Influence of Heating Conditions for Formation of a Thin Apatite Film on Zirconia Using a Molecular Precursor Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zirconia Substrate
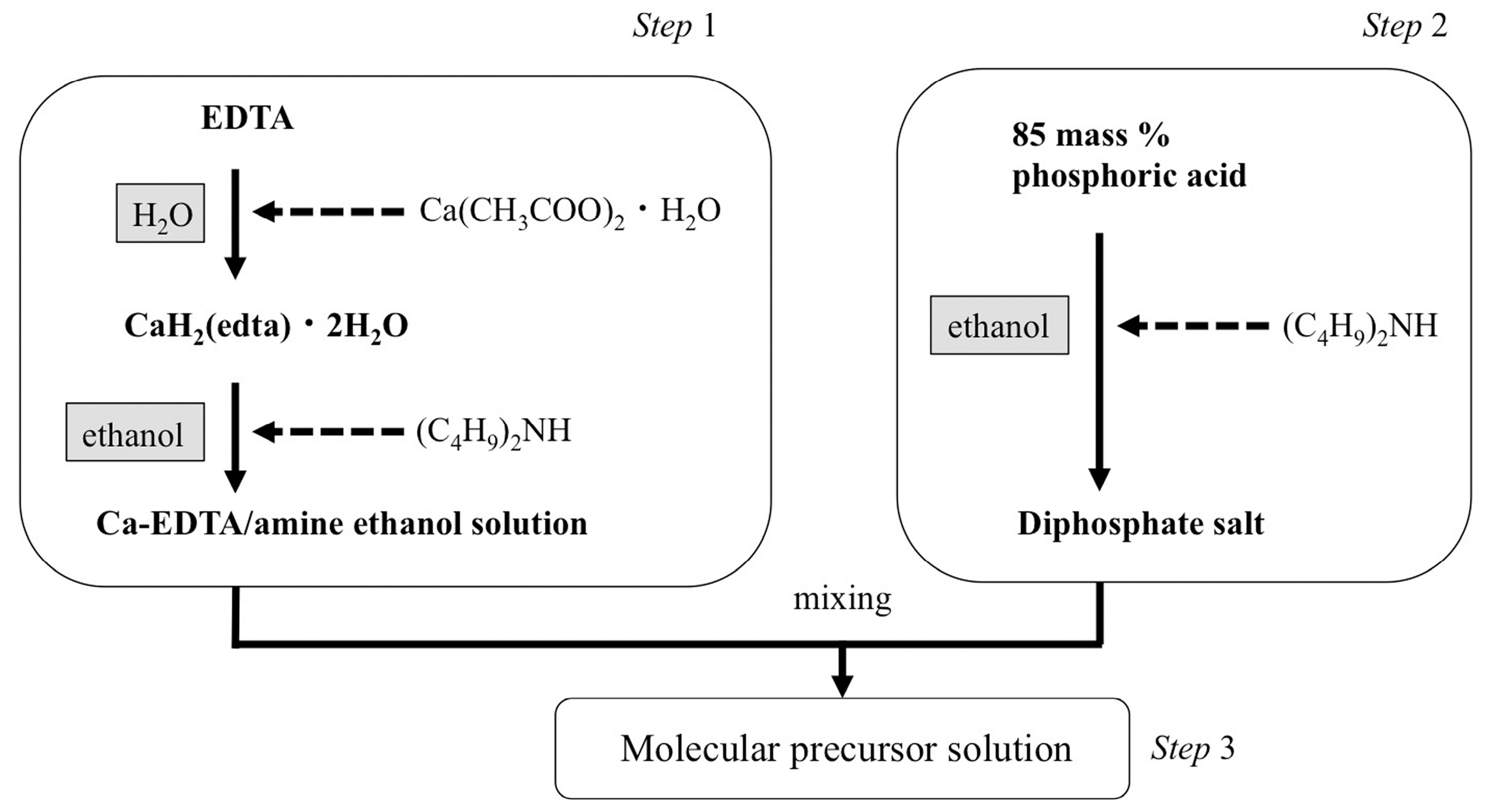
2.2. Apatite Coating Using the Molecular Precursor Method
2.3. Morphology and Surface Roughness
2.4. Surface Wettability
2.5. Crystal Structure of CA Coating
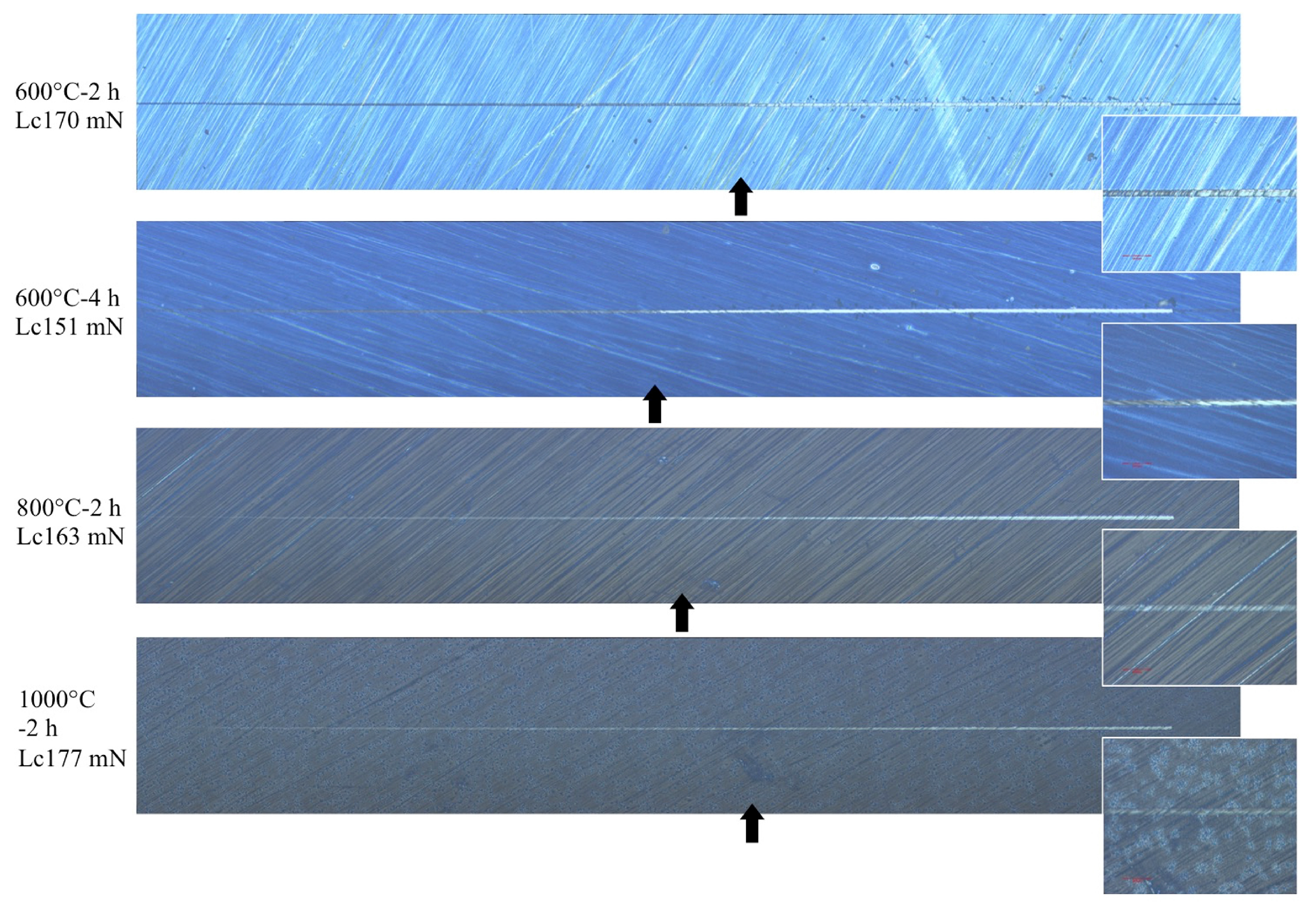
2.6. Adhesiveness of CA Coating Films
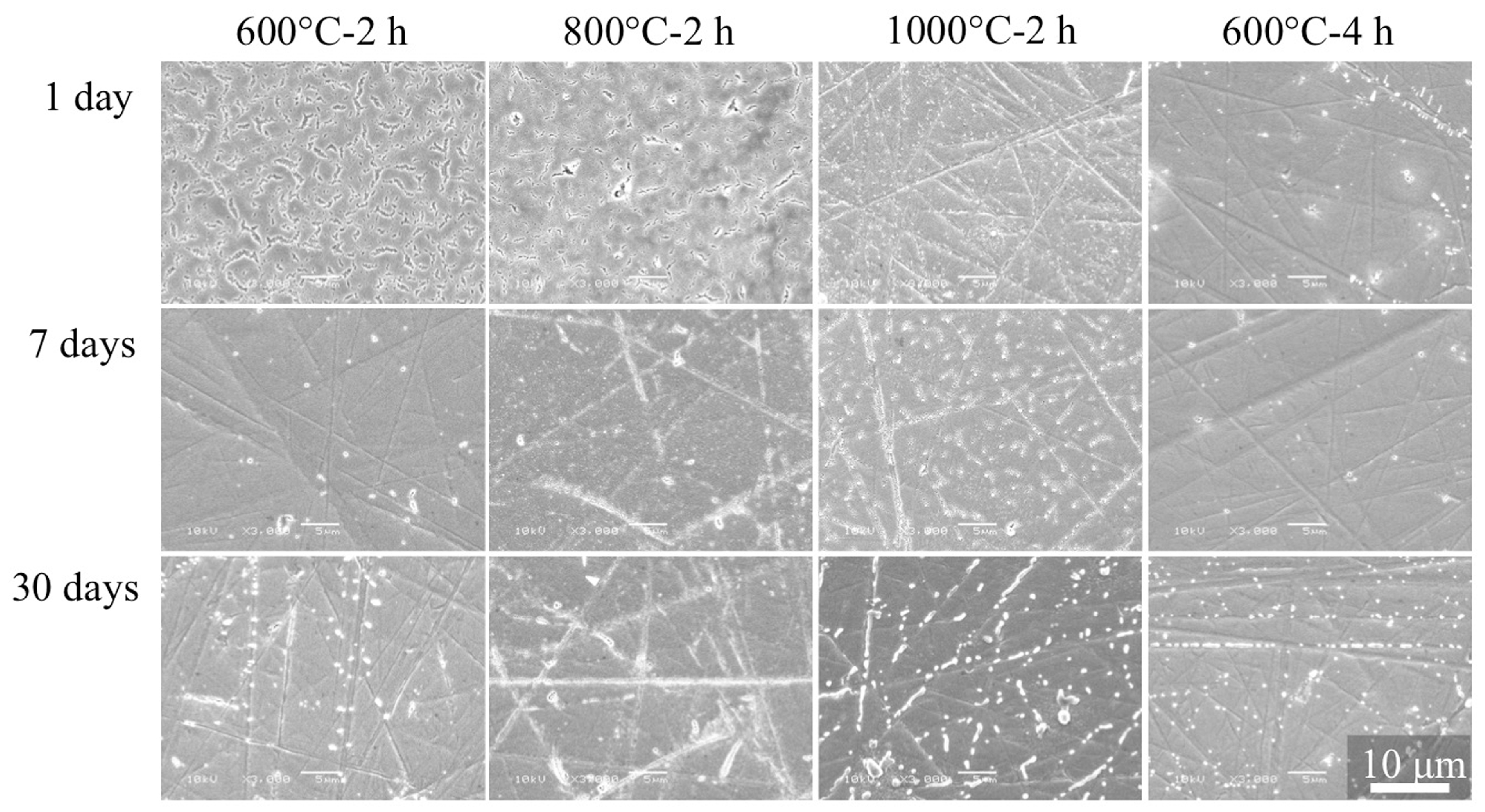
2.7. Durability of CA Coating
2.8. SBF Immersion
2.9. Statistical Analysis
3. Results
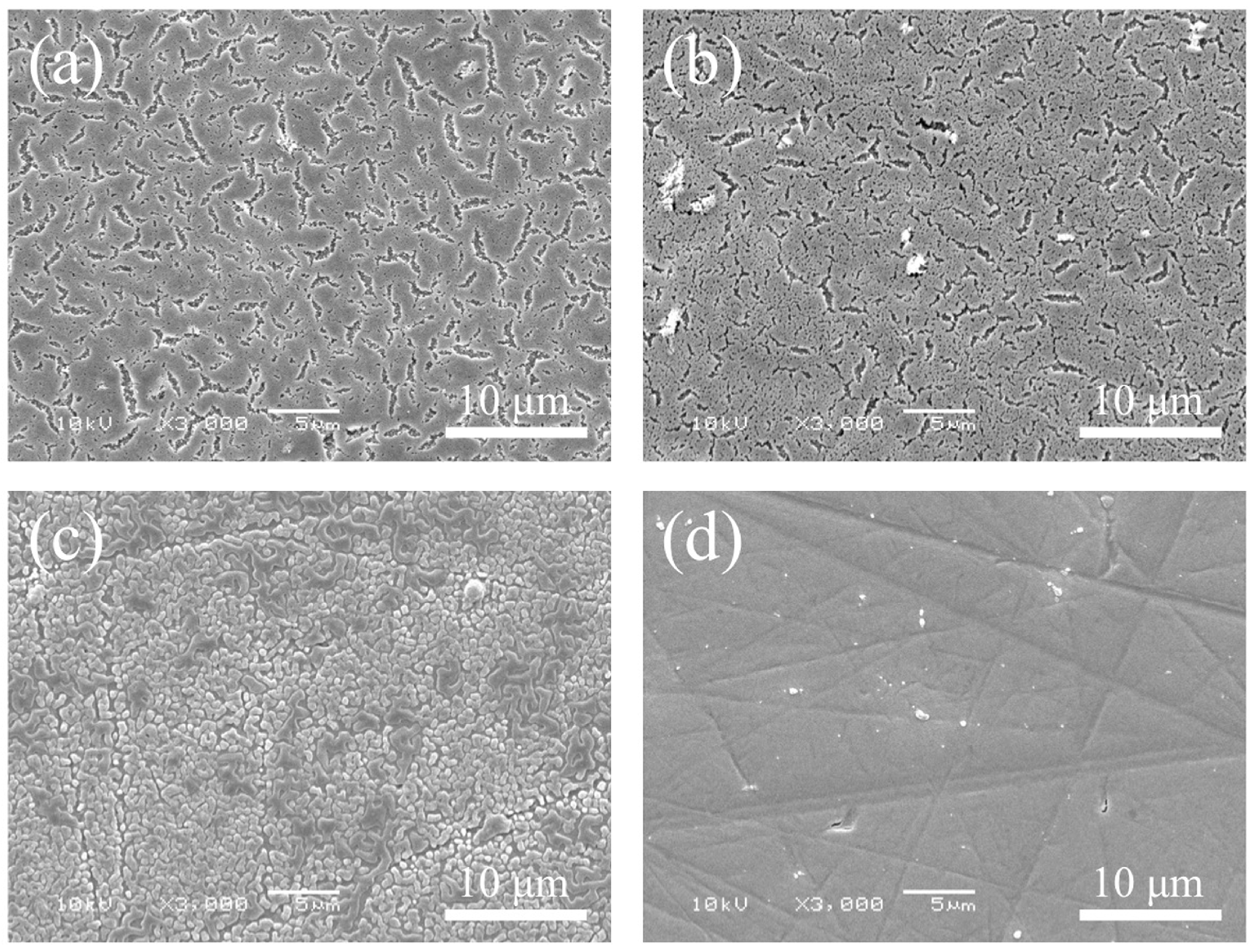
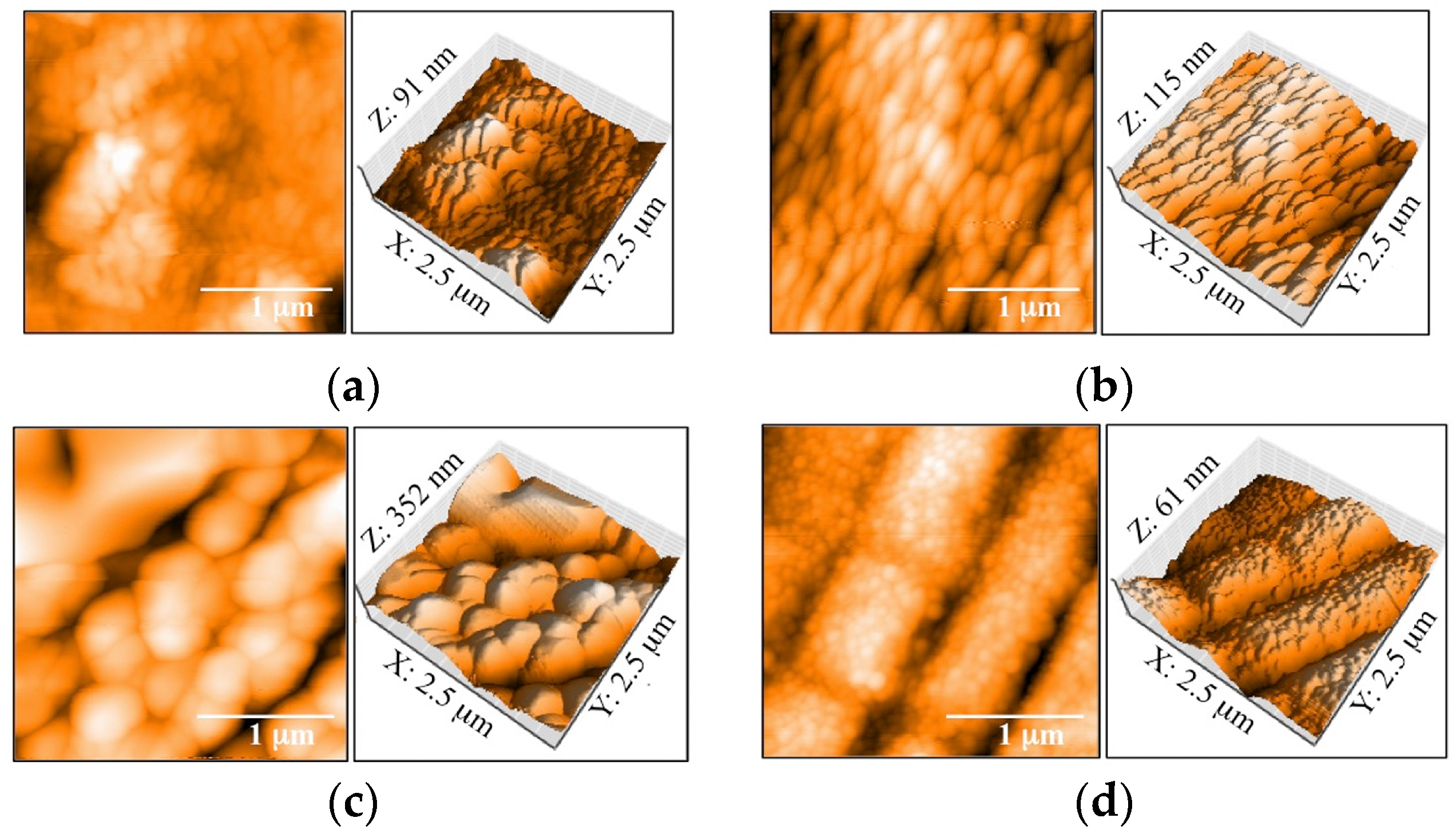
3.1. Morphology and Surface Roughness
3.2. Surface Wettability
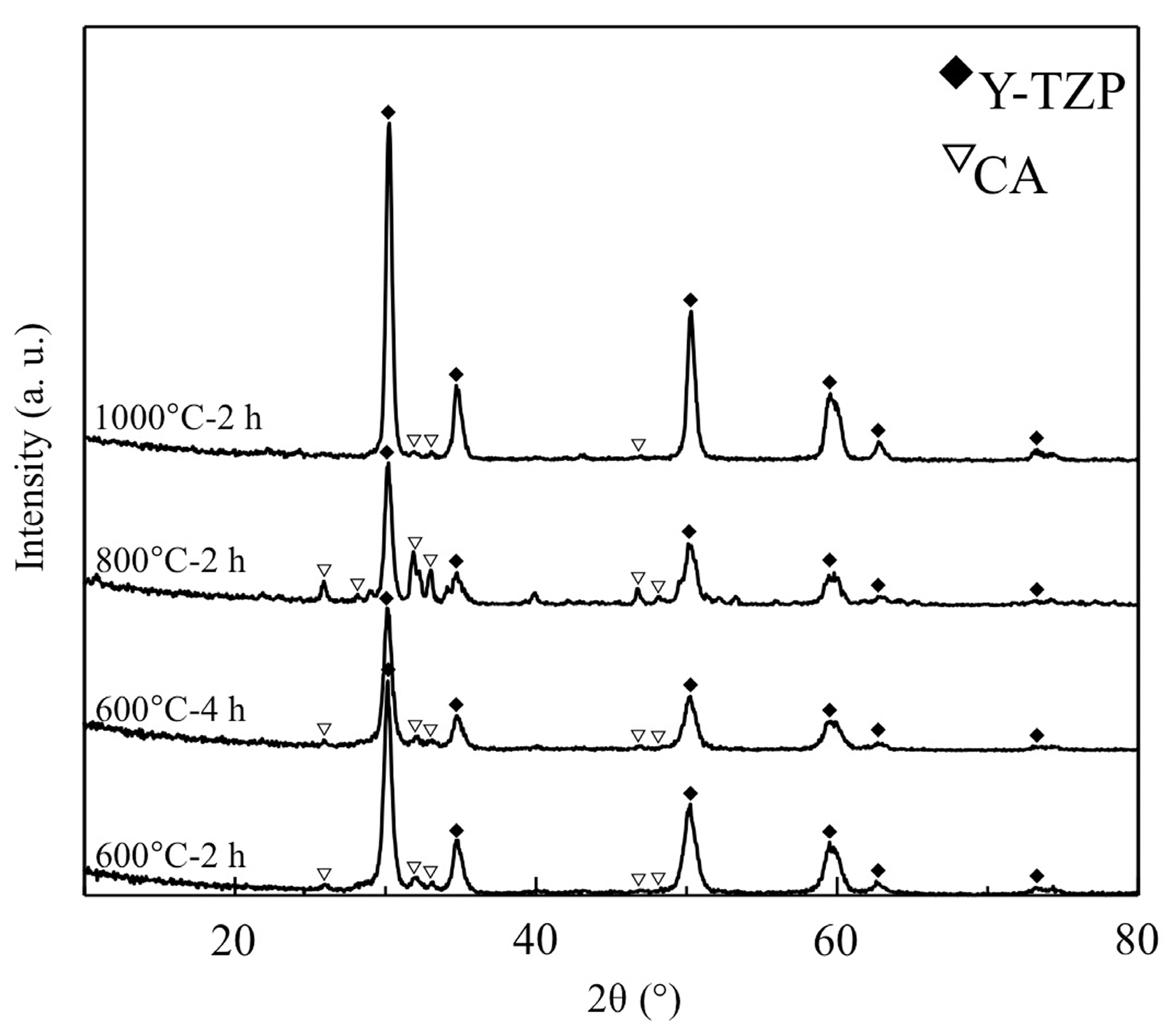
3.3. XRD Analysis of CA Coating Films
3.4. Adhesiveness of CA Coating Films
3.5. Durability of CA Coating Films
3.6. SBF Immersion
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pieralli, S.; Kohal, R.J.; Jung, R.E.; Vach, K.; Spies, B.C. Clinical outcomes of zirconia dental implants: A systematic review. J. Dent. Res. 2017, 96, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Özkurt, Z.; Kazazoğlu, E. Zirconia dental implants: A literature review. J. Oral. Implantol. 2011, 37, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.G. Perspective on the development of high-toughness ceramics. J. Am. Ceram. Soc. 1990, 73, 187–206. [Google Scholar] [CrossRef]
- Scarano, A.; Piattelli, M.; Caputi, S.; Favero, G.A.; Piattelli, A. Bacterial adhesion commercially pure titanium and zirconium oxide disks: An in vivo human study. J. Periodontol. 2004, 75, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Denry, I.; Kelly, J.R. State of the art of zirconia for dental applications. Dent. Mater. 2008, 24, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Bräunemark, P.I. Osseointegration and its experimental background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef]
- Hoffmann, O.; Angelov, N.; Gallez, F.; Jung, R.E.; Weber, F.E. The zirconia implant-bone interface: A preliminary histologic evaluation in rabbits. Int. J. Oral. Maxillofac. Implant. 2008, 23, 691–695. [Google Scholar]
- Hoffmann, O.; Angelov, N.; Zafiropoulos, G.G.; Andreana, S. Osseointergration of zirconia implants with different surface characteristics: An evaluation in rabbits. Int. J. Oral. Maxillofac. Implant. 2012, 27, 352–358. [Google Scholar]
- Hirota, M.; Hayakawa, T.; Ohkubo, C.; Sato, M.; Hara, H.; Toyama, T.; Tanaka, Y. Bone responses to zirconia implants with a thin carbonate-containing hydroxyapatite coating using a molecular precursor method. J. Biomed. Mater. Res. Part B 2014, 102, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Hara, H.; Tomikoshi, H.; Mochizuki, C. Film fabrication of monolithic hydroxyapatite by an application of molecular precursor method. Proceedings of International Symposium on Advance Materials (ISAM/ISAT2), Islamabad, Pakistan, 23–27 September 2013. [Google Scholar]
- Takahashi, K.; Hayakawa, T.; Yoshinari, M.; Hara, H.; Mochizuki, C.; Sato, M.; Nemoto, K. Molecular precursor method for thin calcium phosphate coating on titanium. Thin Solid Films 2005, 484, 1–9. [Google Scholar] [CrossRef]
- Hayakawa, T.; Takahashi, K.; Okada, H.; Yoshinari, M.; Hara, H.; Mochizuki, C.; Yamamoto, H.; Sato, M. Effect of thin carbonate-containing apatite (CA) coating of titanium fiber mesh on trabecular bone response. J. Mater. Sci. Mater. Med. 2008, 19, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, T.; Fukayo, Y.; Nakaoka, K.; Hamada, Y.; Hayakawa, T. Tissue response of surface modified three dimensional titanium fiber structure. J. Hard Tissue Biol. 2014, 23, 137–148. [Google Scholar] [CrossRef]
- Pushkareva, M.; Adrien, J.; Marie, E.; Segurado, J.; Llorca, J.; Weck, A. Three-dimensional investigation of grain orientation effects on void growth in commerciall pure titanium. Mater. Sci. Eng. A 2016, 671, 221–232. [Google Scholar] [CrossRef]
- Steinmann, P.A.; Tardy, Y.; Hintermann, H.E. Adhesion testing by the scratch test method: The influence of intrinsic and extrinsic parameters on the critical load. Thin Solid Films 1987, 154, 333–349. [Google Scholar] [CrossRef]
- Hanawa, T.; Ota, M. Calcium phosphate naturally formed on titanium in electrolyte solution. Biomaterials 1991, 12, 767–774. [Google Scholar] [CrossRef]
- Garvie, R.C.; Hannink, R.H.; Pascoe, R.T. Ceramic steel? Nature 1975, 258, 703–704. [Google Scholar] [CrossRef]
- Rohanizadeh, R.; LeGeros, R.Z.; Fan, D.; Jean, A.; Daculsi, G. Ultrastructural properties of laser-irradiated and heat-treated dentin. J. Dent. Res. 1999, 78, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takamada, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, C.; Sasaki, Y.; Hara, H.; Sato, M.; Hayakawa, T.; Yang, F.; Hu, X.; Shen, H.; Wang, S. Crystallinity controlled apatites through a Ca-EDTA complex and their porous composites with PLGA. J. Biomed. Mater. Res. Part B 2009, 90, 290–301. [Google Scholar]
- Hayakawa, T.; Mochizuki, C.; Hara, H.; Sato, M.; Yang, F.; Shen, H.; Wang, S. In vivo evaluation of composites of PLGA and apatite with two different levels of crystallinity. J. Mater. Sci. Mater. Med. 2010, 21, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Yoshinari, M.; Toyama, T.; Hayakawa, T.; Ohkubo, C. Effects of a multilayered DNA/protamine coating on titanium implants on bone responses. J. Biomed. Mater. Res. Part A 2016, 104, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Maletta, C.; Inchingolo, F.; Alfano, M.; Tatullo, M. Three-point bending tests of zirconia core/veneer ceramics for dental restorations. Int. J. Dent. 2013, 2013, 831976. [Google Scholar] [CrossRef] [PubMed]

| Ion | Concentration (mmol/L) | Ion | Concentration (mmol/L) |
|---|---|---|---|
| Na+ | 142 | Cl− | 145 |
| K+ | 5.81 | HPO42− | 0.778 |
| Mg2+ | 0.811 | SO42− | 0.811 |
| Ca2+ | 1.26 | HCO3− | 4.17 |
| Specimen | Ra (nm) | Sa (nm) |
|---|---|---|
| 600 °C-2 h | 10.31 (3.67) * | 10.44 (2.94) * |
| 800 °C-2 h | 11.93 (3.84) * | 11.12 (3.08) * |
| 1000 °C-2 h | 39.21 (5.71) | 41.94 (4.85) |
| 600 °C-4 h | 6.49 (0.74) * | 6.41 (0.85) * |
| θ (°) | Specimen |
|---|---|
| 41.89 a (4.42) | 600 °C-2 h |
| 18.14 (6.97) | 800 °C-2 h |
| 47.92 a,b (4.39) | 1000 °C-2 h |
| 51.37 b,c (2.80) | 600 °C-4 h |
| 56.73 c (2.96) | Non-Treated Y-TZP |
| Lc Value (mN) | Specimen |
|---|---|
| 169.96 (20.40) | 600 °C-2 h |
| 163.32 (16.09) | 800 °C-2 h |
| 176.67 (2.16) | 1000 °C-2 h |
| 150.56 (9.81) | 600 °C-4 h |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirota, M.; Mochizuki, C.; Sato, M.; Hayakawa, T. Influence of Heating Conditions for Formation of a Thin Apatite Film on Zirconia Using a Molecular Precursor Method. Coatings 2017, 7, 69. https://doi.org/10.3390/coatings7050069
Hirota M, Mochizuki C, Sato M, Hayakawa T. Influence of Heating Conditions for Formation of a Thin Apatite Film on Zirconia Using a Molecular Precursor Method. Coatings. 2017; 7(5):69. https://doi.org/10.3390/coatings7050069
Chicago/Turabian StyleHirota, Masatsugu, Chihiro Mochizuki, Mitsunobu Sato, and Tohru Hayakawa. 2017. "Influence of Heating Conditions for Formation of a Thin Apatite Film on Zirconia Using a Molecular Precursor Method" Coatings 7, no. 5: 69. https://doi.org/10.3390/coatings7050069






