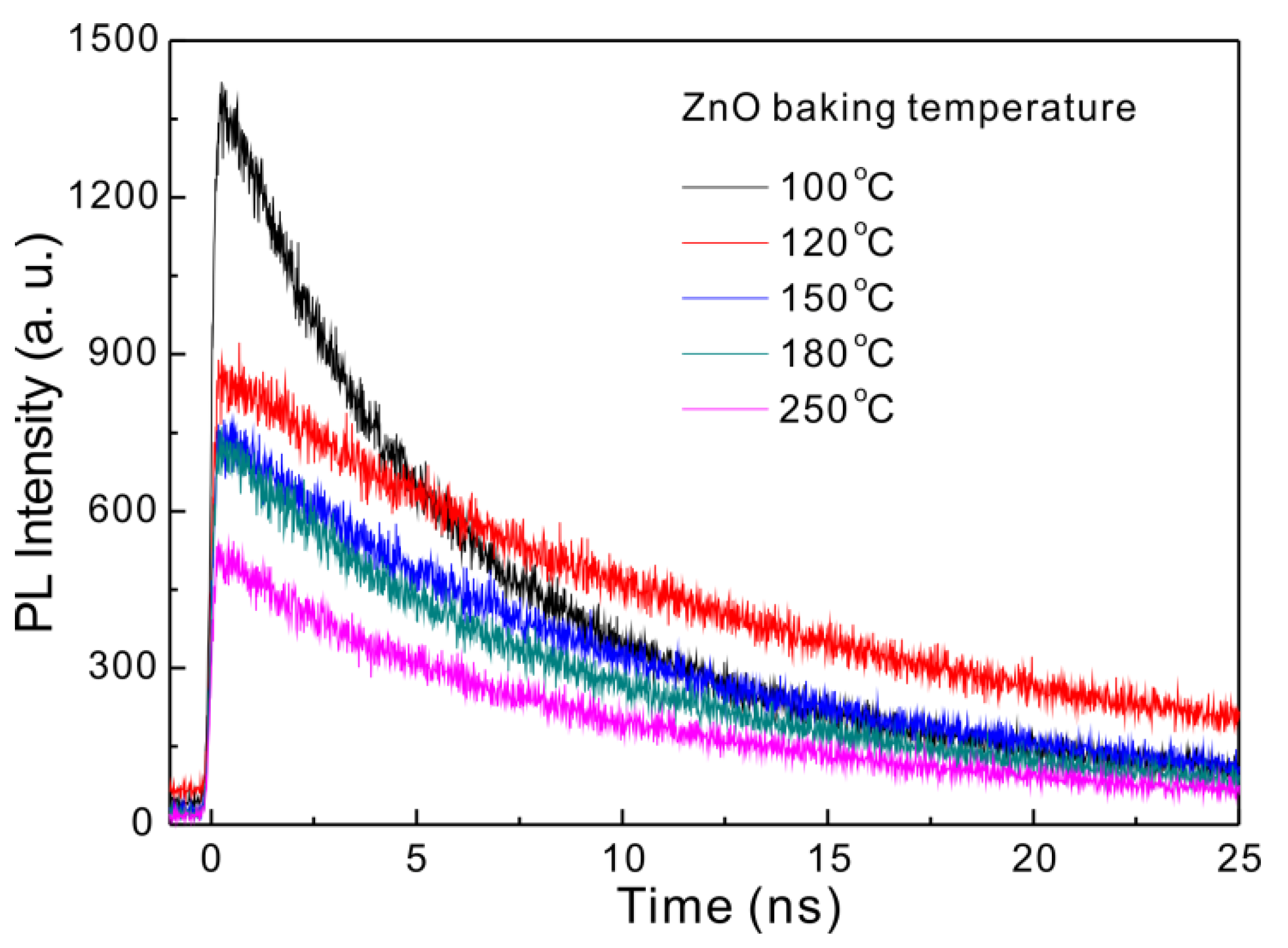
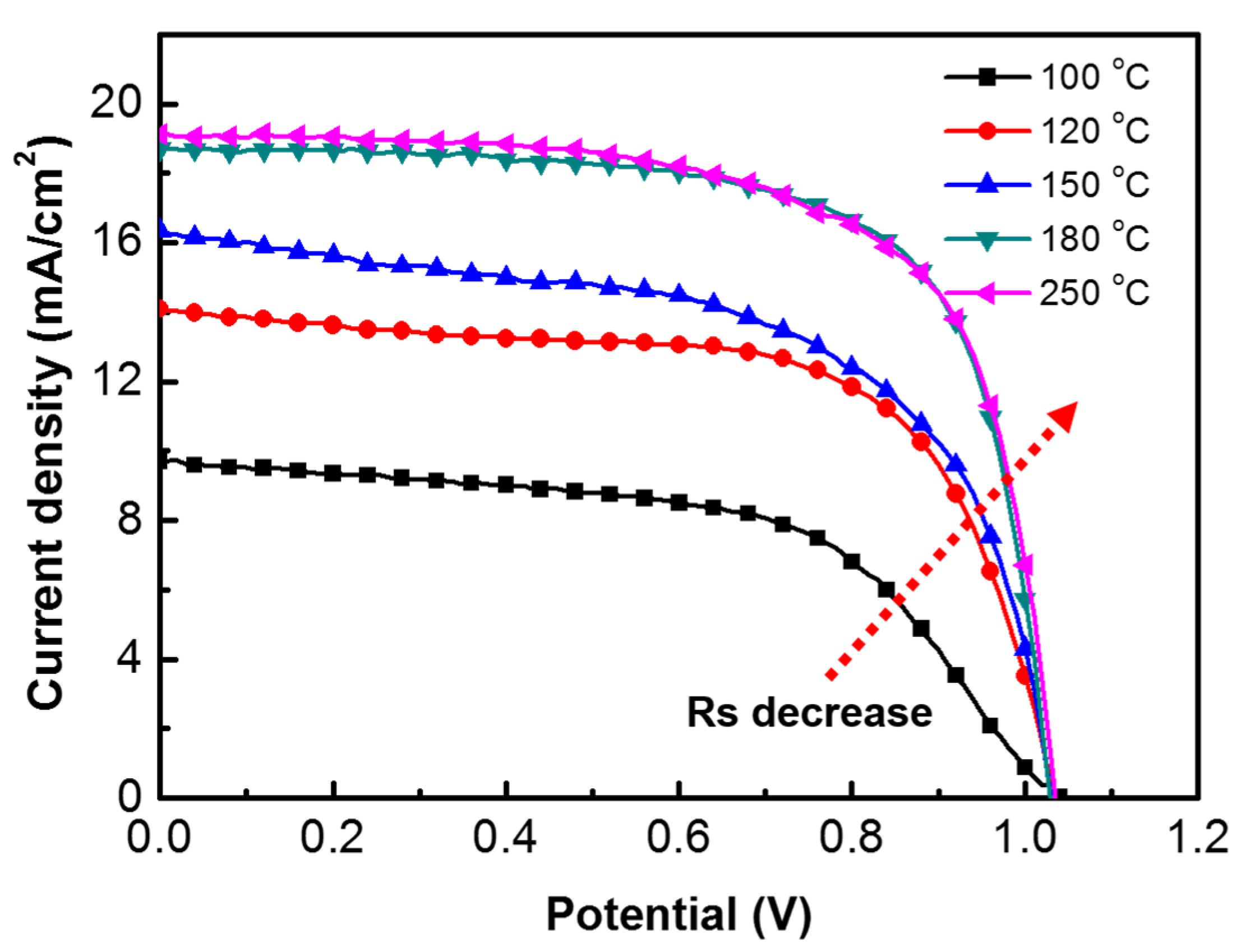
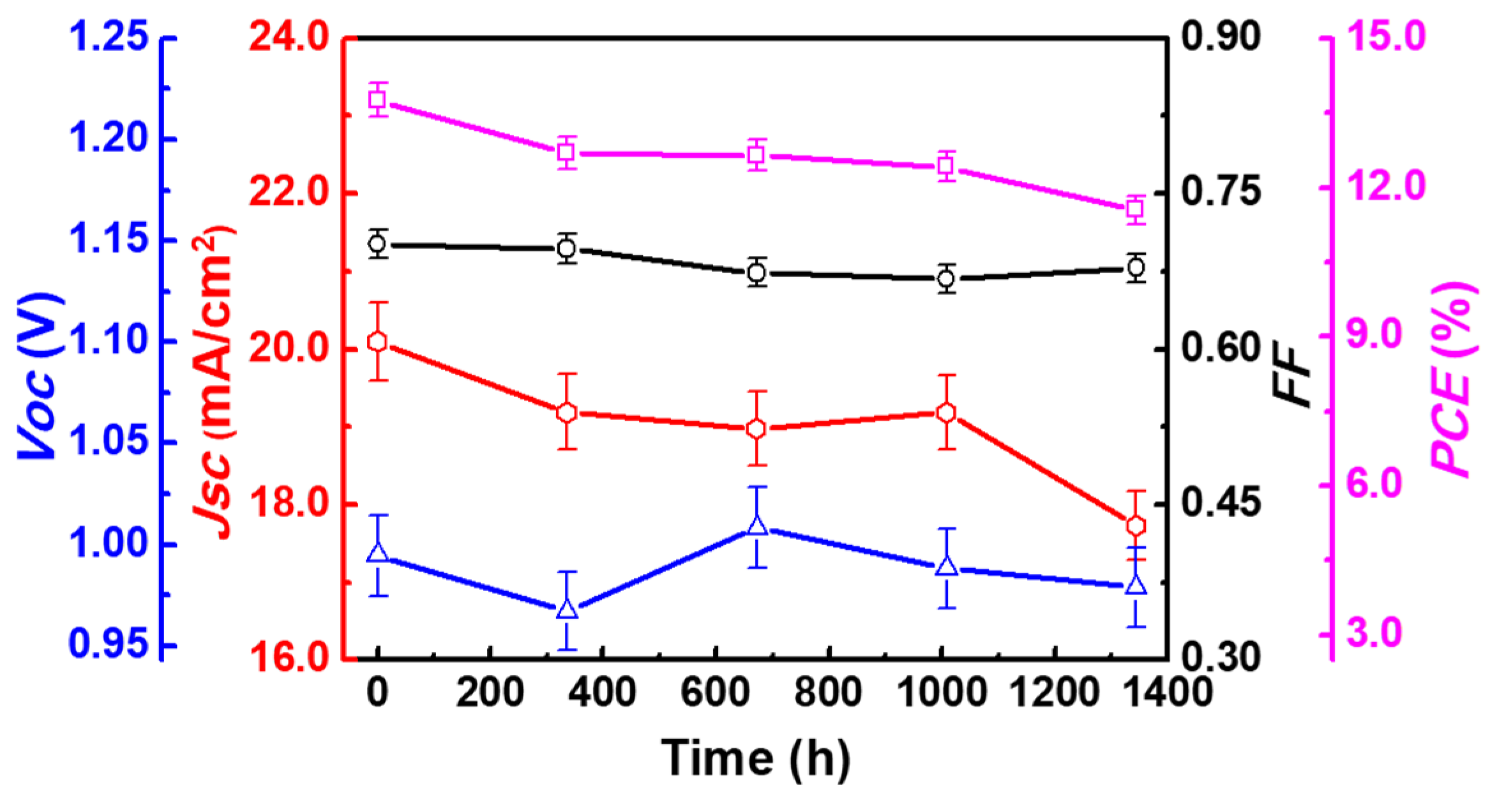
| Baking Temp. (°C) | VOC (V) | JSC (mA/cm2) | FF (%) | Efficiency (%) | Lifetime (ns) |
|---|---|---|---|---|---|
| 100 | 1.05 | 8.81 | 56.0 | 5.18 | 5.81 |
| 120 | 1.03 | 14.09 | 65.3 | 9.47 | 13.81 |
| 150 | 1.03 | 16.35 | 59.2 | 10.49 | 10.45 |
| 180 | 1.04 | 18.71 | 70.2 | 13.66 | 8.66 |
| 250 | 1.03 | 19.12 | 67.8 | 13.34 | 9.36 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-M.; Lin, C.-J.; Chang, Y.-H.; Lin, T.-H.; Suryanarayanan, V.; Wu, M.-C. The Effect of Post-Baking Temperature and Thickness of ZnO Electron Transport Layers for Efficient Planar Heterojunction Organometal-Trihalide Perovskite Solar Cells. Coatings 2017, 7, 215. https://doi.org/10.3390/coatings7120215
Lee K-M, Lin C-J, Chang Y-H, Lin T-H, Suryanarayanan V, Wu M-C. The Effect of Post-Baking Temperature and Thickness of ZnO Electron Transport Layers for Efficient Planar Heterojunction Organometal-Trihalide Perovskite Solar Cells. Coatings. 2017; 7(12):215. https://doi.org/10.3390/coatings7120215
Chicago/Turabian StyleLee, Kun-Mu, Chuan-Jung Lin, Yin-Hsuan Chang, Ting-Han Lin, Vembu Suryanarayanan, and Ming-Chung Wu. 2017. "The Effect of Post-Baking Temperature and Thickness of ZnO Electron Transport Layers for Efficient Planar Heterojunction Organometal-Trihalide Perovskite Solar Cells" Coatings 7, no. 12: 215. https://doi.org/10.3390/coatings7120215







