Multi-Walled Carbon Nanotube-Assisted Electrodeposition of Silver Dendrite Coating as a Catalytic Film
Abstract
:1. Introduction
2. Materials and Methods
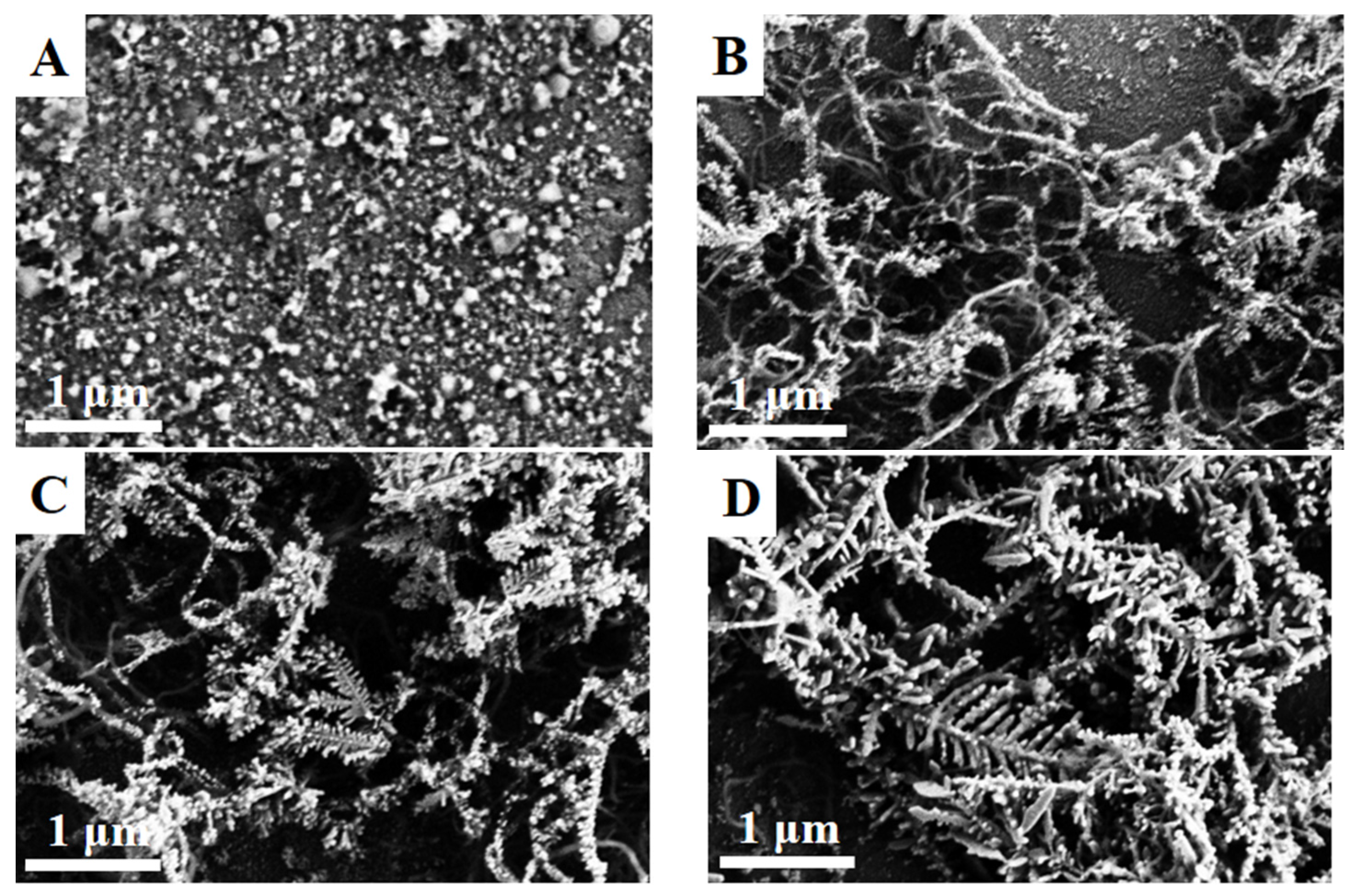
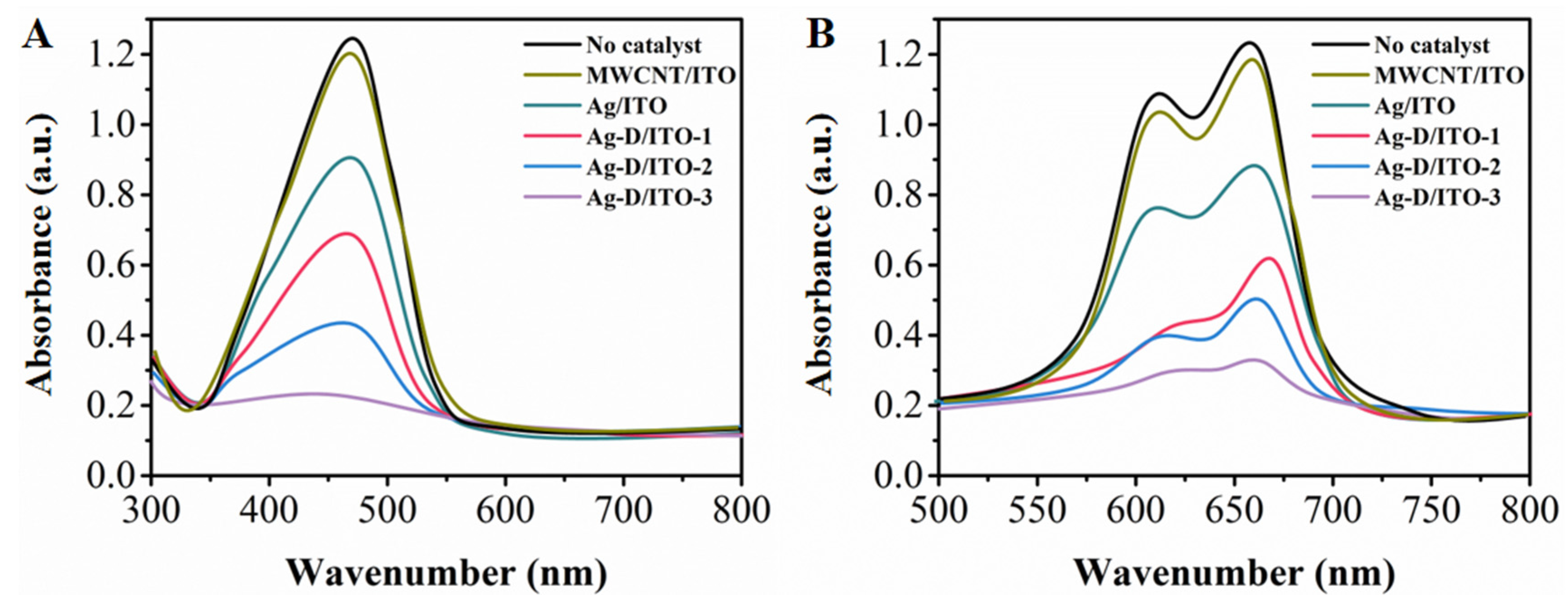
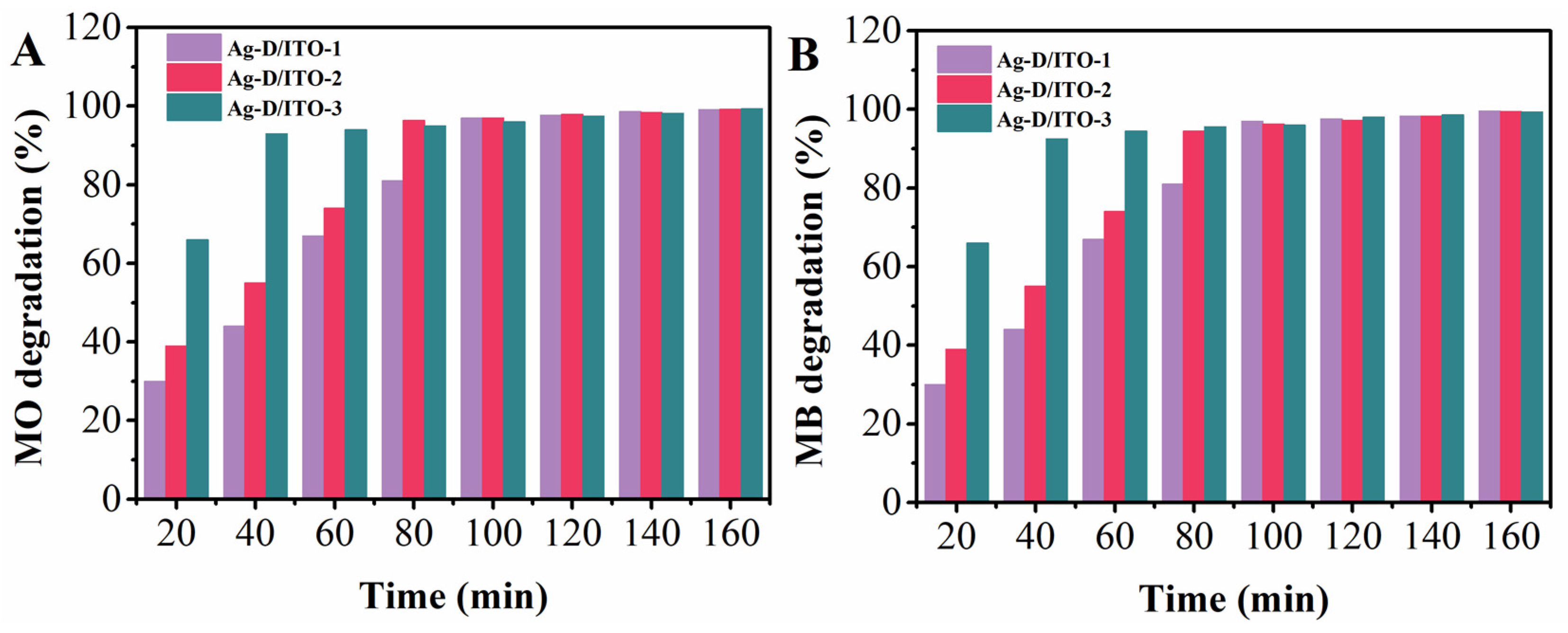
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhu, C.; Du, D.; Eychmüller, A.; Lin, Y. Engineering ordered and nonordered porous noble metal nanostructures: Synthesis, assembly, and their applications in electrochemistry. Chem. Rev. 2015, 115, 8896–8943. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Li, H.; Fu, S.; Du, D.; Lin, Y. Highly efficient nonprecious metal catalysts towards oxygen reduction reaction based on three-dimensional porous carbon nanostructures. Chem. Soc. Rev. 2016, 45, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Jeong, H.; Lee, K.-H. Facile conversion of bulk metal surface to metal oxide single-crystalline nanostructures by microwave irradiation: Formation of pure or Cr-doped hematite nanostructure arrays. Thin Solid Films 2017, 518, 5110–5114. [Google Scholar] [CrossRef]
- Erdem, A.; Meric, B.; Kerman, K.; Dalbasti, T.; Ozsoz, M. Detection of interaction between metal complex indicator and DNA by using electrochemical biosensor. Electroanalysis 2015, 11, 1372–1376. [Google Scholar] [CrossRef]
- Wu, L.-L.; Wang, Z.; Zhao, S.-N.; Meng, X.; Song, X.-Z.; Feng, J.; Song, S.-Y.; Zhang, H.-J. A metal-organic framework/DNA hybrid system as a novel fluorescent biosensor for mercury(II) ion detection. Chemistry 2016, 22, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, D.B.; Adamson, D.; Arndtsen, B.A. A novel example of chiral counteranion induced enantioselective metal catalysis: The importance of ion-pairing in copper-catalyzed olefin aziridination and cyclopropanation. Org. Lett. 2015, 2, 4165–4168. [Google Scholar] [CrossRef]
- Zheng, C.; You, S.-L. Catalytic asymmetric dearomatization by transition-metal catalysis: A method for transformations of aromatic compounds. Dalton Trans. 2016, 25, 1454–1456. [Google Scholar] [CrossRef]
- Shimizu, H. Behavior of metal-induced oxide charge during thermal oxidation in silicon wafers. J. Electrochem. Soc. 2016, 144, 4335–4340. [Google Scholar] [CrossRef]
- Elangovan, D.; Yuzay, I.E.; Selke, S.E.M.; Auras, R. Poly(L-lactic acid) metal organic framework composites: Optical, thermal and mechanical properties. Polym. Int. 2015, 61, 30–37. [Google Scholar] [CrossRef]
- Cong, S.; Yuan, Y.; Chen, Z.; Hou, J.; Yang, M.; Su, Y.; Zhang, Y.; Li, L.; Li, Q.; Geng, F. Noble metal-comparable sers enhancement from semiconducting metal oxides by making oxygen vacancies. Nat. Commun. 2015, 6, 7800. [Google Scholar] [CrossRef] [PubMed]
- Hugall, J.T.; Baumberg, J.J. Demonstrating photoluminescence from au is electronic inelastic light scattering of a plasmonic metal: The origin of sers backgrounds. Nano Lett. 2015, 15, 2600–2604. [Google Scholar] [CrossRef] [PubMed]
- Long, N.V. Engineering of sers substrates based on noble metal nanomaterials for chemical and biomedical applications. Appl. Spectrosc. Rev. 2015, 50, 499–525. [Google Scholar]
- Chen, Y.; Tan, R.; Zhang, Y.; Zhao, G.; Yin, D. Dendritic chiral salen titanium(IV) catalysts enforce the cooperative catalysis of asymmetric sulfoxidation. Chemcatchem 2016, 7, 4066–4075. [Google Scholar] [CrossRef]
- Wang, D.; Deraedt, C.; Ruiz, J.; Astruc, D. Magnetic and dendritic catalysts. Acc. Chem. Res. 2015, 48, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Pan, Y.; Gu, H. Synthesis of Pt dendritic nanocubes with enhanced catalytic properties. RSC Adv. 2015, 5, 16497–16500. [Google Scholar] [CrossRef]
- Vieira, E.G.; Silva, R.O.; Dalbó, A.G.; Frizon, T.E.A.; Filho, N.L.D. Syntheses and catalytic activities of new metallodendritic catalysts. New J. Chem. 2016, 40, 9403–9414. [Google Scholar] [CrossRef]
- Xia, Q.; Jiang, Z.; Wang, J.; Yao, Z. A facile preparation of hierarchical dendritic zero-valent iron for fenton-like degradation of phenol. Catal. Commun. 2017, 100, 57–61. [Google Scholar] [CrossRef]
- Cui, S.; Lu, S.; Xu, W.; Wu, B.; Zhao, N.; He, G.; Hou, X.; Zhang, H. Robust dendritic Ag-Fe2O3/Fe surfaces with exquisite catalytic properties. New J. Chem. 2016, 40, 8897–8904. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Thakur, D.; Panigrahy, B.; Bahadur, D. Highly efficient and simultaneous catalytic reduction of multiple dyes using recyclable RGO/Co dendritic nanocomposites as catalyst for the wastewater treatment. RSC Adv. 2016, 6, 106723–106731. [Google Scholar] [CrossRef]
- Rheiner, P.B.; Seebach, D. Dendritic taddols: Synthesis, characterization and use in the catalytic enantioselective addition of Et2Zn to benzaldehyde. Chem. A Eur. J. 2015, 5, 3221–3236. [Google Scholar] [CrossRef]
- Tajabadi, M.T.; Basirun, W.J.; Lorestani, F.; Zakaria, R.; Baradaran, S.; Amin, Y.M.; Mahmoudian, M.R.; Rezayi, M.; Sookhakian, M. Nitrogen-doped graphene-silver nanodendrites for the non-enzymatic detection of hydrogen peroxide. Electrochim. Acta 2015, 151, 126–133. [Google Scholar] [CrossRef]
- Song, J.; Hou, J.; Tian, L.; Guan, Y.; Zhang, Y.; Zhu, X. Growth of giant silver dendrites on layer-by-layer assembled films. Polymer 2015, 63, 237–243. [Google Scholar] [CrossRef]
- Hu, L.; Liu, Y.J.; Han, Y.; Chen, P.; Zhang, C.; Li, C.; Lu, Z.; Luo, D.; Jiang, S. Graphene oxide-decorated silver dendrites for high-performance surface-enhanced raman scattering applications. J. Mater. Chem. C 2017, 5, 3908–3915. [Google Scholar] [CrossRef]
- Gao, Y.; Xia, B.; Liu, J.; Ding, L.; Li, B.; Zhou, Y. Reversible tuning of the wettability on a silver mesodendritic surface by the formation and disruption of lipid-like bilayers. Appl. Surf. Sci. 2015, 329, 150–157. [Google Scholar] [CrossRef]
- Nersisyan, H.H.; Lee, Y.J.; Joo, S.H.; Han, S.K.; Lee, T.H.; Lee, J.S.; An, Y.S.; Lee, J.H. Iron-assisted electroless deposition reaction for synthesizing copper and silver dendritic structures. Crystengcomm 2015, 17, 7535–7542. [Google Scholar] [CrossRef]
- Guadagnini, L.; Ballarin, B.; Tonelli, D. Dendritic silver nanostructures obtained via one-step electrosynthesis: Effect of nonanesulfonic acid and polyvinylpyrrolidone as additives on the analytical performance for hydrogen peroxide sensing. J. Nanopart. Res. 2013, 15, 1971. [Google Scholar] [CrossRef]
- Fu, L.; Sokiransky, M.M.; Wang, J.; Lai, G.; Yu, A. Development of ag dendrites-reduced graphene oxide composite catalysts via galvanic replacement reaction. Phys. E Low-dimens. Syst. Nanostruct. 2016, 83, 146–150. [Google Scholar] [CrossRef]
- Fu, L.; Lai, G.; Mahon, P.J.; Wang, J.; Zhu, D.; Jia, B.; Malherbe, F.; Yu, A. Carbon nanotube and graphene oxide directed electrochemical synthesis of silver dendrites. RSC Adv. 2014, 4, 39645–39650. [Google Scholar] [CrossRef]
- Fu, L.; Yong, J.; Lai, G.; Tamanna, T.; Notley, S.; Yu, A. Nanocomposite coating of multilayered carbon nanotube–titania. Mater. Manuf. Process. 2014, 29, 1030–1036. [Google Scholar] [CrossRef]
- Witten, T.A., Jr.; Sander, L.M. Diffusion-limited aggregation, a kinetic critical phenomenon. Phys. Rev. Lett. 1981, 47, 1400–1403. [Google Scholar] [CrossRef]
- Ben-Jacob, E.; Garik, P. The formation of patterns in non-equilibrium growth. Nature 1990, 343, 523–530. [Google Scholar] [CrossRef]
- Huang, Y.F.; Zhang, M.; Zhao, L.B.; Feng, J.M.; Wu, D.Y.; Ren, B.; Tian, Z.Q. Activation of oxygen on gold and silver nanoparticles assisted by surface plasmon resonances. Angew. Chem. Int. Ed. 2014, 53, 2353–2357. [Google Scholar] [CrossRef] [PubMed]
- Kaniyankandy, S.; Nuwad, J.; Thinaharan, C.; Dey, G.K.; Pillai, C.G.S. Electrodeposition of silver nanodendrites. Nanotechnology 2007, 18, 125610. [Google Scholar] [CrossRef]
- Rashid, M.H.; Mandal, T.K. Synthesis and catalytic application of nanostructured silver dendrites. J. Phys. Chem. C 2007, 111, 16750–16760. [Google Scholar] [CrossRef]
- Wang, X.; Itoh, H.; Naka, K.; Chujo, Y. Tetrathiafulvalene-assisted formation of silver dendritic nanostructures in acetonitrile. Langmuir 2003, 19, 6242–6246. [Google Scholar] [CrossRef]
- Vidhu, V.K.; Philip, D. Catalytic degradation of organic dyes using biosynthesized silver nanoparticles. Micron 2014, 56, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Frelink, T.; Visscher, W.; Van Veen, J. Particle size effect of carbon-supported platinum catalysts for the electrooxidation of methanol. J. Electroanal. Chem. 1995, 382, 65–72. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, Q.; Zhu, Z.; Zhang, J. Preparation and properties of vanadium-doped TiO2 photocatalysts. J. Phys. D Appl. Phys. 2010, 43, 035301. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation pathway of methylene blue in water. Appl. Ctatl. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Ji, Z.; Shen, X.; Yang, J.; Zhu, G.; Chen, K. A novel reduced graphene oxide/Ag/CeO2 ternary nanocomposite: Green synthesis and catalytic properties. Appl. Ctatl. B Environ. 2014, 144, 454–461. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, H.; Zhou, L.; Bao, C.; Zhu, H.; Zhang, Y. Synthesis and application of RGO/CoFe2O4 composite for catalytic degradation of methylene blue on heterogeneous fenton-like oxidation. J. Taiwan Inst. Chem. Eng. 2016, 67, 484–494. [Google Scholar] [CrossRef]
- Li, K.; Luo, X.; Lin, X.; Qi, F.; Wu, P. Novel nicomno4 thermocatalyst for low-temperature catalytic degradation of methylene blue. J. Mol. Catal. A Chem. 2014, 383–384, 1–9. [Google Scholar] [CrossRef]
- Suvith, V.S.; Philip, D. Catalytic degradation of methylene blue using biosynthesized gold and silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Lee, J.; Cho, M.H. Au@TiO2 nanocomposites for the catalytic degradation of methyl orange and methylene blue: An electron relay effect. J. Ind. Eng. Chem. 2014, 20, 1584–1590. [Google Scholar] [CrossRef]

| Catalyst | MO Rate Constant (min−1) | MB Rate Constant (min−1) | Reference |
|---|---|---|---|
| RGO/CoFe2O4 | – | 0.2651 | [41] |
| NiCoMnO4 | – | 0.0096 | [42] |
| Biosynthesized Au/Ag | – | 0.668 | [43] |
| Au@TiO2 | 0.0177 | 0.0482 | [44] |
| Ag-D/ITO-3 | 0.06648 | 0.06475 | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, L.; Xie, K.; Zhang, H.; Zheng, Y.; Su, W.; Liu, Z. Multi-Walled Carbon Nanotube-Assisted Electrodeposition of Silver Dendrite Coating as a Catalytic Film. Coatings 2017, 7, 232. https://doi.org/10.3390/coatings7120232
Fu L, Xie K, Zhang H, Zheng Y, Su W, Liu Z. Multi-Walled Carbon Nanotube-Assisted Electrodeposition of Silver Dendrite Coating as a Catalytic Film. Coatings. 2017; 7(12):232. https://doi.org/10.3390/coatings7120232
Chicago/Turabian StyleFu, Li, Kefeng Xie, Huaiwei Zhang, Yuhong Zheng, Weitao Su, and Zhong Liu. 2017. "Multi-Walled Carbon Nanotube-Assisted Electrodeposition of Silver Dendrite Coating as a Catalytic Film" Coatings 7, no. 12: 232. https://doi.org/10.3390/coatings7120232






