Effect of Hydrogen Concentration on the Growth of Carbon Nanotube Arrays for Gecko-Inspired Adhesive Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. VACNTs Preparation
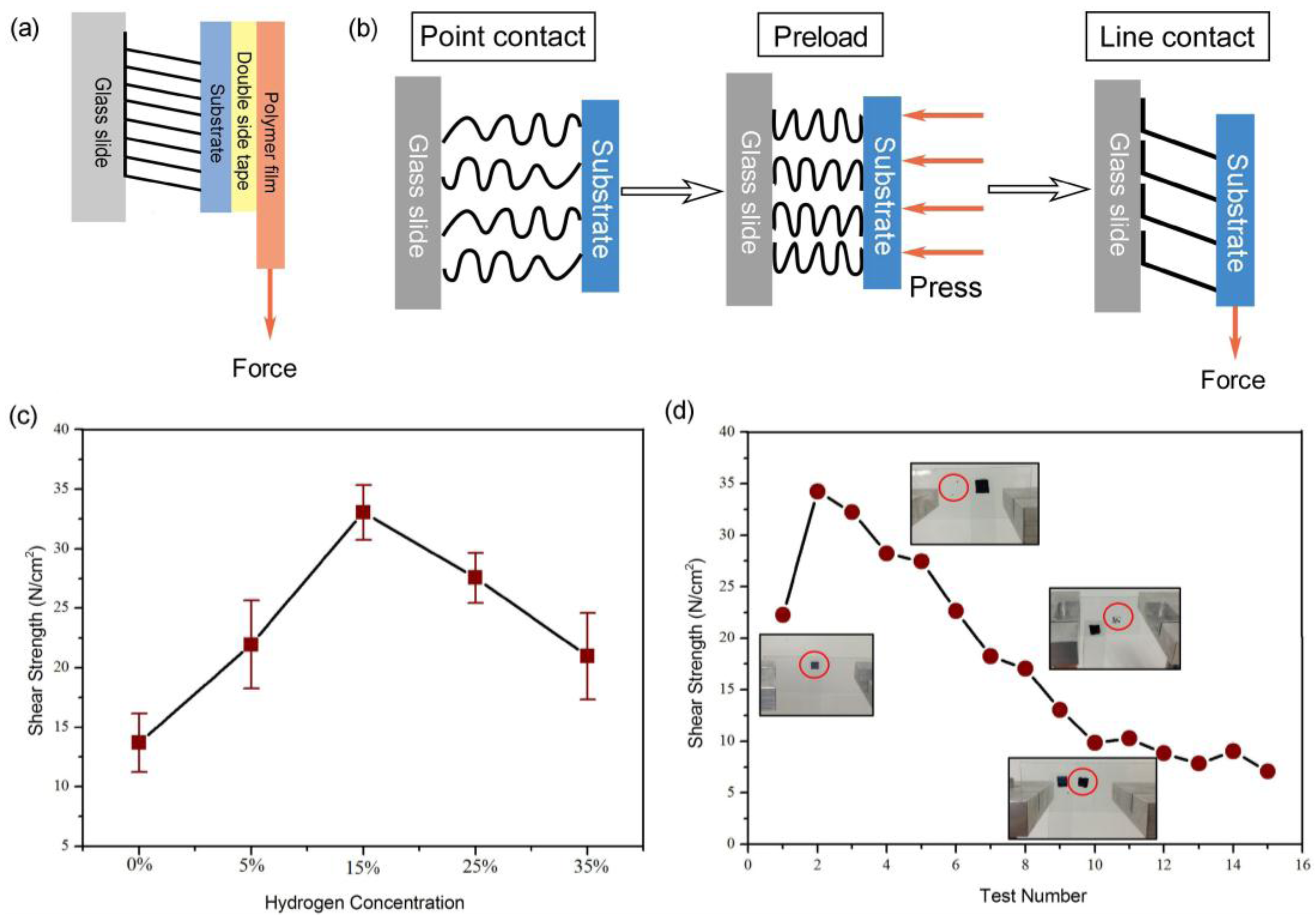
2.2. Characterization and Test Methods
3. Results and Discussion
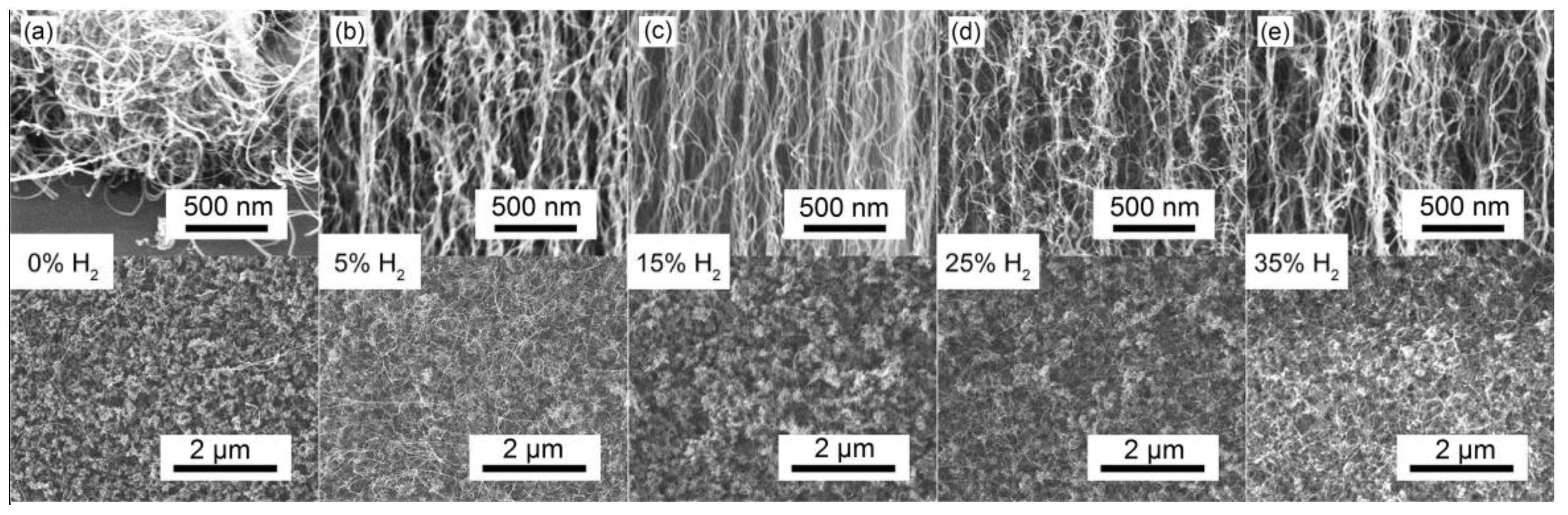
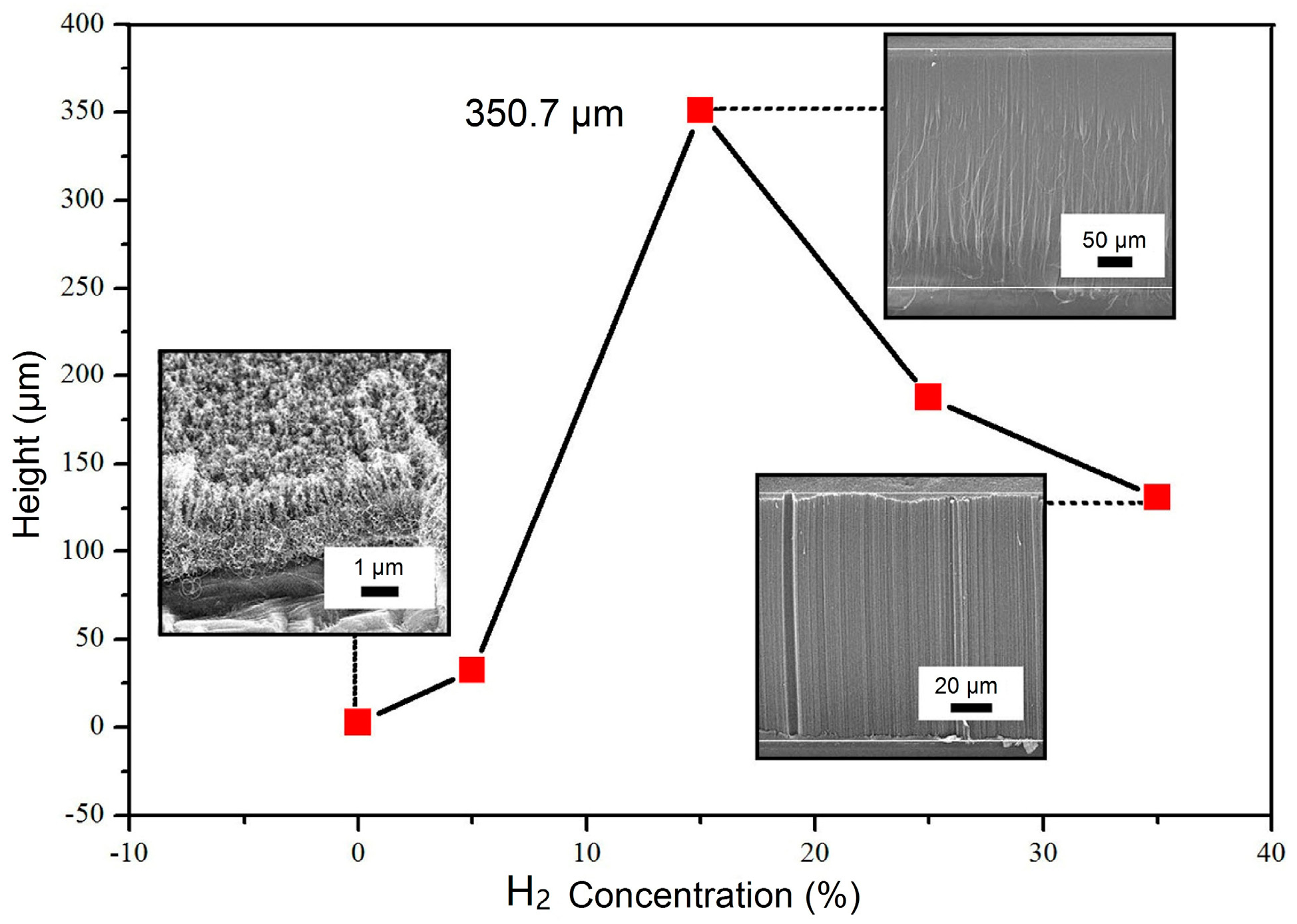
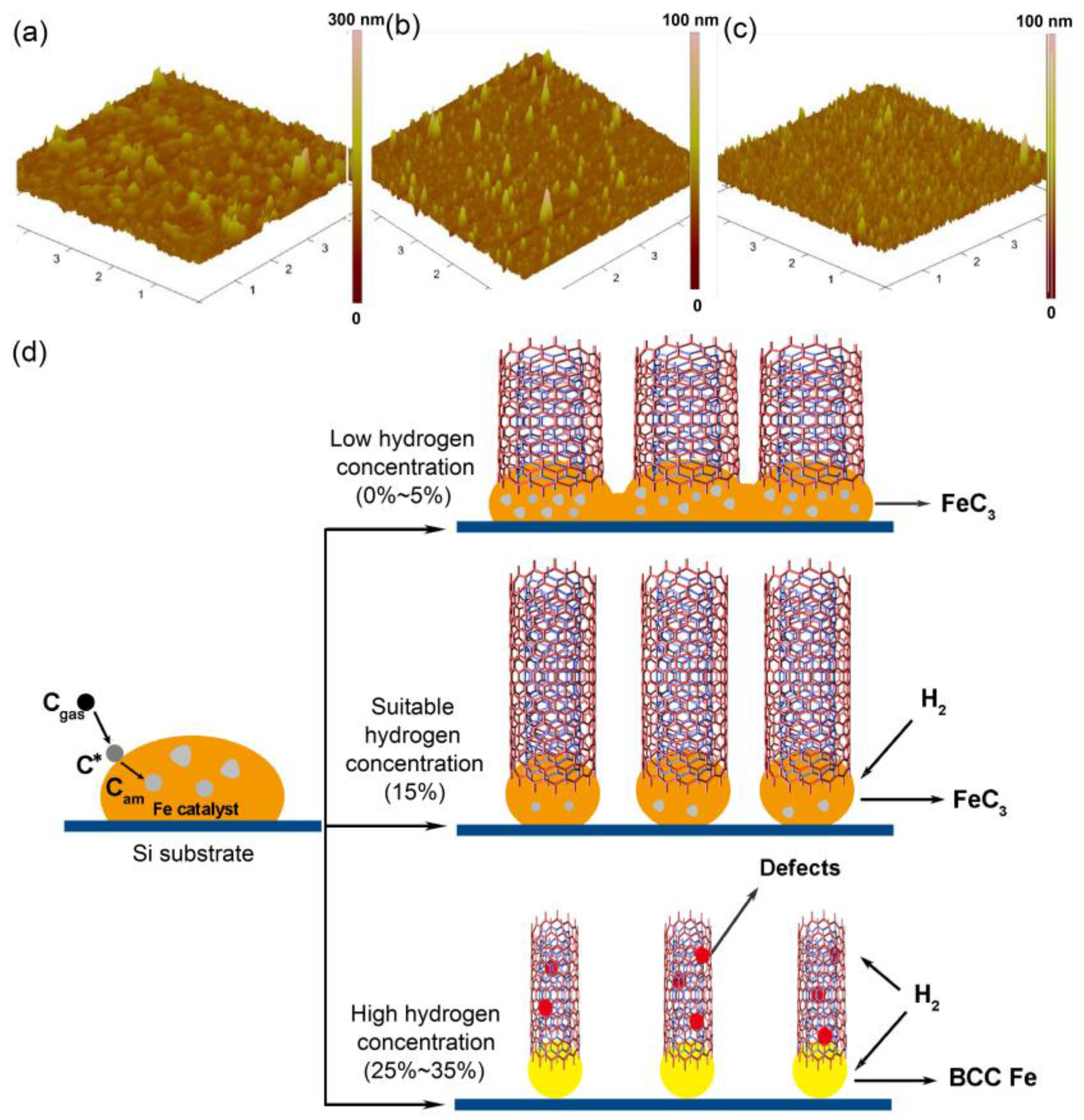
3.1. Morphological Analysis of VACNTs
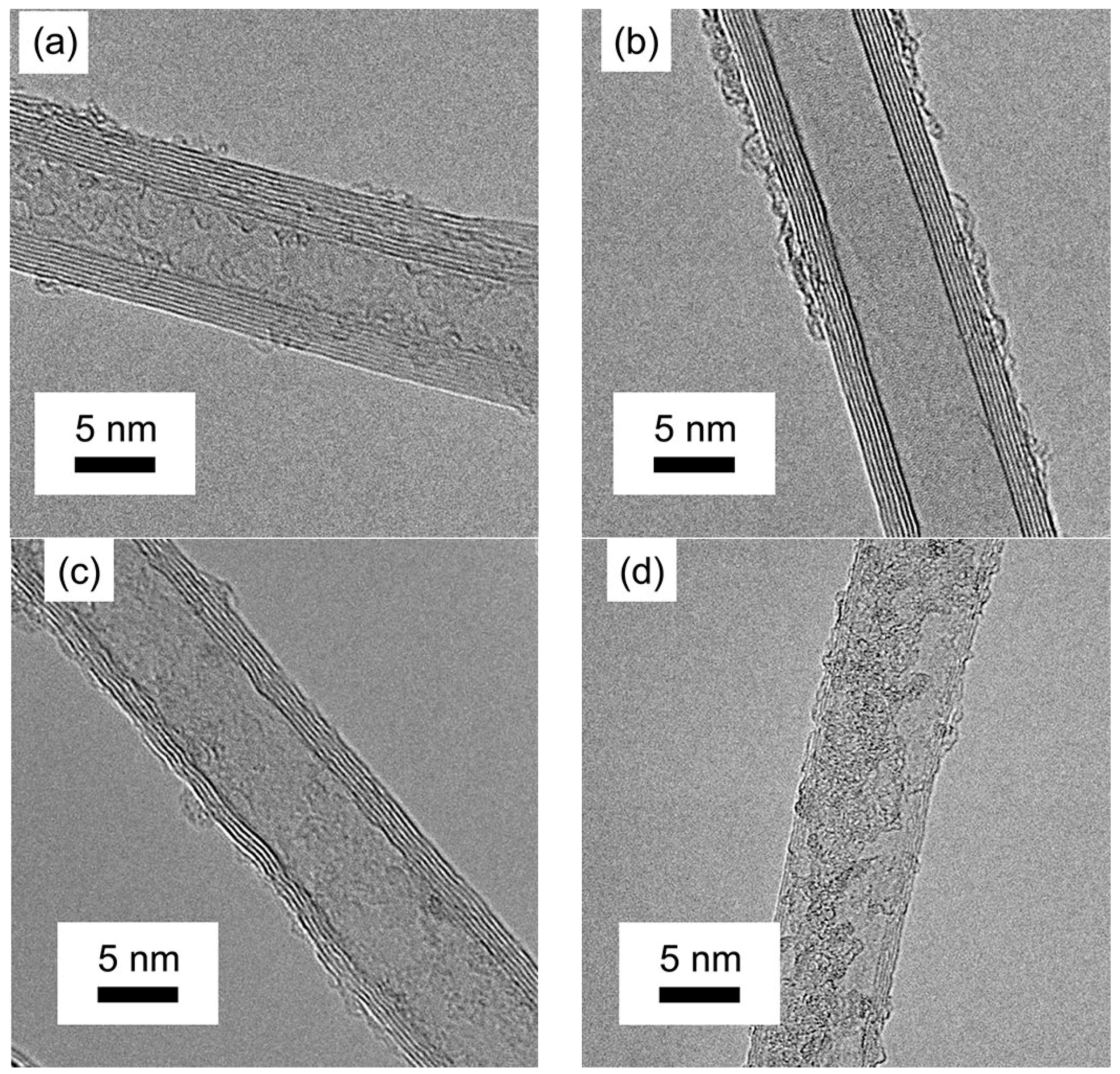
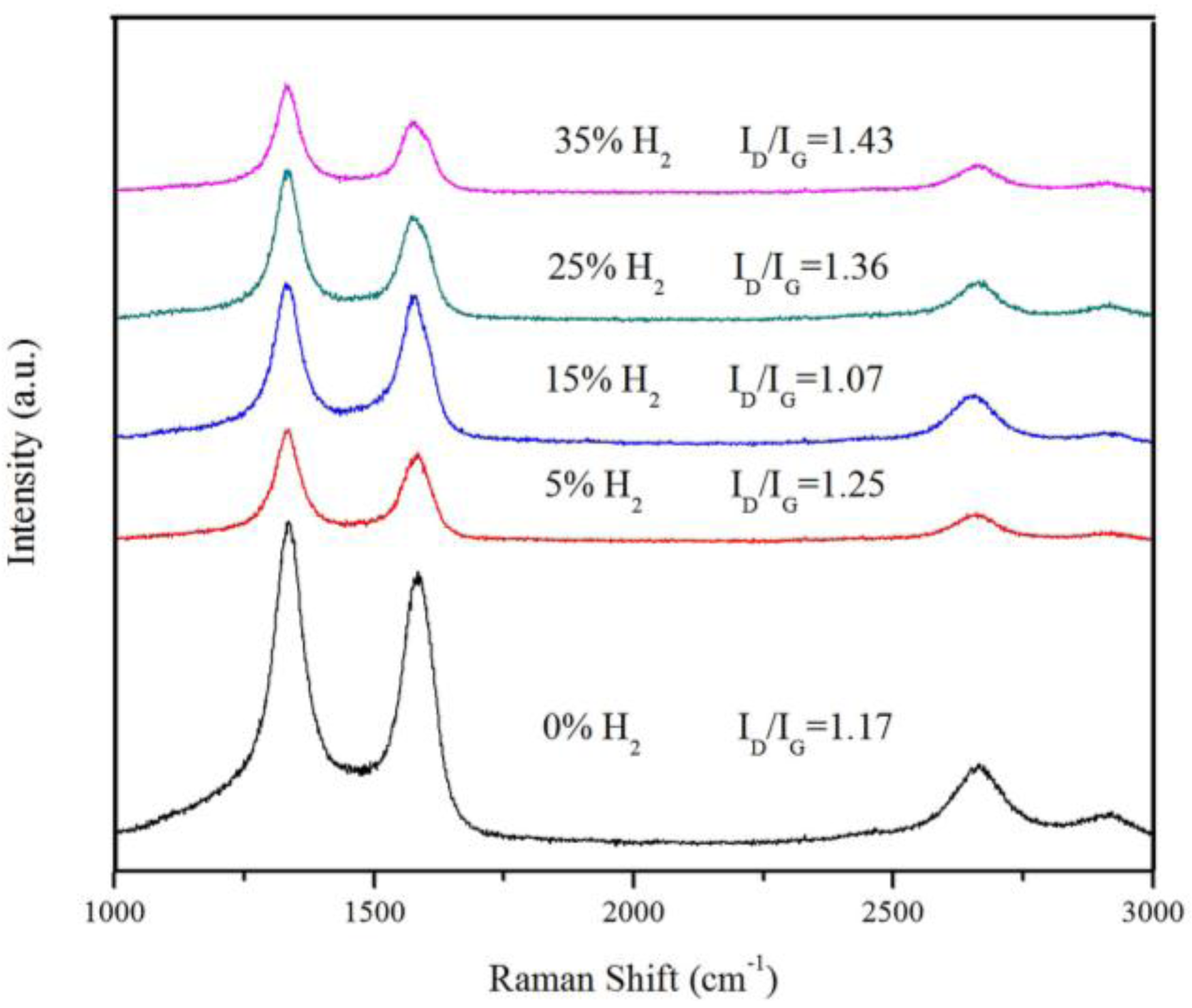
3.2. The Quality Analysis of VACNTs
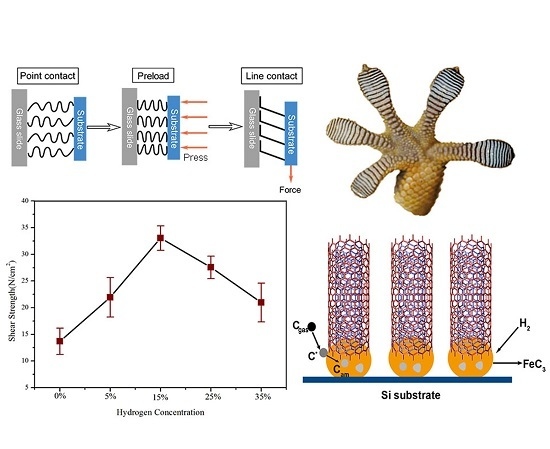
3.3. Influence Mechanism of Hydrogen Concentration on VACNTs Synthesis
3.4. Adhesive Performance Test of VACNTs
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Autumn, K.; Liang, Y.A.; Hsieh, S.T.; Zesch, W. Adhesive force of a single gecko foot-hair. Nature 2000, 405, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Autumn, K.; Sitti, M.; Liang, Y.A.; Peattie, A.M.; Hansen, W.R.; Sponberg, S.; Kenny, T.W.; Fearing, R.; Israelachvili, J.N.; Full, R.J. Evidence for van der waals adhesion in gecko setae. Proc. Natl. Acad. Sci. USA 2002, 99, 12252–12256. [Google Scholar] [CrossRef] [PubMed]
- Brodoceanu, D.; Bauer, C.T.; Kroner, E.; Arzt, E.; Kraus, T. Hierarchical bioinspired adhesive surfaces—A review. Bioinspir. Biomim. 2016, 11, 51001. [Google Scholar] [CrossRef] [PubMed]
- Autumn, K.; Niewiarowski, P.H.; Puthoff, J.B. Gecko adhesion as a model system for integrative biology, interdisciplinary science, and bioinspired engineering. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 445–470. [Google Scholar] [CrossRef]
- Kizilkan, E.; Strueben, J.; Staubitz, A.; Gorb, S.N. Bioinspired photocontrollable microstructured transport device. Sci. Robot. 2017, 2, 9454. [Google Scholar] [CrossRef]
- Jiang, H.; Hawkes, E.W.; Fuller, C.; Estrada, M.A.; Suresh, S.A.; Abcouwer, N.; Han, A.K.; Wang, S.; Ploch, C.J.; Parness, A. A robotic device using gecko-inspired adhesives can grasp and manipulate large objects in microgravity. Sci. Robot. 2017, 2, 4545. [Google Scholar] [CrossRef]
- Xu, Q.; Wan, Y.; Hu, T.S.; Liu, T.X.; Tao, D.; Niewiarowski, P.H.; Tian, Y.; Liu, Y.; Dai, L.; Yang, Y. Robust self-cleaning and micromanipulation capabilities of gecko spatulae and their bio-mimics. Nat. Commun. 2015, 6, 8949. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.E.; Suh, K.Y. Nanohairs and nanotubes: Efficient structural elements for gecko-inspired artificial dry adhesives. Nano Today 2009, 4, 335–346. [Google Scholar] [CrossRef]
- Jin, K.; Cremaldi, J.C.; Erickson, J.S.; Tian, Y.; Israelachvili, J.N.; Pesika, N.S. Biomimetic bidirectional switchable adhesive inspired by the gecko. Adv. Funct. Mater. 2014, 24, 574–579. [Google Scholar] [CrossRef]
- Hawkes, E.W.; Eason, E.V.; Asbeck, A.T.; Cutkosky, M.R. The gecko’s toe: Scaling directional adhesives for climbing applications. IEEE/ASME Trans. Mechatron. 2013, 18, 518–526. [Google Scholar] [CrossRef]
- Qu, L.; Dai, L.; Stone, M.; Xia, Z.; Wang, Z.L. Carbon nanotube arrays with strong shear binding-on and easy normal lifting-off. Science 2008, 322, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Du, F.; Ganguli, S.; Roy, A.; Dai, L. Carbon nanotube dry adhesives with temperature-enhanced adhesion over a large temperature range. Nat. Commun. 2016, 7, 13450. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Xu, G.; Gong, L.; Yong, Z.; Li, Q.; Dai, Z. Adhesion performance of gecko-inspired flexible carbon nanotubes dry adhesive. In Bioinspiration, Biomimetics, and Bioreplication 2013, Proceedings of SPIE Smart Structures and Materials + Nondestructive Evaluation and Health Monitoring, San Diego, CA, USA, 10–14 March 2013; Martín-Palma, R.J., Ed.; SPIE: Bellingham, WA, USA, 2013. [Google Scholar]
- Li, Y.; Zhang, H.; Yao, Y.; Li, T.; Zhang, Y.; Li, Q.; Dai, Z. Transfer of vertically aligned carbon nanotube arrays onto flexible substrates for gecko-inspired dry adhesive application. RSC Adv. 2015, 5, 46749–46759. [Google Scholar] [CrossRef]
- Zhao, Y.; Tong, T.; Delzeit, L.; Kashani, A.; Meyyappan, M.; Majumdar, A. Interfacial energy and strength of multiwalled-carbon-nanotube-based dry adhesive. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2006, 24, 331–335. [Google Scholar] [CrossRef]
- Dhinojwala, A. Carbon nanotube-based synthetic gecko tapes. Proc. Natl. Acad. Sci. USA 2007, 104, 10792–10795. [Google Scholar]
- Tian, Y.; Pesika, N.; Zeng, H.; Rosenberg, K.; Zhao, B.; McGuiggan, P.; Autumn, K.; Israelachvili, J. Adhesion and friction in gecko toe attachment and detachment. Proc. Natl. Acad. Sci. USA 2006, 103, 19320–19325. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Majidi, C.; Schubert, B.; Fearing, R.S. Sliding-induced adhesion of stiff polymer microfibre arrays. I. Macroscale behaviour. J. R. Soc. Interface 2008, 5, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Maeno, Y.; Nakayama, Y. Geckolike high shear strength by carbon nanotube fiber adhesives. Appl. Phys. Lett. 2009, 94, 012103. [Google Scholar] [CrossRef]
- Wirth, C.T.; Hofmann, S.; Robertson, J. Surface properties of vertically aligned carbon nanotube arrays. Diam. Relat. Mater. 2008, 17, 1518–1524. [Google Scholar] [CrossRef]
- Cui, Y.; Ju, Y.; Xu, B.; Wang, P.; Kojima, N.; Ichioka, K.; Hosoi, A. Mimicking a gecko foot with strong adhesive strength based on a spinnable vertically aligned carbon nanotube array. RSC Adv. 2013, 4, 9056–9060. [Google Scholar] [CrossRef]
- Li, P.; Zhang, J. CVD growth of carbon nanotube forest with selective wall-number from Fe-Cu catalyst. J. Phys. Chem. C 2016, 120, 11163–11169. [Google Scholar] [CrossRef]
- Hoecker, C.; Smail, F.; Bajada, M.; Pick, M.; Boies, A. Catalyst nanoparticle growth dynamics and their influence on product morphology in a CVD process for continuous carbon nanotube synthesis. Carbon 2016, 96, 116–124. [Google Scholar] [CrossRef]
- Esconjauregui, S.; Fouquet, M.; Bayer, B.C.; Ducati, C.; Smajda, R.; Hofmann, S.; Robertson, J. Growth of ultrahigh density vertically aligned carbon nanotube forests for interconnects. ACS Nano 2010, 4, 7431–7436. [Google Scholar] [CrossRef] [PubMed]
- Wirth, C.T.; Hofmann, S.; Robertson, J. ChemInform Abstract: State of the catalyst during carbon nanotube growth. Cheminform 2010, 40, 940–945. [Google Scholar] [CrossRef]
- Hofmann, S.; Cantoro, M.; Kleinsorge, B.; Casiraghi, C.; Parvez, A.; Robertson, J.; Ducati, C. Effects of catalyst film thickness on plasma-enhanced carbon nanotube growth. J. Appl. Phys. 2005, 98, 034308. [Google Scholar] [CrossRef]
- Wei, B.Q.; Vajtai, R.; Ajayan, P.M. Reliability and current carrying capacity of carbon nanotubes. Appl. Phys. Lett. 2001, 79, 1172–1174. [Google Scholar] [CrossRef]
- Jung, Y.J.; Vajtai, R.; Ajayan, P.M.; Homma, Y.; Prabhakaran, K.; Ogino, T. Mechanism of selective growth of carbon nanotubes on SiO2/Si patterns. Nano Lett. 2003, 3, 561–564. [Google Scholar] [CrossRef]
- Homma, Y.; Kobayashi, Y.; Ogino, T. Role of transition metal catalysts in single-walled carbon nanotube growth in chemical vapor deposition. J. Phys. Chem. B 2003, 107, 12161–12164. [Google Scholar] [CrossRef]
- Jourdain, V.; Bichara, C. Current understanding of the growth of carbon nanotubes in catalytic chemical vapour deposition. Carbon 2013, 58, 2–39. [Google Scholar] [CrossRef] [Green Version]
- Schneider, J.J. Vertically aligned carbon nanotubes as platform for biomimetically inspired mechanical sensing, bioactive surfaces, and electrical cell interfacing. Adv. Biosyst. 2017, 1, 1700101. [Google Scholar] [CrossRef]
- Plata, D.L.; Hart, A.J.; Reddy, C.M.; Gschwend, P.M. Early evaluation of potential environmental impacts of carbon nanotube synthesis by chemical vapor deposition. Environ. Sci. Technol. 2009, 43, 8367–8373. [Google Scholar] [CrossRef] [PubMed]
- Shukla, B.; Saito, T.; Ohmori, S.; Koshi, M.; Yumura, M.; Iijima, S. Interdependency of gas phase intermediates and chemical vapor deposition growth of single wall carbon nanotubes. Chem. Mater. 2010, 22, 6035–6043. [Google Scholar] [CrossRef]
- Shah, K.A.; Tali, B.A. Synthesis of carbon nanotubes by catalytic chemical vapour deposition: A review on carbon sources, catalysts and substrates. Mater. Sci. Semicond. Process. 2016, 41, 67–82. [Google Scholar] [CrossRef]
- Stadermann, M.; Sherlock, S.P.; In, J.-B.; Fornasiero, F.; Park, H.G.; Artyukhin, A.B.; Wang, Y.; de Yoreo, J.J.; Grigoropoulos, C.P.; Bakajin, O.; et al. Mechanism and kinetics of growth termination in controlled chemical vapor deposition growth of multiwall carbon nanotube arrays. Nano Lett. 2009, 9, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Douven, S.; Pirard, S.L.; Heyen, G.; Toye, D.; Pirard, J. Kinetic study of double-walled carbon nanotube synthesis by catalytic chemical vapour deposition over an Fe–Mo/MgO catalyst using methane as the carbon source. Chem. Eng. J. 2011, 175, 396–407. [Google Scholar] [CrossRef]
- Joshi, R.; Schneider, J.J.; Yilmazoglu, O.; Pavlidis, D. Patterned growth of ultra long carbon nanotubes. Properties and systematic investigation into their growth process. J. Mater. Chem. 2010, 20, 1717–1721. [Google Scholar] [CrossRef]
- Meyyappan, M. A review of plasma enhanced chemical vapour deposition of carbon nanotubes. J. Phys. D Appl. Phys. 2009, 42, 213001. [Google Scholar] [CrossRef]
- Li, Y.; Xu, G.; Zhang, H.; Li, T.; Yao, Y.; Li, Q.; Dai, Z. Alcohol-assisted rapid growth of vertically aligned carbon nanotube arrays. Carbon 2015, 91, 45–55. [Google Scholar] [CrossRef]
- Zhang, G.; Mann, D.; Zhang, L.; Javey, A.; Li, Y.; Yenilmez, E.; Wang, Q.; McVittie, J.P.; Nishi, Y.; Gibbons, J. Ultra-high-yield growth of vertical single-walled carbon nanotubes: Hidden roles of hydrogen and oxygen. Proc. Natl. Acad. Sci. USA 2005, 102, 16141–16145. [Google Scholar] [CrossRef] [PubMed]
- In, J.B.; Grigoropoulos, C.P.; Chernov, A.A.; Noy, A. Growth kinetics of vertically aligned carbon nanotube arrays in clean oxygen-free conditions. ACS Nano 2011, 5, 9602–9610. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Engstler, J.; Houben, L.; Sadan, M.B.; Weidenkaff, A.; Mandaliev, P.; Issanin, A.; Schneider, J.J. Catalyst composition, morphology and reaction pathway in the growth of “super-long” carbon nanotubes. ChemCatChem 2010, 2, 1069–1073. [Google Scholar] [CrossRef]
- Joshi, R.; Waldschmidt, B.; Engstler, J.; Schäfer, R.; Schneider, J.J. Generation and agglomeration behaviour of size-selected sub-nm iron clusters as catalysts for the growth of carbon nanotubes. Beilstein J. Nanotech. 2011, 2, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Schaber, C.F.; Heinlein, T.; Keeley, G.; Schneider, J.J.; Gorb, S.N. Tribological properties of vertically aligned carbon nanotube arrays. Carbon 2015, 94, 396–404. [Google Scholar] [CrossRef]
- Schaber, C.F.; Filippov, A.E.; Heinlein, T.; Schneider, J.J.; Gorb, S.N. Modelling clustering of vertically aligned carbon nanotube arrays. Interface Focus 2015, 5, 20150026. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhong, G.; Goldberg Oppenheimer, P.; Zhang, C.; Tornatzky, H.; Esconjauregui, S.; Hofmann, S.; Robertson, J. Influence of packing density and surface roughness of vertically-aligned carbon nanotubes on adhesive properties of gecko-inspired mimetics. ACS Appl. Mater. Interfaces 2015, 7, 3626–3632. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.J.; Engstler, J.; Franzka, S.; Hofmann, K.; Albert, B.; Ensling, J.; Gütlich, P.; Hildebrandt, P.; Döpner, S.; Pfleging, W. Carbon nanotube bags: Catalytic formation, physical properties, two-dimensional alignment and geometric structuring of densely filled carbon tubes. Chemistry 2001, 7, 2888–2895. [Google Scholar] [CrossRef]
- Mahanandia, P.; Schneider, J.J.; Engel, M.; Stühn, B.; Subramanyam, S.V.; Nanda, K.K. Studies towards synthesis, evolution and alignment characteristics of dense, millimeter long multiwalled carbon nanotube arrays. Beilstein J. Nanotech. 2011, 2, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.H.; Futaba, D.N.; Yumura, M.; Hata, K. Direct wall number control of carbon nanotube forests from engineered iron catalysts. J. Nanosci. Nanotechnol. 2013, 13, 2745–2751. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Futaba, D.N.; Sakurai, S.; Yumura, M.; Hata, K. Interplay of wall number and diameter on the electrical conductivity of carbon nanotube thin films. Carbon 2014, 67, 318–325. [Google Scholar] [CrossRef]
- Yamada, T.; Namai, T.; Hata, K.; Futaba, D.N.; Mizuno, K.; Fan, J.; Yudasaka, M.; Yumura, M.; Iijima, S. Size-selective growth of double-walled carbon nanotube forests from engineered iron catalysts. Nat. Nanotechnol. 2006, 1, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Mattevi, C.; Wirth, C.T.; Hofmann, S.; Blume, R.; Cantoro, M.; Ducati, C.; Cepek, C.; Knop-Gericke, A.; Milne, S.; Castellarin-Cudia, C. In-situ X-ray photoelectron spectroscopy study of catalyst—Support interactions and growth of carbon nanotube forests. J. Phys. Chem. C 2008, 112, 12207–12213. [Google Scholar] [CrossRef]
- Kumar, M.; Ando, Y. Chemical vapor deposition of carbon nanotubes: A review on growth mechanism and mass production. J. Nanosci. Nanotechnol. 2010, 10, 3739–3758. [Google Scholar] [CrossRef] [PubMed]
- Behr, M.J.; Gaulding, E.A.; Mkhoyan, K.A.; Aydil, E.S. Effect of hydrogen on catalyst nanoparticles in carbon nanotube growth. J. Appl. Phys. 2010, 108, 053303. [Google Scholar] [CrossRef]
- Maeno, Y.; Nakayama, Y. Experimental investigation of adhesive behavior in carbon nanotube based gecko tape. J. Adhes. 2012, 88, 243–252. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Ji, K.; Duan, Y.; Meng, G.; Dai, Z. Effect of Hydrogen Concentration on the Growth of Carbon Nanotube Arrays for Gecko-Inspired Adhesive Applications. Coatings 2017, 7, 221. https://doi.org/10.3390/coatings7120221
Li Y, Ji K, Duan Y, Meng G, Dai Z. Effect of Hydrogen Concentration on the Growth of Carbon Nanotube Arrays for Gecko-Inspired Adhesive Applications. Coatings. 2017; 7(12):221. https://doi.org/10.3390/coatings7120221
Chicago/Turabian StyleLi, Yang, Keju Ji, Yali Duan, Guiyun Meng, and Zhendong Dai. 2017. "Effect of Hydrogen Concentration on the Growth of Carbon Nanotube Arrays for Gecko-Inspired Adhesive Applications" Coatings 7, no. 12: 221. https://doi.org/10.3390/coatings7120221